Phenyl-C61-Butyric Acid Methyl Ester Hybrid Solution for Efficient CH3NH3PbI3 Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
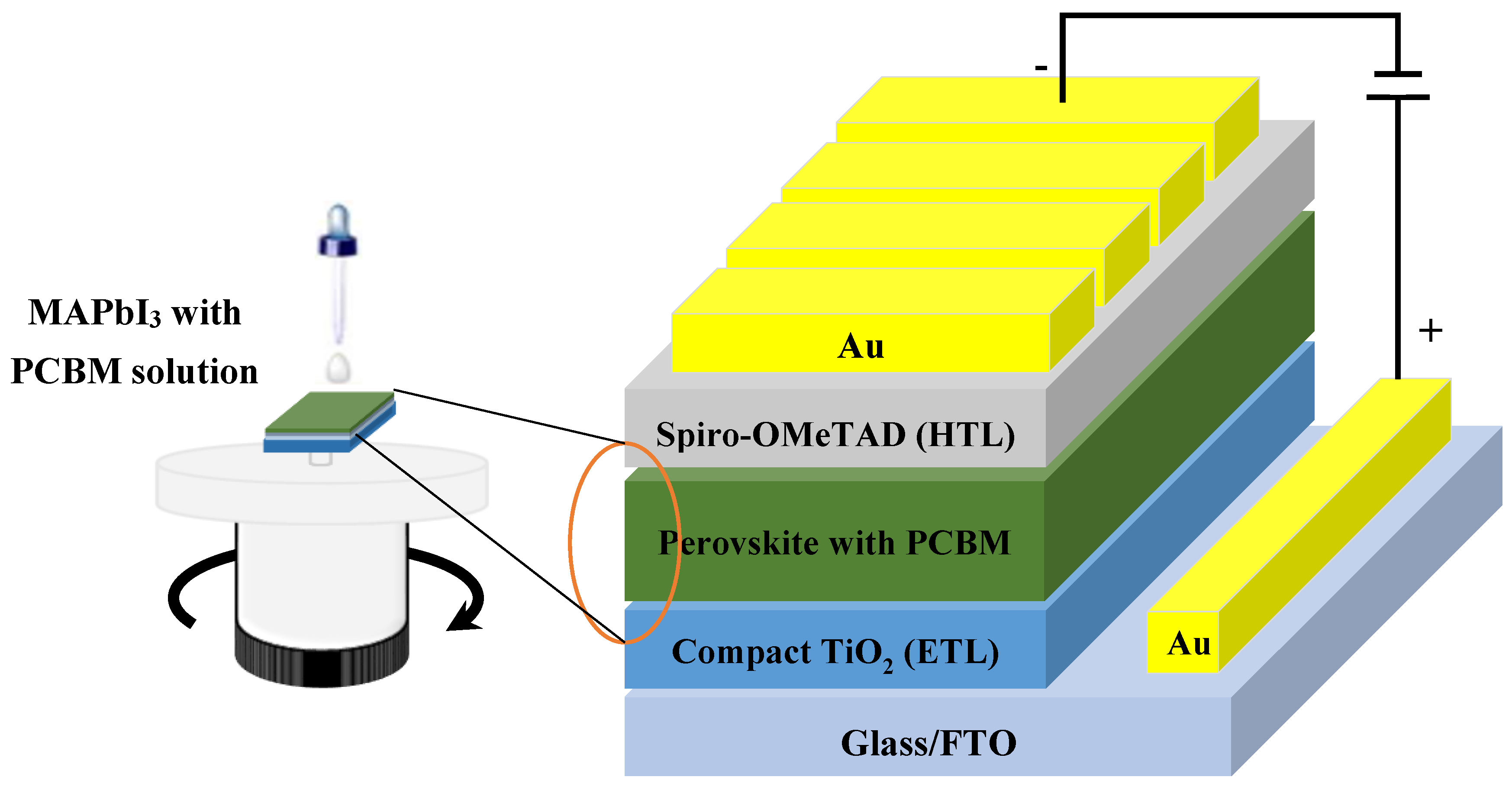
2.2. Film Formation and Device Fabrication Procedure of n-i-p Perovskite Solar Cells (PSCs)
2.3. Characterizations
3. Results and Discussion
3.1. Crystal Structure and FTIR Spectra of Phenyl-C61-Butyric Acid Methyl Ester– Methyl Ammonium Lead Iodide (PCBM-MAPbI3) Hybrid Film
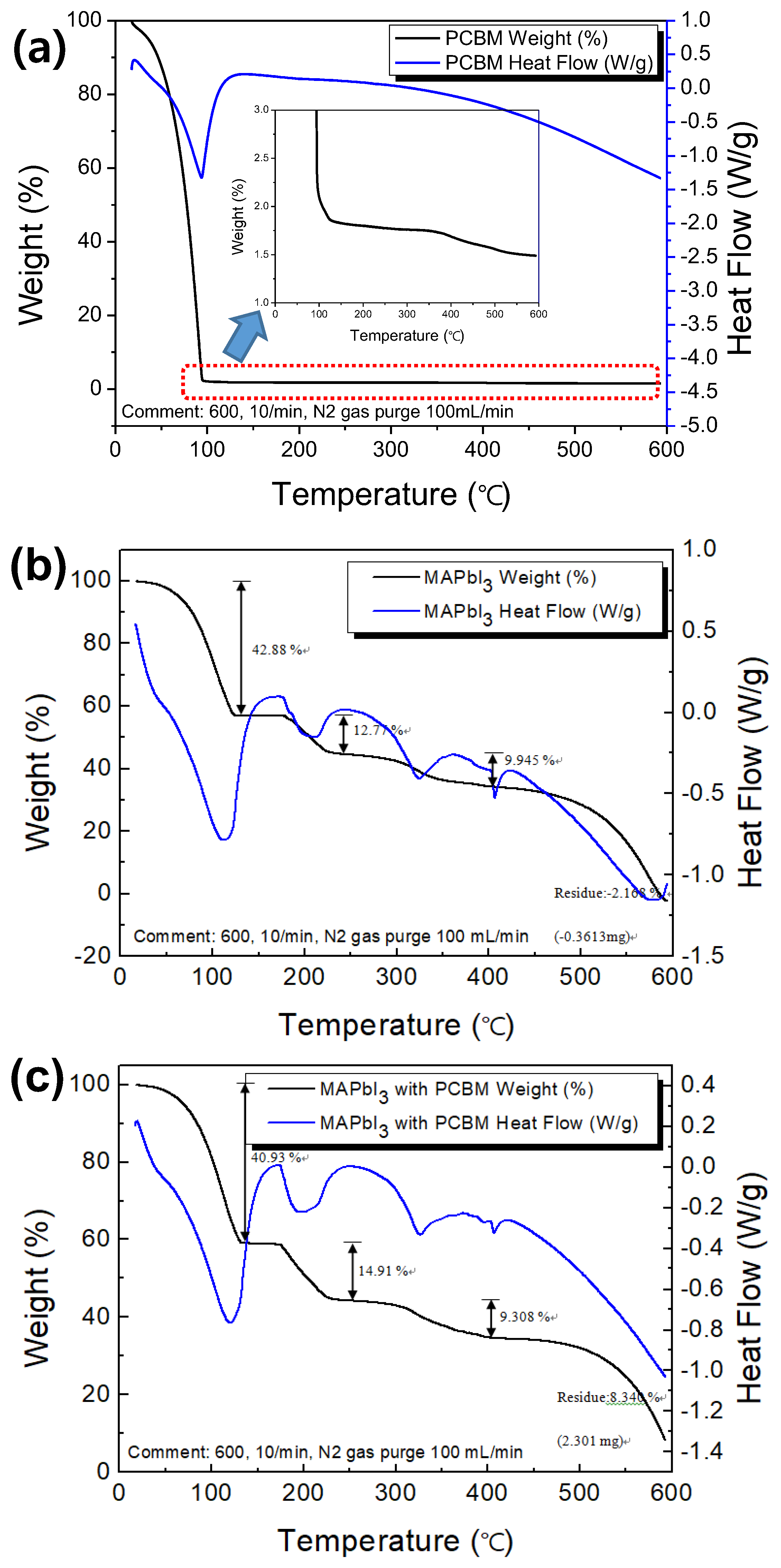
3.2. Thermal Behavior of MAPbI3, PCBM, and MAPbI3 with PCBM
3.3. Photovoltaic Performance of Normal (n-i-p) Planar PSCs
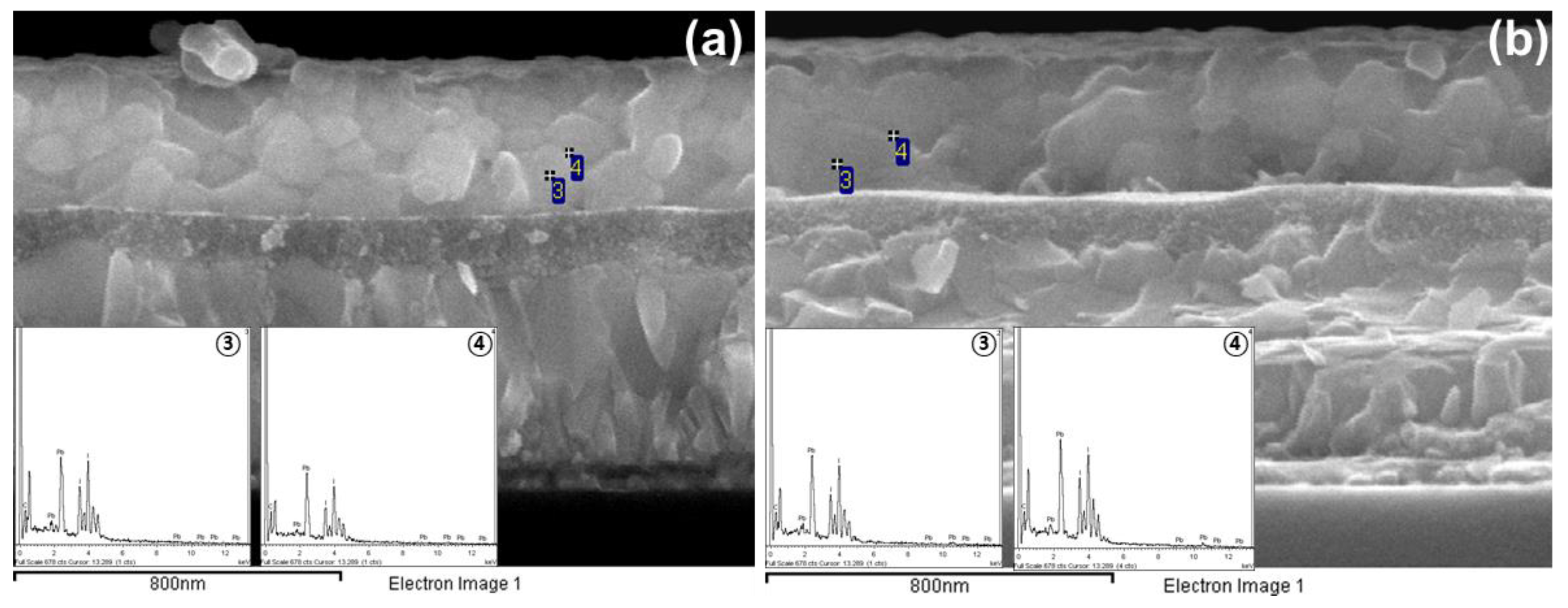
3.4. Difference in C Content Between Grain Interior and Grain Boundaries
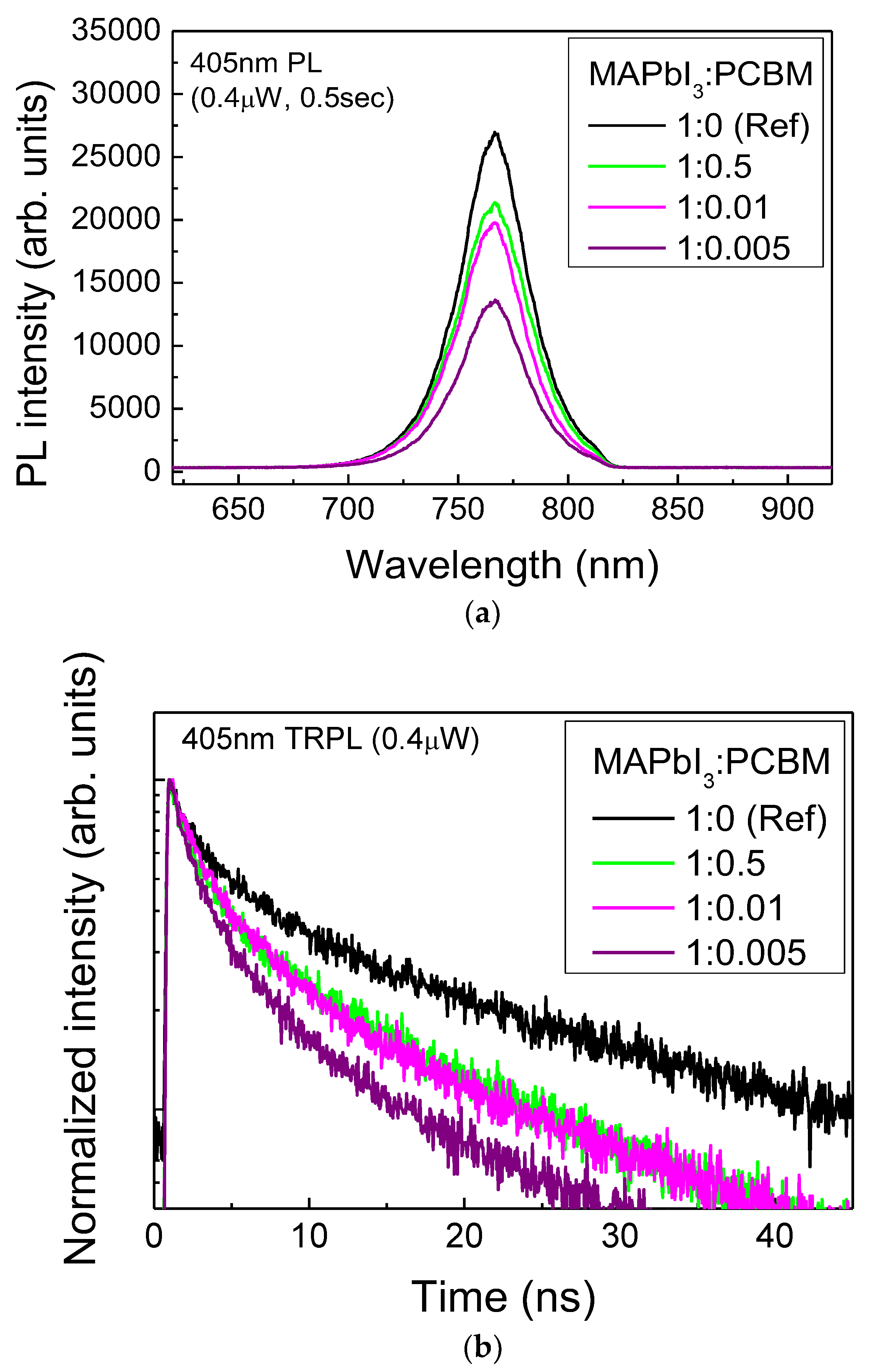
3.5. Steady-State and TRPL Decay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tonui, P.; Oseni, S.O.; Sharm, G.; Yan, Q.; Mola, G.T. Perovskites photovoltaic solar cells: An overview of current status. Renew. Sust. Energ. Rev. 2018, 91, 1025–1044. [Google Scholar] [CrossRef]
- Fang, F.; Chen, J.; Wu, G.; Chen, H. Highly efficient perovskite solar cells fabricated by simplified one-step deposition method with non-halogenated anti-solvents. Org. Electron. 2018, 59, 330–336. [Google Scholar] [CrossRef]
- Cao, W.; Xue, J. Recent progress in organic photovoltaics: Device architecture and optical design. Energy Environ. Sci. 2014, 7, 2123–2144. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- D’Innocenzo, V.; Grancini, G.; Alcocer, M.J.; Kandada, A.R.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons versus free charges in organo-lead trihalide perovskites. Nat. Commun. 2014, 5, 3586. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Si, D.; Song, X.; Wang, K.; Wang, S.; Zhao, Z.; Hao, C.; Wei, L.; Shi, Y. Pseudo halogen-based 2D perovskite: A more complex thermal degradation mechanism than 3D perovskite. Inorg. Chem. 2018, 57, 2045–2050. [Google Scholar] [CrossRef]
- Liu, D.B.; Wang, G.; Wu, F.; Wu, R.; Chen, T.; Ding, B.F.; Song, Q.L. Crystallization process of perovskite modified by adding lead acetate in precursor solution for better morphology and higher device efficiency. Org. Electron. 2017, 43, 189–195. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, T.; Leng, C.; Zang, Z.; Wang, M.; Hu, W.; Tang, X.; Lu, S.; Fang, L.; Zhou, M. Performance improvement of perovskite solar cells by employing a CdSe quantum dot/PCBM composite as an electron transport layer. J. Mater. Chem. A 2017, 5, 17499–17505. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; Wang, H.; Hu, W.; Yu, W.; Sheikh, A.D.; Wu, T. Ambipolar solution-processed hybrid perovskite phototransistors. Nat. Commun. 2015, 6, 8238. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Chen, W.; Yue, Y.; Cai, M.; Xie, F.; Bi, E.; Islam, A.; Han, L. Perovskite solar cells with 18.21% efficiency and area over 1 cm2 fabricated by heterojunction engineering. Nat. Energy 2016, 1, 16148. [Google Scholar] [CrossRef]
- Rafael, S.T.; Jorge, P.; Inés, G.B.; Silvia, C.; Ivet, K.; Ramón, T.Z.; Nazario, M.; Juan, L.D. Modified fullerenes for efficient electron transport layer-free perovskite/fullerene blend-based solar cells. ChemSusChem 2017, 10, 2023–2029. [Google Scholar]
- Silvia, C.; Michael, S.; Wolfgang, R.T.; Philippe, J.H.; Sebastian, F.W.; Konrad, D.; Silver, H.T.-C.; Amita, U.; Shaik, M.Z.; Anders, H.; et al. Poly(ethylene glycol)–[60]Fullerene-based materials for perovskite solar cells with improved moisture resistance and reduced hysteresis. ChemSusChem 2018, 11, 1032–1039. [Google Scholar]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.Y.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.S.; Park, N.G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via Lewis base adduct of lead(II) iodide. JACS 2015, 137, 8696–8699. [Google Scholar] [CrossRef]
- Son, D.Y.; Lee, J.W.; Choi, Y.J.; Jang, I.H.; Lee, S.H.; Yoo, P.J.; Shin, H.J.; Ahn, N.Y.; Choi, M.S.; Kim, D.H.; et al. Self-formed grain boundary healing layer for highly efficient CH3NH3PbI3 perovskite solar cells. Nat. Energy 2016, 1, 16081. [Google Scholar] [CrossRef]
- Xu, J.; Buin, A.; Ip, A.H.; Li, W.; Voznyy, O.; Comin, R.; Yuan, M.; Jeon, S.; Ning, Z.; McDowell, J.J.; et al. Perovskite–fullerene hybrid materials suppress hysteresis in planar diodes. Nat. Commun. 2015, 6, 7081. [Google Scholar] [CrossRef]
- Luo, S.; Daoud, W.A.; Luo. Crystal structure formation of CH3NH3PbI3-xClx perovskite. Materials 2016, 9, 123. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-Ray Diffraction, 3rd ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 2001; pp. 167–171. ISBN 0-201-61091-4. [Google Scholar]
- Tian, C.; Castro, E.; Wang, T.; German, B.S.; Gloria, R.; Luis, E. Improved performance and stability of inverted planar perovskite solar cells using fulleropyrrolidine Layers. ACS Appl. Mater. Interfaces 2016, 8, 31426–31432. [Google Scholar] [CrossRef]
- Jorge, P.; Juan, L.D.; Ramόn, T.Z. Physicochemical phenomena and application in solar cells of perovskite:fullerene films. J. Phys. Chem. Lett. 2018, 9, 2893–2902. [Google Scholar]
- Liu, X.; Huang, P.; Dong, Q.; Wang, Z.; Zhang, K.; Yu, H.; Lei, M.; Zhou, Y.; Song, B.; Li, Y. Enhancement of the efficiency and stability of planar p-i-n perovskite solar cells via incorporation of an amine-modified fullerene derivative as a cathode buffer layer. Sci. China Chem. 2016, 60, 136–143. [Google Scholar] [CrossRef]
- Redinger, A.; Levcenko, S.; Hages, C.J.; Greiner, D.; Kaufmann, C.A.; Unold, T. Time resolved photoluminescence on Cu(In, Ga)Se2 absorbers: Distinguishing degradation and trap states. Appl. Phys. Lett. 2017, 110, 122104. [Google Scholar] [CrossRef]


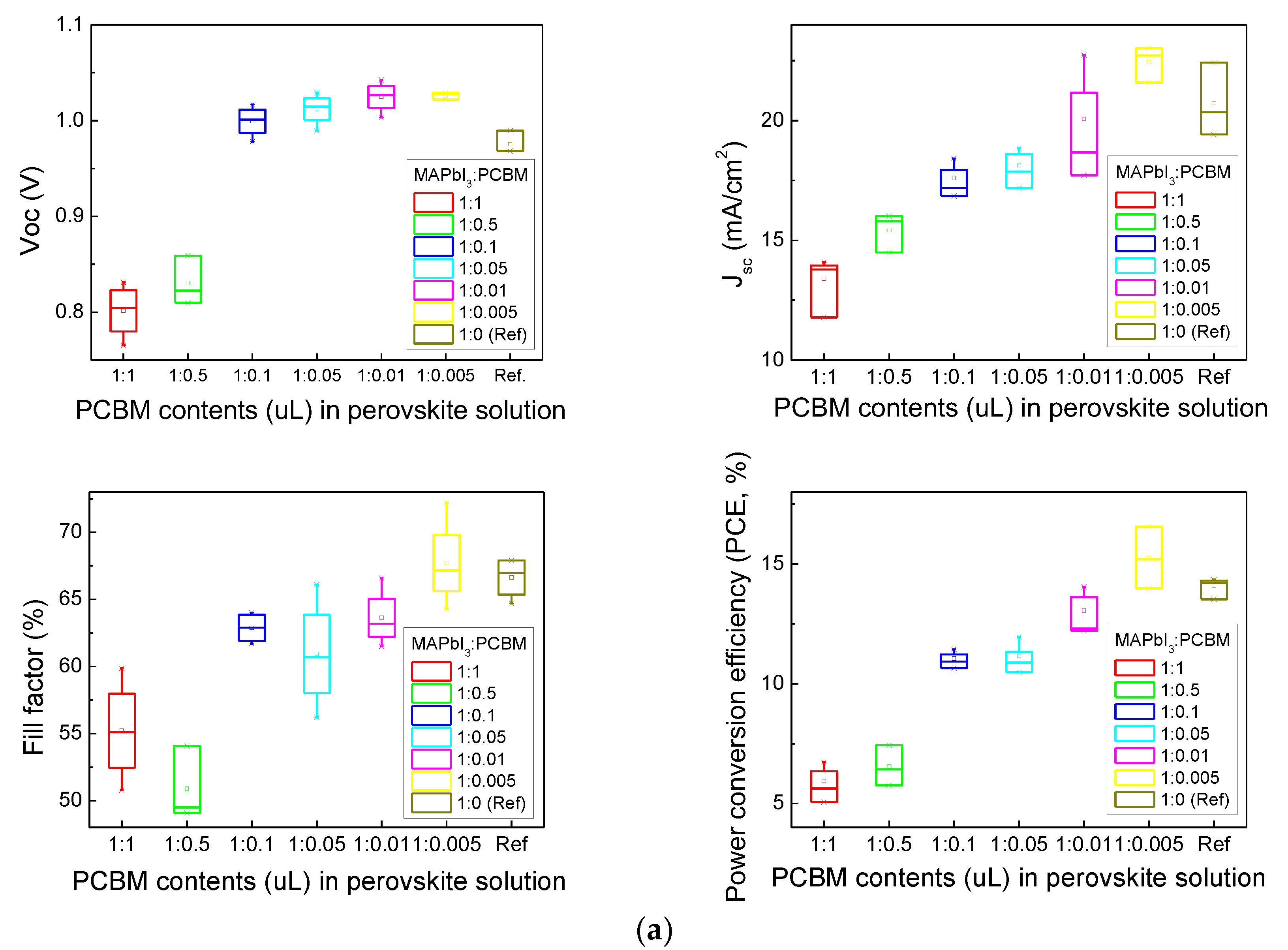

| Structure | MAPbI3:PCBM Ratio (volume %) | Jsc (mA/cm2) | Voc (V) | FF (%) | Efficiency (%) |
|---|---|---|---|---|---|
| FTO/TiO2/PCBM-MAPbI3/Spiro-OMeTAD/Au | 1:1 | 13.79 | 0.8150 | 59.85 | 6.73 |
| 1:0.5 | 16.01 | 0.8591 | 54.07 | 7.43 | |
| 1:0.1 | 17.37 | 1.006 | 61.7 | 11.43 | |
| 1:0.05 | 17.87 | 1.012 | 66.1 | 11.95 | |
| 1:0.01 | 22.74 | 1.0032 | 61.5 | 14.04 | |
| 1:0.005 | 22.76 | 1.0089 | 72.2 | 16.55 | |
| FTO/TiO2/MAPbI3/Spiro-OMeTAD/Au | 1:0 (Ref) | 21.82 | 0.9683 | 67.9 | 14.34 |
| Samples | MAPBI3:PCBM = 1:0.005 | Only MAPbI3 | ||||
|---|---|---|---|---|---|---|
| Element | Intra-Grain (wt%) | Grain Boundary (wt%) | Difference | Intra-Grain (wt%) | Grain Boundary (wt%) | Difference |
| C K | 21.29 | 23.83 | +2.54 | 20.75 | 17.53 | –3.22 |
| I L | 48.48 | 44.69 | +3.79 | 48.72 | 50.61 | +1.89 |
| Pb M | 30.23 | 31.48 | +1.25 | 30.53 | 31.87 | +1.34 |
| Totals | 100.00 | 100.00 | 100.00 | 100.00 | ||
| 1:0.5 | 1:0.01 | 1:0.005 | 1:0 (Ref) | |
|---|---|---|---|---|
| A1 | 0.44276 | 0.41395 | 0.37235 | 0.70634 |
| t1 | 13.18063 | 12.4614 | 10.11754 | 1.96384 |
| A2 | 0.94474 | 0.93156 | 1.1608 | 0.48923 |
| t2 | 1.67703 | 2.06318 | 1.62432 | 16.78579 |
| τ | 10.72439151 | 9.63879019 | 7.284585908 | 14.64394564 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, M.; Oh, J.; Kwon, N.; Kang, Y.; Yang, J. Phenyl-C61-Butyric Acid Methyl Ester Hybrid Solution for Efficient CH3NH3PbI3 Perovskite Solar Cells. Sustainability 2019, 11, 3867. https://doi.org/10.3390/su11143867
Kim M, Kim M, Oh J, Kwon N, Kang Y, Yang J. Phenyl-C61-Butyric Acid Methyl Ester Hybrid Solution for Efficient CH3NH3PbI3 Perovskite Solar Cells. Sustainability. 2019; 11(14):3867. https://doi.org/10.3390/su11143867
Chicago/Turabian StyleKim, MiJoung, MoonHoe Kim, JungSeock Oh, NamHee Kwon, Yoonmook Kang, and JungYup Yang. 2019. "Phenyl-C61-Butyric Acid Methyl Ester Hybrid Solution for Efficient CH3NH3PbI3 Perovskite Solar Cells" Sustainability 11, no. 14: 3867. https://doi.org/10.3390/su11143867
APA StyleKim, M., Kim, M., Oh, J., Kwon, N., Kang, Y., & Yang, J. (2019). Phenyl-C61-Butyric Acid Methyl Ester Hybrid Solution for Efficient CH3NH3PbI3 Perovskite Solar Cells. Sustainability, 11(14), 3867. https://doi.org/10.3390/su11143867






