Comparison between Daytime and Nighttime Scenery Focusing on Restorative and Recovery Effect
Abstract
1. Introduction
1.1. Background
1.2. Landscape Preference and Landscape Restorative and Recovery Potential
1.3. Research Hypotheses
- H1: Daytime scenes will be rated more positively than nighttime scenes in terms of self-reported perceived restorative and recovery scale.
- H2: The signals and power spectrum of participants’ EEG will show significantly different results on daytime and nighttime sceneries.
- H3: There will be a significant correlation between the EEG band depending on the region of the brain.
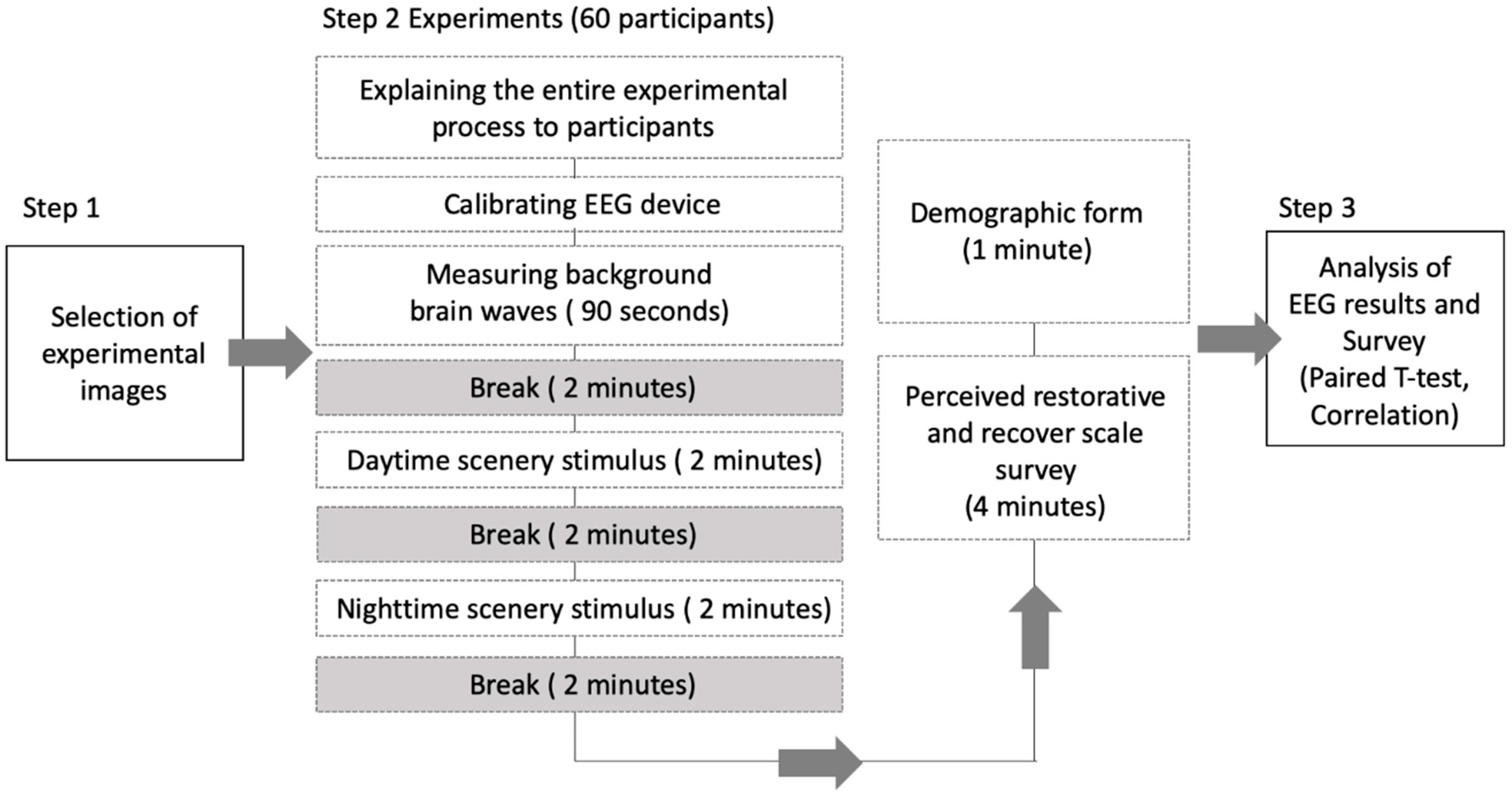
2. Methods and Data
2.1. Participants
2.2. Experimental Images
2.3. Research Procedure
2.4. Outcome Measures
2.4.1. Questionnaire for Subjective Preferences
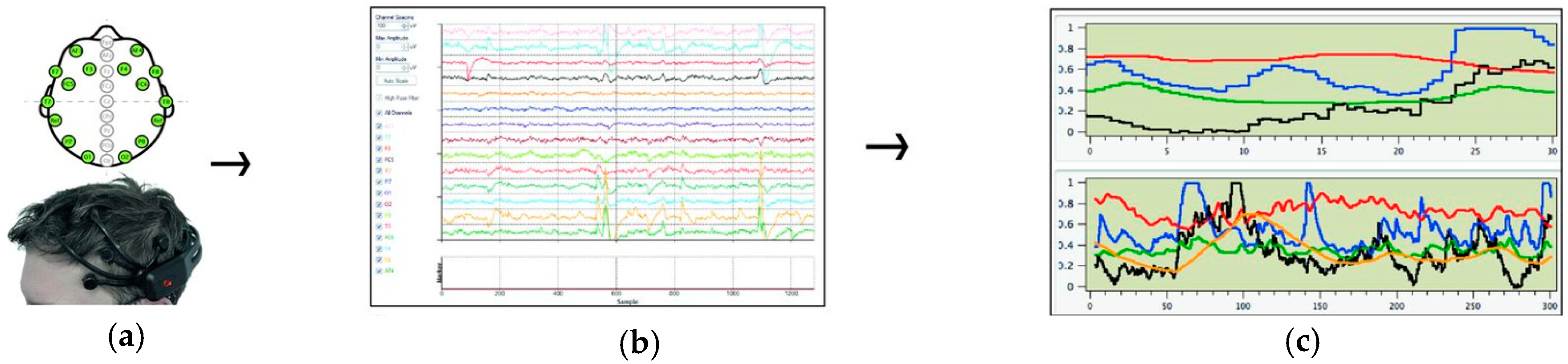
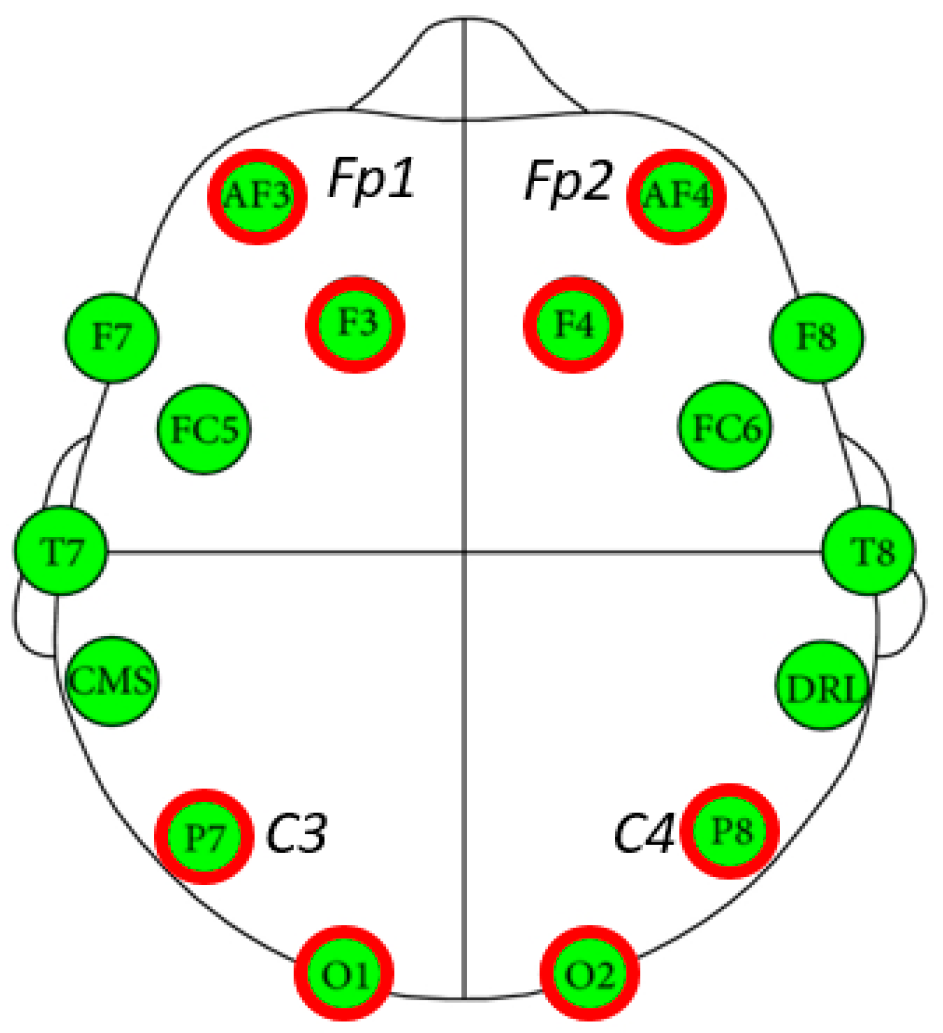
2.4.2. EEG outcome Measures
2.5. Data Analysis
3. Results
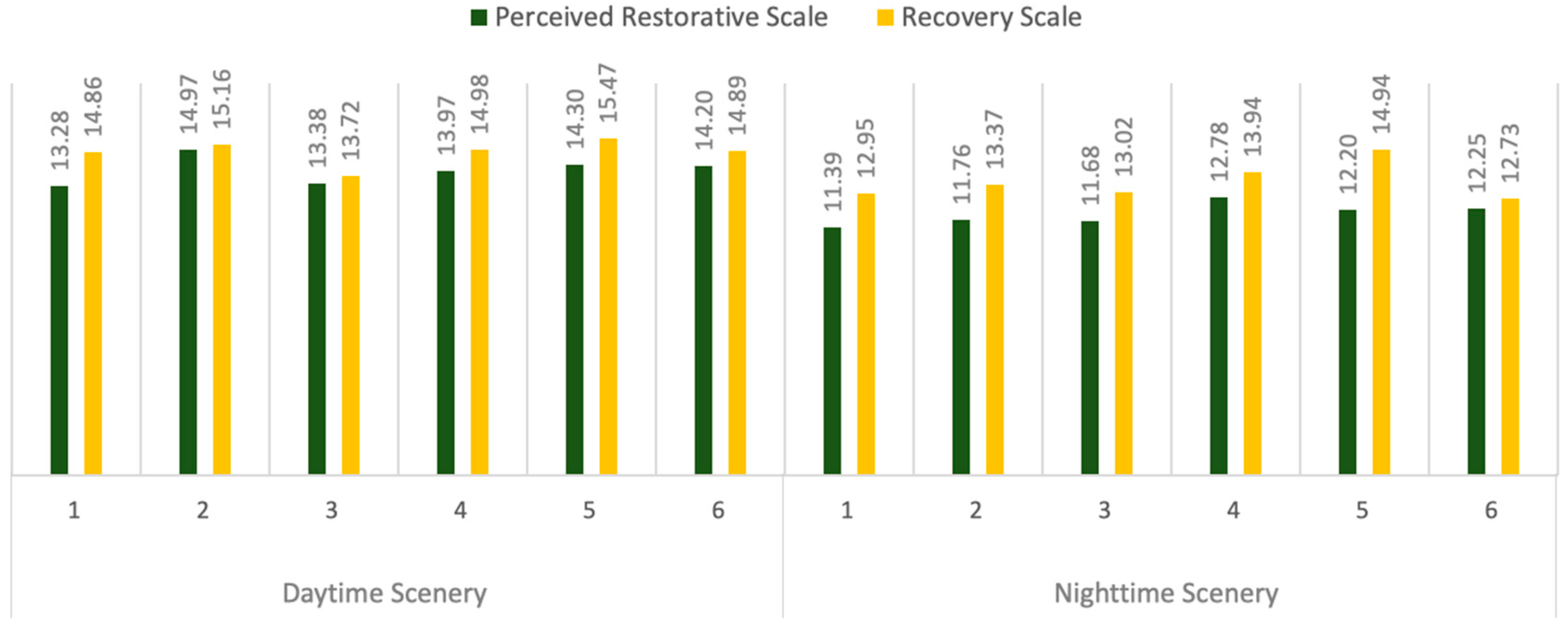
3.1. Subjective Preferences
3.2. Activity of EEG Bands
3.3. Comparison of EEG Activity on the Brain Region
3.4. Correlation Analysis of EEG Bands
3.4.1. Daytime Scenery Stimuli
3.4.2. Nighttime Scenery Stimuli
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Appleton, J. Landscape evaluation: The theoretical vacuum. Trans. Inst. Br. Geogr. 1975, 66, 120–123. [Google Scholar] [CrossRef]
- Appleton, J. The Experience of Landscape; Wiley: Chichester, UK, 1996. [Google Scholar]
- Hartig, T. Nature experience in transactional perspective. Landsc. Urban Plan. 1993, 25, 17–36. [Google Scholar] [CrossRef]
- Kaplan, R. The nature of the view from home: Psychological benefits. Environ. Behav. 2001, 33, 507–542. [Google Scholar] [CrossRef]
- Lu, D.; Burley, J.; Crawford, P.; Schutzki, R.; Loures, L. Quantitative methods in environmental and visual quality mapping and assessment: A Muskegon, Michigan watershed case study with urban planning implications. In Advances in Spatial Planning; IntechOpen: London, UK, 2012. [Google Scholar]
- Partin, S.; Burley, J.B.; Schutzki, R.; Crawford, P. Concordance between Photographs and Computer Generated 3D Models in a Michigan Highway Transportation Setting. Anhalt University of Applied Sciences, Wichmann: Köthen, Germany. 2012, pp. 482–489. Available online: http://193.25.34.143/landschaftsinformatik-4.2.6/fileadmin/user_upload/_temp_/2012/Proceedings/Buhmann_2012_56_Partin_et_al.pdf (accessed on 7 June 2019).
- Joliet, F.; Landon, W.; Yu, W.; Burley, J.B. The silent language of artistic representations in landscape: Alentejo (Portugal), Yellowstone (USA) and Kaifeng (PR of China). Int. J. Energy Environ. 2011, 5, 618–628. [Google Scholar]
- Mo, F.; Le Cléach, G.; Sales, M.; Deyoung, G.; Burley, J. Visual and environmental quality perception and preference in the People’s Republic of China, France, and Portugal. Int. J. Energy Environ. 2011, 5, 549–555. [Google Scholar]
- Mazure, A.; Burley, J. An aesthetic, economic, and ecological equation/theories for predicting environmental quality: Including a GIS-based remote access application. In Proceedings of the Our Shared Landscape: Integrating Ecological, Socio-Economic and Aesthetics Aspects in Landscape Planning and Management, Ascona, Switzerland, 26 May 2005; pp. 68–69. [Google Scholar]
- Kaplan, R.; Kaplan, S.; Ryan, R. With People in Mind: Design and Management of Everyday Nature; Island Press: London, UK, 1998. [Google Scholar]
- Ulrich, R.S. View through a window may influence recovery from surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; CUP Archive: Cambridge, UK, 1989. [Google Scholar]
- Han, K.-T. A reliable and valid self-rating measure of the restorative quality of natural environments. Landsc. Urban Plan. 2003, 64, 209–232. [Google Scholar] [CrossRef]
- Hull IV, R.B.; Michael, S.E. Nature-based recreation, mood change, and stress restoration. Leis. Sci. 1995, 17, 1–14. [Google Scholar] [CrossRef]
- Kyoung, Y.-Y. Beneficial effect of forest landscape on relieving stress based on psychological and physiological measures. J. Korean Inst. Landsc. Archit. 2003, 31, 70–82. [Google Scholar]
- Yi, Y. Characteristic analysis of natural landscape: Based on the assessments of naturalness by landscape professionals and laypersons. J. Korean Inst. Landsc. Archit. 2004, 31, 1–14. [Google Scholar]
- Yi, Y.-K.; Yi, P.-I. The Impact of Landscape Type on Urban Office Workers’ Stress and Cognitive Performance-Comparison between Natural and Urban Landscape. J. Korean Inst. Landsc. Archit. 2006, 33, 1–11. [Google Scholar]
- Ulrich, R.S. Natural versus urban scenes: Some psychophysiological effects. Environ. Behav. 1981, 13, 523–556. [Google Scholar] [CrossRef]
- Ulrich, R.S. Visual landscapes and psychological well-being. Landsc. Res. 1979, 4, 17–23. [Google Scholar] [CrossRef]
- Parsons, R.J. Recovery from Stress during Exposure to Videotaped Outdoor Environments; The University of Arizona: Tucson, AZ, USA, 1991. [Google Scholar]
- Parsons, R.; Tassinary, L.G.; Ulrich, R.S.; Hebl, M.R.; Grossman-Alexander, M. The view from the road: Implications for stress recovery and immunization. J. Environ. Psychol. 1998, 18, 113–140. [Google Scholar] [CrossRef]
- Ulrich, R.S. Influences of passive experiences with plants on individual well-being and health. Role Hortic. Hum. Well-Being Soc. Dev. 1992. Available online: http://tci.ncl.edu.tw/cgi-bin/gs32/gsweb.cgi?o=dnclret&s=id=%22RF10006648536%22.&searchmode=basic&tcihsspage=tcisearch_opt2_search (accessed on 15 June 2019).
- Van den Berg, A.E.; Koole, S.L.; Van der Wulp, N.Y. Environmental preference and restoration:(How) are they related? J. Environ. Psychol. 2003, 23, 135–146. [Google Scholar] [CrossRef]
- Park, J.-M.; Yoon, C.-S.; Park, E.-S. A Study on the characteristics of preferred housing interior image and sensibility of university students. J. Korean Hous. Assoc. 2011, 22, 97–106. [Google Scholar] [CrossRef]
- Roe, J.J.; Aspinall, P.A.; Mavros, P.; Coyne, R. Engaging the brain: The impact of natural versus urban scenes using novel EEG methods in an experimental setting. Environ. Sci. 2013, 1, 93–104. [Google Scholar] [CrossRef]
- Küller, R. A semantic test for use in cross-cultural studies. Man-Environ. Syst. 1979, 9, 253–256. [Google Scholar]
- Zuckerman, M. Development of a situation-specific trait-state test for the prediction and measurement of affective responses. J. Consult. Clin. Psychol. 1977, 45, 513. [Google Scholar] [CrossRef]
- Hartig, T.; Kaiser, F.G.; Bowler, P.A. Further Development of a Measure of Perceived Environmental Restorativeness; Institutet för bostads-och urbanforskning: Uppsala, Sweden, 1997. [Google Scholar]
- Tilley, S.; Neale, C.; Patuano, A.; Cinderby, S. Older people’s experiences of mobility and mood in an urban environment: A mixed methods approach using electroencephalography (EEG) and interviews. Int. J. Environ. Res. Public Health 2017, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Choi, Y.-R.; Chun, C.-Y. Effect of indoor air temperature on the occupants’ attention ability based on the electroencephalogram analysis. J. Archit. Inst. Korea 2012, 28, 217–225. [Google Scholar]
- Kim, J.-Y.; Lee, H.-S. A study on interior wall color based on measurement of emotional responses. Korean J. Sci. Emot. Sensib. 2009, 12, 205–214. [Google Scholar]
- Hwang, Y.-S.; Kim, S.-Y.; Kim, J.-Y. An analysis of youth EEG based on the emotional color scheme images by different space of community facilities. Korean Inst. Inter. Des. J. 2013, 22, 171–178. [Google Scholar]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Ottosson, J.; Grahn, P. The role of natural settings in crisis rehabilitation: How does the level of crisis influence the response to experiences of nature with regard to measures of rehabilitation? Landsc. Res. 2008, 33, 51–70. [Google Scholar] [CrossRef]
- Herzog, T.R.; Maguire, P.; Nebel, M.B. Others Assessing the restorative components of environments. J. Environ. Psychol. 2003, 23, 159–170. [Google Scholar] [CrossRef]
- Ajzen, I.; Fishbein, M. Understanding Attitudes and Predicting Social Behavior; Prentice Hall: Upper Saddle Riever, NJ, USA, 1980. [Google Scholar]
- Natori, Y.; Chenoweth, R. Differences in rural landscape perceptions and preferences between farmers and naturalists. J. Environ. Psychol. 2008, 28, 250–267. [Google Scholar] [CrossRef]
- Ivarsson, C.T.; Hagerhall, C.M. The perceived restorativeness of gardens–Assessing the restorativeness of a mixed built and natural scene type. Urban For. Urban Green. 2008, 7, 107–118. [Google Scholar] [CrossRef]
- Ulrich, R.S. Aesthetic and affective response to natural environment. In Behavior and the Natural Environment; Springer: Berlin, Germany, 1983; pp. 85–125. [Google Scholar]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Herzog, T.R.; Black, A.M.; Fountaine, K.A.; Knotts, D.J. Reflection and attentional recovery as distinctive benefits of restorative environments. J. Environ. Psychol. 1997, 17, 165–170. [Google Scholar] [CrossRef]
- Purcell, T.; Peron, E.; Berto, R. Why do preferences differ between scene types? Environ. Behav. 2001, 33, 93–106. [Google Scholar] [CrossRef]
- Staats, H.; Kieviet, A.; Hartig, T. Where to recover from attentional fatigue: An expectancy-value analysis of environmental preference. J. Environ. Psychol. 2003, 23, 147–157. [Google Scholar] [CrossRef]
- Herzog, T.R.; Bosley, P.J. Tranquility and preference as affective qualities of natural environments. J. Environ. Psychol. 1992, 12, 115–127. [Google Scholar] [CrossRef]
- Lee, S.H.; Hyun, M.H. The factor structure of the Korean version of the Perceived Restorativeness Scale (PRS). Korean J. Health Psychol. 2003, 8, 229–241. [Google Scholar]
- Lee, S.; Hyun, M. The comparison of natural environment and restorative environment in stress-buffering effects. Korean J. Psychol 2004, 9, 609–632. [Google Scholar]
- Hartig, T.; Kaiser, F.G.; Bowler, P.A. Psychological restoration in nature as a positive motivation for ecological behavior. Environ. Behav. 2001, 33, 590–607. [Google Scholar] [CrossRef]
- Ohly, H.; White, M.P.; Wheeler, B.W.; Bethel, A.; Ukoumunne, O.C.; Nikolaou, V.; Garside, R. Attention restoration theory: A systematic review of the attention restoration potential of exposure to natural environments. J. Toxicol. Environ. Health Part B 2016, 19, 305–343. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-Y.; Kim, S.-H. A Study on the Attention Concentration Properties in Convergent Exploration Situations in Cafe Space-Focusing on Gaze and Brain wave Data Analysis. Korean Inst. Inter. Des. J. 2016, 25, 30–40. [Google Scholar] [CrossRef]
- Emotiv Homepage. Available online: https://www.emotiv.com/ (accessed on 28 May 2019).
- Mavros, P.; Coyne, R.; Roe, J.; Aspinall, P. Engaging the brain: Implications of mobile EEG for spatial representation. In Proceedings of the 30th International Conference on Education and Research in Computer Aided Architectural Design in Europe, Prague, Czech Republic, 12–14 September 2012; Volume 2, pp. 657–665. [Google Scholar]
- White, M.; Smith, A.; Humphryes, K.; Pahl, S.; Snelling, D.; Depledge, M. Blue space: The importance of water for preference, affect, and restorativeness ratings of natural and built scenes. J. Environ. Psychol. 2010, 30, 482–493. [Google Scholar] [CrossRef]
- Klimesch, W. Memory processes described as brain oscillations in the EEG-alpha and theta bands. Psycoloquy 1995, 6. Available online: https://psycnet.apa.org/record/2000-03258-002 (accessed on 15 June 2019).
- Hutchinson, M. Mega Brain: New Tools and Techniques for Brain Growth and Mind Expansion; Beech Tree; William Morrow: New York, NY, USA, 1986. [Google Scholar]
- Levine, P.H. Transcendental meditation and the science of creative intelligence. Phi Delta Kappan 1972, 54, 231–235. [Google Scholar]
- Vecchiato, G.; Astolfi, L.; Tabarrini, A.; Salinari, S.; Mattia, D.; Cincotti, F.; Bianchi, L.; Sorrentino, D.; Aloise, F.; Soranzo, R.; et al. EEG analysis of the brain activity during the observation of commercial, political, or public service announcements. Comput. Intell. Neurosci. 2010, 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.; Thorson, E.; Rothschild, M.L.; McDonald, D.; Hirsch, J.; Goldstein, R. Attention to television: Intrastimulus effects of movement and scene changes on alpha variation over time. Int. J. Neurosci. 1985, 27, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Miller, W. A view from the inside: Brainwaves and television viewing. J. Q. 1985, 62, 508–514. [Google Scholar] [CrossRef]
- Weinstein, S.; Appel, V.; Weinstein, C. Brain-activity responses to magazine and television advertising. J. Advert. Res. 1980. Available online: https://psycnet.apa.org/record/1980-33676-001 (accessed on 15 June 2019).
- Mulholland, T.; Runnals, S. Increased occurrence of EEG alpha during increased attention. J. Psychol. 1962, 54, 317–330. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Chen, P.-K.; Hammitt, W.E.; Machnik, L. Psychophysiological responses and restorative values of wilderness environments. In Proceedings of the Science and Stewardship to Protect and Sustain Wilderness Values: Eighth World Wilderness Congress Symposium, Anchorage, AK, USA, 30 September–6 October 2005; Proceedings RMRS-P-49. Watson, A., Sproull, J., Dean, L., Eds.; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007; Volme 49, pp. 479–484. [Google Scholar]
- Fernández, T.; Harmony, T.; Rodríguez, M.; Bernal, J.; Silva, J.; Reyes, A.; Marosi, E. EEG activation patterns during the performance of tasks involving different components of mental calculation. Electroencephalogr. Clin. Neurophysiol. 1995, 94, 175–182. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, K.-W.; Lee, S.-R.; Jung, I.-C. The Study of Cognitive Functional Difference and EEG Spectrum Difference among Sasang Constitutions. J. Orient. Neuropsychiatry 2007, 18, 89–100. [Google Scholar]
- Kim, M.; Cheon, S.; Kang, Y. Use of Electroencephalography (EEG) for the Analysis of Emotional Perception and Fear to Nightscapes. Sustainability 2019, 11, 233. [Google Scholar] [CrossRef]
- Fitzgibbon, S.; Pope, K.; Mackenzie, L.; Clark, C.; Willoughby, J. Cognitive tasks augment gamma EEG power. Clin. Neurophysiol. 2004, 115, 1802–1809. [Google Scholar] [CrossRef]
- Tao, J.X.; Ray, A.; Hawes-Ebersole, S.; Ebersole, J.S. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia 2005, 46, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-J.; Lim, J.-H. Effect of Pilates Gymball Exercises on the Electroencephalogram and Cognitive Function in Mentally Disabled Persons. Phys. Korea 2017, 29, 227–233. [Google Scholar] [CrossRef][Green Version]
- Simons, R.F.; Detenber, B.H.; Cuthbert, B.N.; Schwartz, D.D.; Reiss, J.E. Attention to television: Alpha power and its relationship to image motion and emotional content. Media Psychol. 2003, 5, 283–301. [Google Scholar] [CrossRef]
| Set1: enclosed setting near stairs | Set2: open setting | Set3: enclosed setting surrounded by buildings | |
| Daytime |  |  |  |
| Nighttime |  |  |  |
| Set4: enclosed setting surrounded by trees | Set5: crooked path setting | Set6: enclosed setting surrounded by architectural features | |
| Daytime |  |  |  |
| Nighttime |  |  |  |
| Daytime Scenery | Nighttime Scenery | t | p | |
|---|---|---|---|---|
| Perceived Restorative Scale | 14.20 ± 1.30 | 14.85 ± 1.43 | 6.12 | 0.000 ** |
| Recovery Scale | 14.89 ± 1.25 | 13.15 ± 1.67 | 5.11 | 0.000 ** |
| Band | Mean of EEG Frequency (SD) (Background Stimuli—Photo Stimuli) | t | p | |
|---|---|---|---|---|
| Daytime | Nighttime | |||
| Relative Theta | 0.024 ± 0.188 | 0.036 ± 0.160 | −0.218 | 0.267 |
| Relative Alpha | −0.020 ± 0.194 | −0.027 ± 0.102 | 1.429 | 0.403 |
| Relative Beta | −0.010 ± 0.097 | −0.001 ± 0.086 | −1.136 | 0.162 |
| Relative Gamma | −0.021 ± 0.132 | −0.013 ± 0.101 | −1.305 | 0.268 |
| Band | Mean Value of the EEG (SD) (Background Stimuli—Photo Stimuli) | t | p | ||
|---|---|---|---|---|---|
| Daytime | Nighttime | ||||
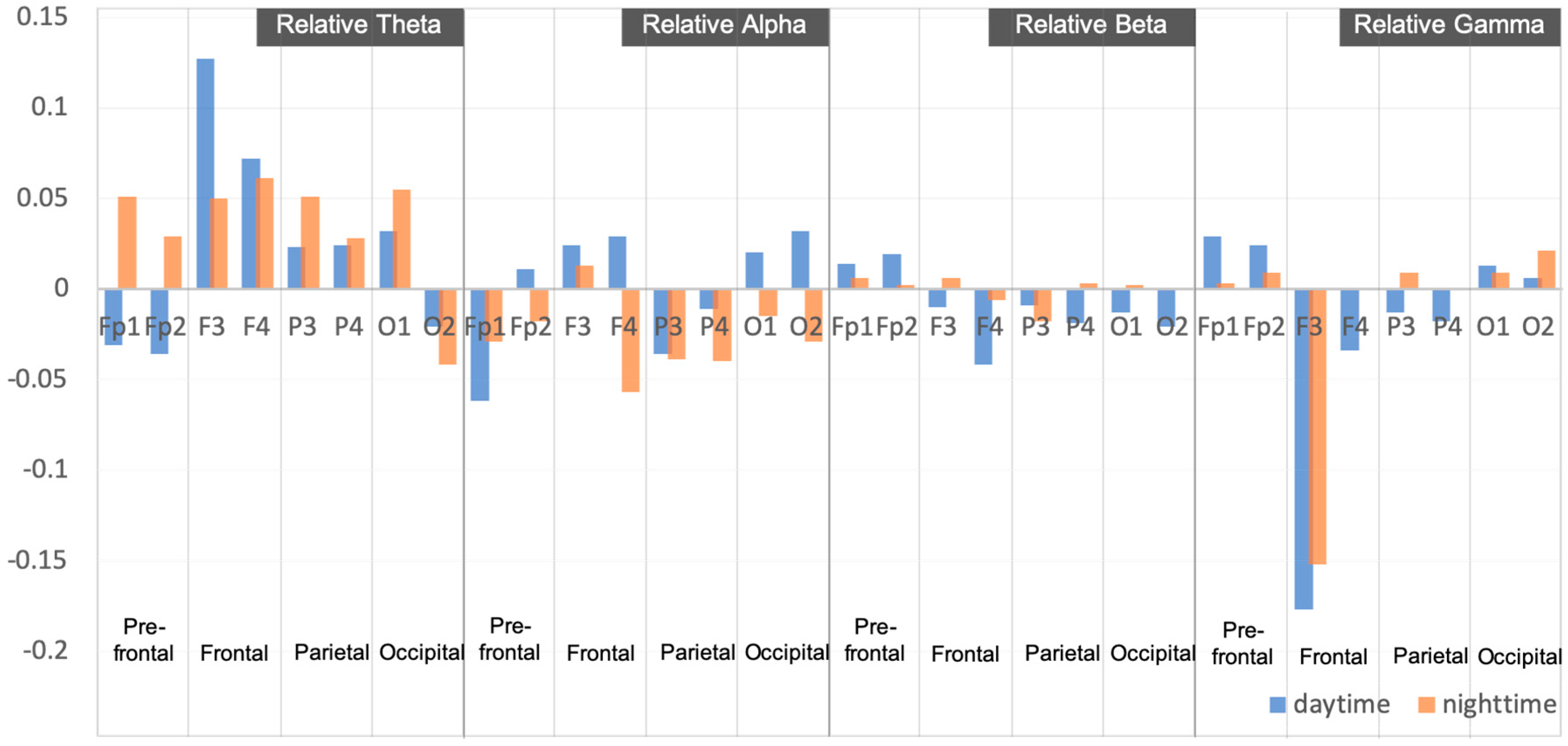
| Relative Theta | Fp1 | −0.031 ± 0.095 | 0.051 ± 0.101 | 0.172 | 0.000 ** |
| Fp2 | −0.036 ± 0.193 | 0.029 ± 0.194 | 1.199 | 0.235 | |
| F3 | 0.127 ± 0.250 | 0.050 ± 0.217 | −0.038 | 0.970 | |
| F4 | 0.072 ± 0.225 | 0.061 ± 0.167 | 2.049 | 0.045 * | |
| P3 | 0.023 ± 0.218 | 0.051 ± 0.149 | 0.748 | 0.458 | |
| P4 | 0.024 ± 0.018 | 0.028 ± 0.141 | −0.337 | 0.737 | |
| O1 | 0.032 ± 0.172 | 0.055 ± 0.126 | 0.916 | 0.363 | |
| O2 | −0.021 ± 0.097 | −0.042 ± 0.140 | 3.858 | 0.000 ** | |
| Relative Alpha | Fp1 | −0.062 ± 0.62 | −0.029 ± 0.114 | 0.172 | 0.000 ** |
| Fp2 | 0.011 ± 0.145 | −0.018 ± 0.097 | 1.199 | 0.015 * | |
| F3 | 0.024 ± 0.095 | 0.013 ± 0.110 | −0.038 | 0.970 | |
| F4 | 0.029 ± 0.100 | −0.057 ± 0.106 | 2.049 | 0.045 * | |
| P3 | −0.036 ± 0.107 | −0.039 ± 0.101 | 0.748 | 0.458 | |
| P4 | −0.011 ± 0.078 | −0.040 ± 0.063 | −0.337 | 0.737 | |
| O1 | 0.020 ± 0.071 | −0.015 ± 0.013 | 0.916 | 0.363 | |
| O2 | 0.032 ± 0.066 | −0.029 ± 0.105 | 3.858 | 0.864 | |
| Relative Beta | Fp1 | 0.014 ± 0.942 | 0.006 ± 0.105 | −0.242 | 0.809 |
| Fp2 | 0.019 ± 0.095 | 0.002 ± 0.091 | 1.962 | 0.035 * | |
| F3 | −0.010 ± 0.087 | 0.006 ± 0.101 | 0.557 | 0.580 | |
| F4 | −0.042 ± 0.081 | −0.006 ± 0.104 | 0.369 | 0.714 | |
| P3 | −0.009 ± 0.104 | −0.018 ± 0.837 | 0.224 | 0.824 | |
| P4 | −0.019 ± 0.098 | 0.003 ± 0.839 | 0.591 | 0.557 | |
| O1 | −0.013 ± 0.105 | 0.002 ± 0.008 | −1.264 | 0.211 | |
| O2 | −0.021 ± 0.098 | −0.001 ± 0.073 | 0.378 | 0.707 | |
| Relative Gamma | Fp1 | 0.029 ± 0.128 | 0.003 ± 0.112 | 0.193 | 0.847 |
| Fp2 | 0.024 ± 0.114 | 0.009 ± 0.116 | −7.163 | 0.762 | |
| F3 | −0.177 ± 0.117 | −0.152 ± 0.132 | 0.240 | 0.011 * | |
| F4 | −0.034 ± 0.126 | −0.001 ± 0.075 | 0.363 | 0.718 | |
| P3 | −0.013 ± 0.139 | 0.009 ± 0.093 | −0.325 | 0.746 | |
| P4 | −0.018 ± 0.101 | 0.000 ± 0.014 | −7.693 | 0.800 | |
| O1 | 0.013 ± 0.090 | 0.009 ± 0.108 | 3.728 | 0.435 | |
| O2 | 0.006 ± 0.110 | 0.021 ± 0.098 | −0.873 | 0.386 | |
| Correlation | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Relative Theta | Prefrontal | 1 | |||||||||||||||
| Frontal | 0.318 ** | 1 | |||||||||||||||
| Parietal | 0.137 | 0.480 ** | 1 | ||||||||||||||
| Occipital | −0.010 | 0.258 ** | 0.712 ** | 1 | |||||||||||||
| Relative Alpha | Prefrontal | −0.310 ** | −0.464 | −0.155 | 0.055 | 1 | |||||||||||
| Frontal | −0.233 | −0.419 | −0.477 ** | −0.225 | 0.601 ** | 1 | |||||||||||
| Parietal | −0.208 * | −0.233 * | −0.315 ** | −0.260 * | 0.607 ** | 0.701 ** | 1 | ||||||||||
| Occipital | −0.242 * | −0.334 * | −0.126 ** | −0.213 | 0.439 ** | 0.708 ** | 0.793 ** | 1 | |||||||||
| Relative beta | Prefrontal | −0.982 ** | −0.299 * | −0.464 | −0.072 | 0.156 | −0.061 | 0.174 | 0.132 | 1 | |||||||
| Frontal | −0.266 * | −0.631 ** | −0.453 ** | −0.071 | 0.258 * | −0.231 | −0.481 | −0.021 | 0.404 ** | 1 | |||||||
| Parietal | −0.102 | −0.310 * | −0.143 * | −0.238 * | −0.229 ** | −0.315 ** | −0.463 ** | −0.308 ** | 0.148 | 0.529 ** | 1 | ||||||
| Occipital | 0.198 | 0.162 | −0.062 | −0.226 ** | −0.495 ** | −0.322 ** | −0.324 * | −0.321 ** | −0.209 | 0.140 | 0.505 ** | 1 | |||||
| Relative gamma | Prefrontal | −0.922 ** | −0.472 * | 0.039 | −0.195 | −0.013 | −0.013 | 0.089 | 0.149 | 0.959 ** | 0.319 ** | 0.130 | −0.148 | 1 | |||
| Frontal | −0.080 | −0.414 ** | −0.145 | −0.342 ** | −0.213 | −0.300 ** | −0.409 * | −0.221 * | 0.140 | 0.162 | 0.396 * | 0.108 | 0.114 | 1 | |||
| Parietal | 0.168 | −0.117 | −0.134 | −0.111 | −0.400 ** | −0.572 ** | −0.454 ** | −0.417 ** | −0.074 | −0.028 ** | 0.583 ** | 0.315 * | 0.105 | 0.443 * | 1 | ||
| Occipital | 0.209 | −0.137 | −0.143 | −0.343 * | −0.443 ** | −0.558 ** | −0.413 ** | −0.714 ** | −0.008 | 0.094 | 0.360* | 0.212 * | −0.015 | 0.351 ** | 0.497 ** | 1 | |
| Correlation | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
| Relative Theta | Prefrontal | 1 | |||||||||||||||
| Frontal | 0.662 ** | 1 | |||||||||||||||
| Parietal | 0.447 ** | 0.558 ** | 1 | ||||||||||||||
| Occipital | 0.170 | 0.292 | 0.796 ** | 1 | |||||||||||||
| Relative Alpha | Prefrontal | −3.297 ** | −0.388 ** | −0.321 ** | −0.207 * | 1 | |||||||||||
| Frontal | −0.554 ** | −0.278 | −0.371 * | −0.299 * | 0.828 ** | 1 | |||||||||||
| Parietal | −0.199 | −0.126 | −0.170 | −0.195 | 0.641 ** | 0.706 ** | 1 | ||||||||||
| Occipital | −0.093 | −0.102 | −0.203 | −0.029 | 0.691 ** | 0.634 ** | 0.778 ** | 1 | |||||||||
| Relative beta | Prefrontal | −0.944 ** | −0.490 ** | −0.247 * | −0.071 | 0.128 | 0.146 | 0.121 | 0.332 * | 1 | |||||||
| Frontal | −0.444 ** | −0.536 ** | −0.360 ** | −0.058 | −0.071 | −0.207 | −0.316 ** | −0.172 | 0.316 ** | 1 | |||||||
| Parietal | 0.120 | −0.004 | −0.385 ** | −0.011 | −0.332 ** | −0.417 ** | −0.587 ** | −0.514 ** | 0.232 | 0.360 ** | 1 | ||||||
| Occipital | 0.024 | 0.096 | −0.313 * | −0.054 | −0.355 ** | −0.376 ** | −0.569 ** | −0.769 ** | 0.211 | 0.375 * | 0.575 ** | 1 | |||||
| Relative gamma | Prefrontal | −0.731 * | −0.314 ** | −0.305 * | −0.022 | 0.052 | 0.067 | −0.189 | −0.022 | 0.812 ** | 0.474 ** | 0.397 | 0.098 | 1 | |||
| Frontal | −0.071 | −0.597 ** | −0.091 | −0.063 | −0.174 | −0.584 | −0.502 | −0.185 | 0.022 | 0.233 * | 0.496 | 0.183 | 0.125 | 1 | |||
| Parietal | −0.201 | −0.029 | −0.317 ** | −0.319* | −0.101 | −0.378 | −0.322 ** | −0.476 ** | 0.304 * | 0.429 ** | 0.552 ** | 0.461 ** | 0.361 * | 0.361 * | 1 | ||
| Occipital | 0.050 | 0.265 * | −0.042 * | −0.342 ** | −0.355 * | −0.312 * | −0.370 ** | −0.797 ** | −0.043 | 0.109 | 0.526 ** | 0.576 ** | −0.076 | 0.104 | 0.582 ** | 1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, S.; Han, S.; Kim, M.; Kwon, Y. Comparison between Daytime and Nighttime Scenery Focusing on Restorative and Recovery Effect. Sustainability 2019, 11, 3326. https://doi.org/10.3390/su11123326
Cheon S, Han S, Kim M, Kwon Y. Comparison between Daytime and Nighttime Scenery Focusing on Restorative and Recovery Effect. Sustainability. 2019; 11(12):3326. https://doi.org/10.3390/su11123326
Chicago/Turabian StyleCheon, SangHyun, Soyoung Han, Mintai Kim, and Yoonku Kwon. 2019. "Comparison between Daytime and Nighttime Scenery Focusing on Restorative and Recovery Effect" Sustainability 11, no. 12: 3326. https://doi.org/10.3390/su11123326
APA StyleCheon, S., Han, S., Kim, M., & Kwon, Y. (2019). Comparison between Daytime and Nighttime Scenery Focusing on Restorative and Recovery Effect. Sustainability, 11(12), 3326. https://doi.org/10.3390/su11123326





