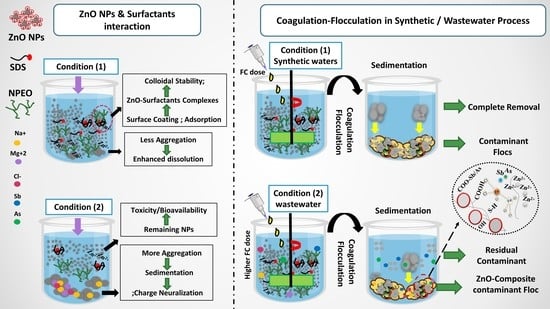
Removal of ZnO Nanoparticles from Natural Waters by Coagulation-Flocculation Process: Influence of Surfactant Type on Aggregation, Dissolution and Colloidal Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of NPs and Surfactants Stock Solutions
2.3. Preparation and Characteristic of Environmental Water Samples
2.4. Laboratory Batch Experiments
2.4.1. Adsorption Study and Isotherm Modelling
2.4.2. Sedimentation and Dissolution Kinetics in Different Waters
2.4.3. Coagulation-Flocculation Experiments
2.5. Characterization and Measurement of ZnO NPs Suspension
3. Results and Discussions
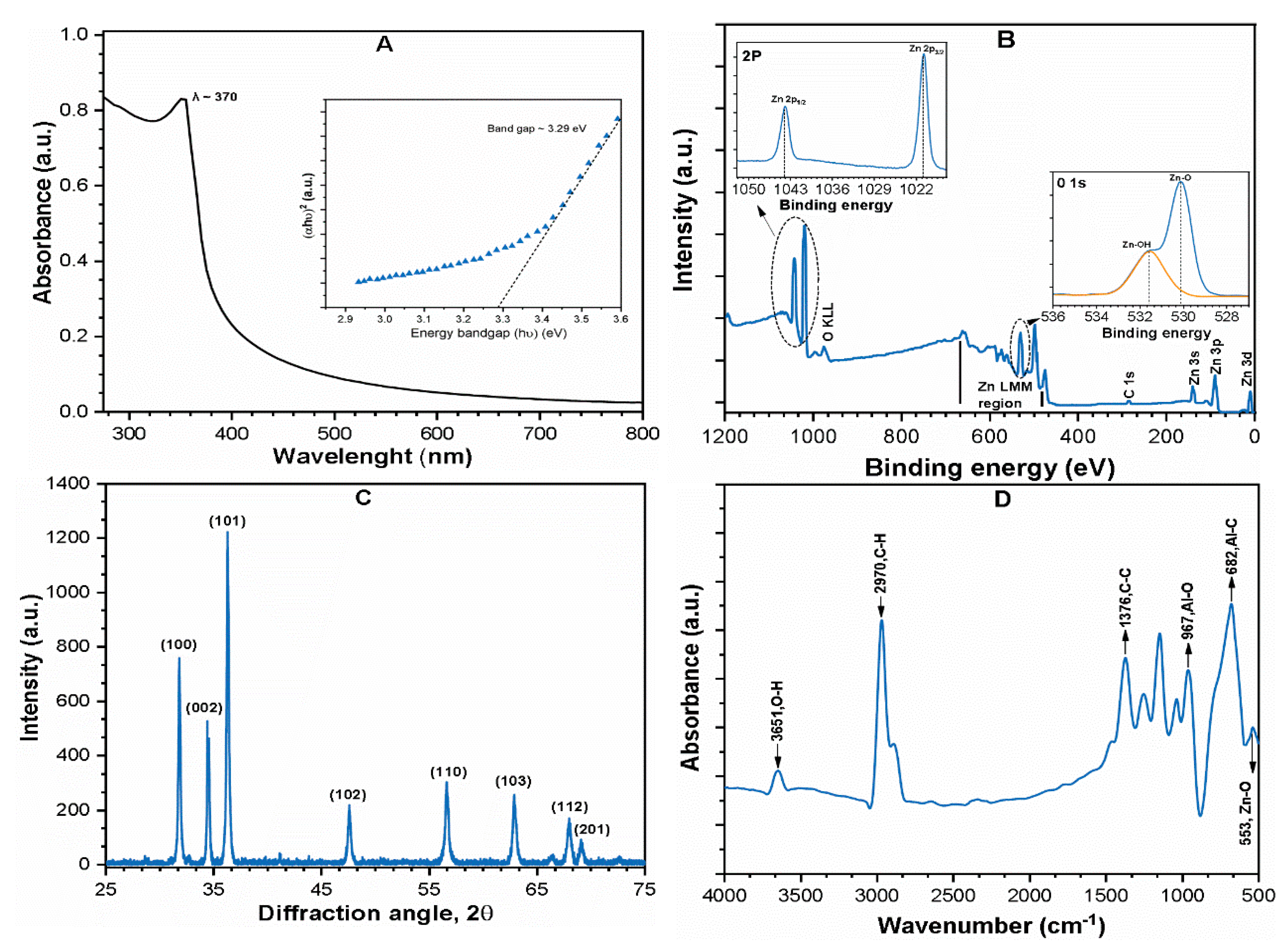
3.1. NPs Characterization and Properties
3.2. Effects of pH and Surfactants on ζ-potential and HDD of ZnO NPs
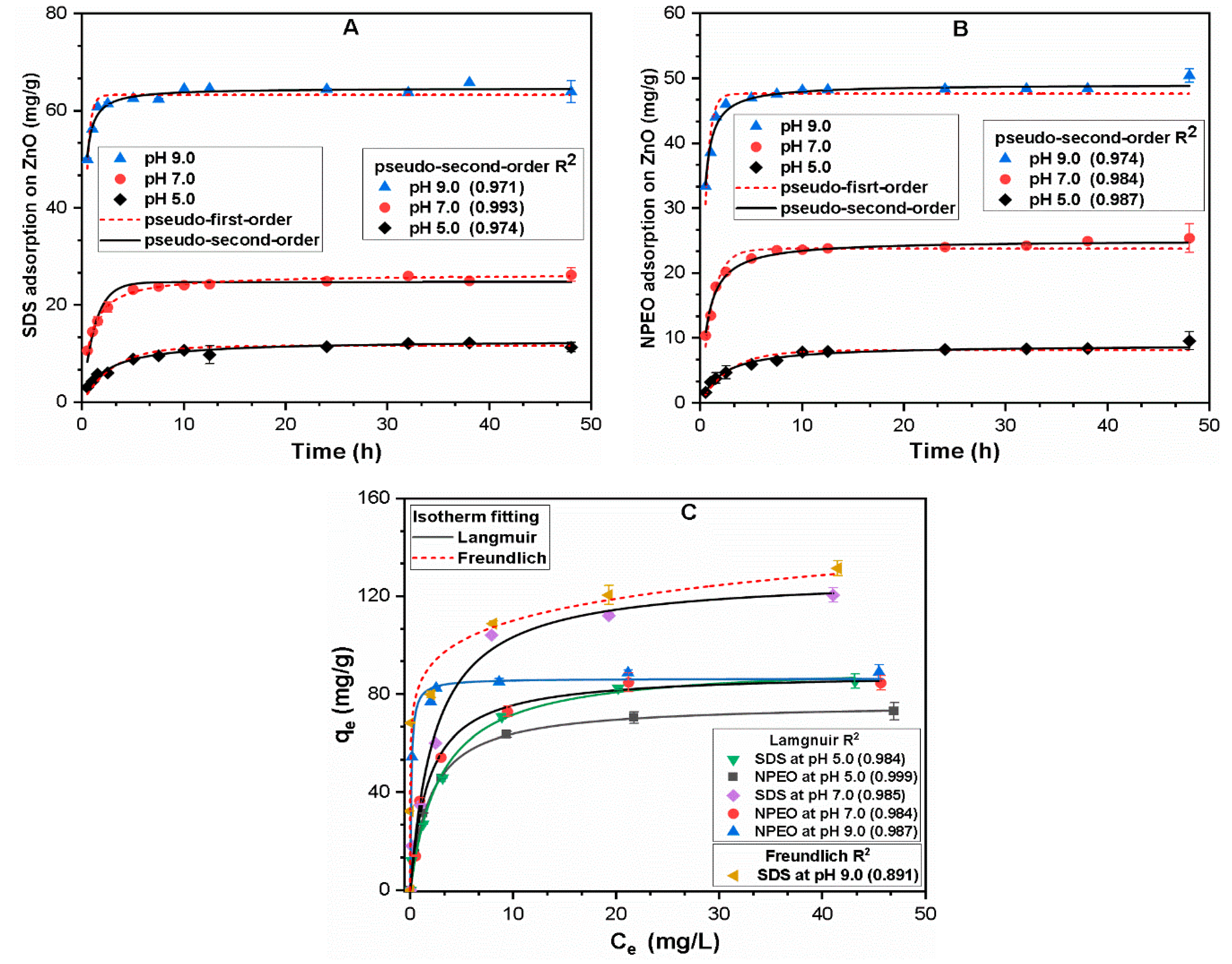
3.3. Surfactants Adsorption onto NPs Surface
Characteristics of ZnO-Surfactant Complexes
3.4. Sedimentation and Aggregation of ZnO NPs in Different Waters
Dissolution of ZnO NPs in Environmental Waters
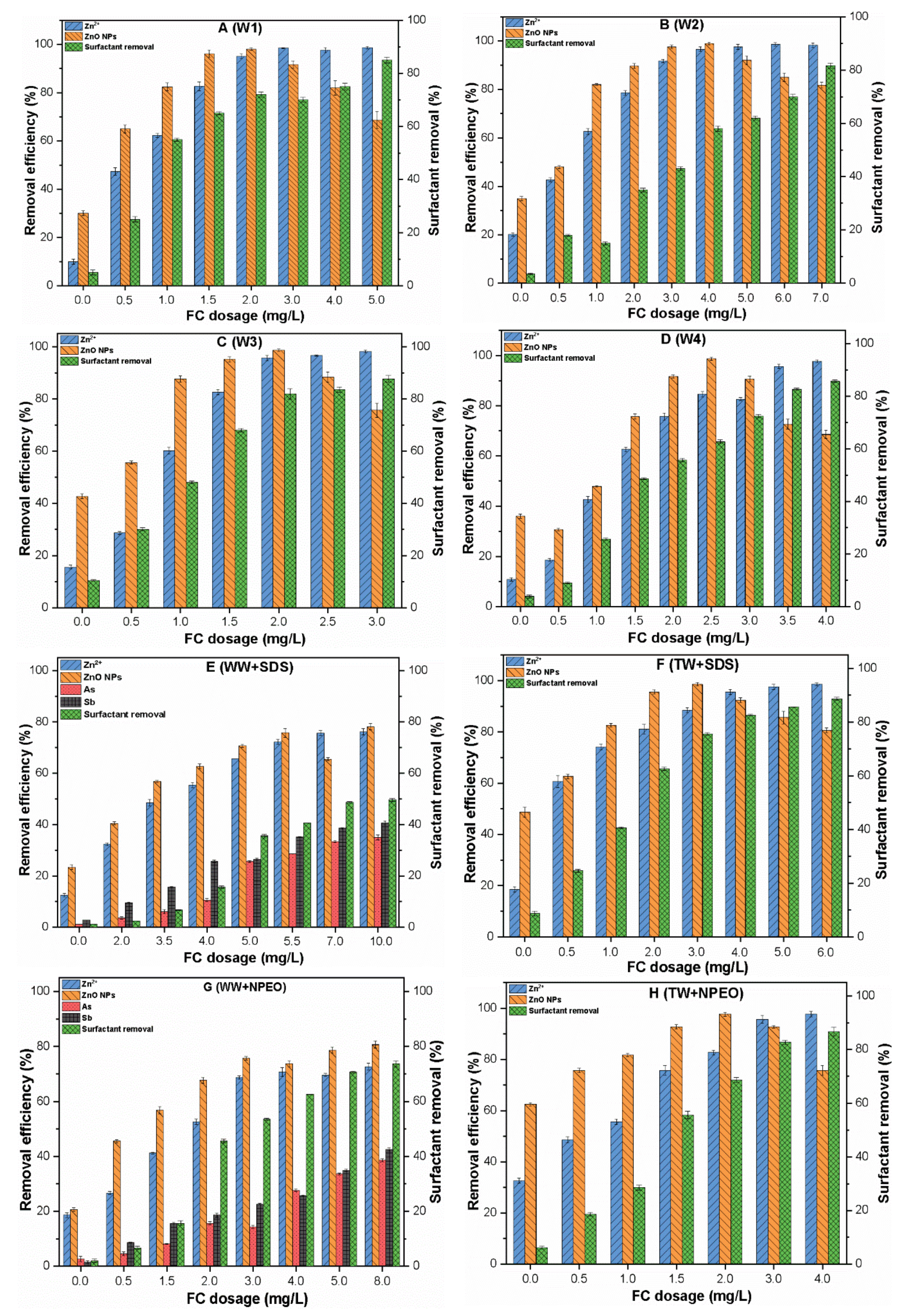
3.5. Removal of ZnO NPs and Zn2+ from Synthetic Waters
3.5.1. Removal of ZnO NPs and Zn2+ from Environmental Waters
3.5.2. Removal of Surfactant from Synthetic and Environmental Waters
3.6. Study Significance and Limitation
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Adam, N.; Leroux, F.; Knapen, D.; Bals, S.; Blust, R. The uptake and elimination of ZnO and CuO nanoparticles in Daphnia magna under chronic exposure scenarios. Water Res. 2015, 68, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Dreher, K.L. Health and environmental impact of nanotechnology: Toxicological assessment of manufactured nanoparticles. Toxicol. Sci. 2004, 77, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Keller, A.A. Role of morphology in the aggregation kinetics of ZnO nanoparticles. Water Res. 2010, 44, 2948–2956. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Tso, C.; Tsai, Y.; Zhuang, C.; Shih, Y. The effect of electrolytes on the aggregation kinetics of three different ZnO nanoparticles in water. Sci. Total Environ. 2015, 530, 183–190. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.; Zam, S.; Park, D.; Yeom, I. Assessment of Key Environmental Factors Influencing the Sedimentation and Aggregation Behavior of Zinc Oxide Nanoparticles in Aquatic Environment. Water 2018, 10, 660. [Google Scholar] [CrossRef]
- Khan, R.; Inam, M.; Park, D.; Zam Zam, S.; Shin, S.; Khan, S.; Akram, M.; Yeom, I. Influence of Organic Ligands on the Colloidal Stability and Removal of ZnO Nanoparticles from Synthetic Waters by Coagulation. Processes 2018, 6, 170. [Google Scholar] [CrossRef]
- Narkis, N.; Ben-David, B. Adsorption of non-ionic surfactants on activated carbon and mineral clay. Water Res. 1985, 19, 815–824. [Google Scholar] [CrossRef]
- Bouchard, D.; Zhang, W.; Powell, T.; Rattanaudompol, U. Aggregation kinetics and transport of single-walled carbon nanotubes at low surfactant concentrations. Environ. Sci. Technol. 2012, 46, 4458–4465. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A. Chronic and sublethal toxicities of surfactants to aquatic animals: A review and risk assessment. Water Res. 1991, 25, 101–113. [Google Scholar] [CrossRef]
- Godinez, I.G.; Darnault, C.J.G. Aggregation and transport of nano-TiO2 in saturated porous media: Effects of pH, surfactants and flow velocity. Water Res. 2011, 45, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yoneda, M.; Shimada, Y.; Matsui, Y. Effect of surfactants on the aggregation and stability of TiO2 nanomaterial in environmental aqueous matrices. Sci. Total Environ. 2017, 574, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Crittenden, J. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res. 2009, 43, 4249–4257. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Zhaoxia, J.I. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Liu, L.; Gao, B.; Wu, L.; Sun, Y.; Zhou, Z. Effects of surfactant type and concentration on graphene retention and transport in saturated porous media. Chem. Eng. J. 2015, 262, 1187–1191. [Google Scholar] [CrossRef]
- Tkachenko, N.H.; Yaremko, Z.M.; Bellmann, C.; Soltys, M.M. The influence of ionic and nonionic surfactants on aggregative stability and electrical surface properties of aqueous suspensions of titanium dioxide. J. Colloid Interface Sci. 2006, 299, 686–695. [Google Scholar] [CrossRef]
- Li, X.; Yoneda, M.; Shimada, Y.; Matsui, Y. Effect of surfactants on the aggregation and sedimentation of zinc oxide nanomaterial in natural water matrices. Sci. Total Environ. 2017, 581, 649–656. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, K.; Fang, J.; Lin, D.; Wang, M.; Han, J. Transport of TiO2 nanoparticles in soil in the presence of surfactants. Sci. Total Environ. 2015, 527, 420–428. [Google Scholar] [CrossRef]
- Apha, A. WPCF, Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Wagner, C.D.; Gale, L.H.; Raymond, R.H. Two-dimensional chemical state plots: A standardized data set for use in identifying chemical states by x-ray photoelectron spectroscopy. Anal. Chem. 1979, 51, 466–482. [Google Scholar] [CrossRef]
- Rupasinghe, R.A. Dissolution and Aggregation of Zinc Oxide Nanoparticles at Circumneutral pH: A Study of Size Effects in the Presence and Absence of Citric Acid. Master’s Thesis, University of Iowa, Iowa City, IA, USA, 2011. [Google Scholar]
- Chibowski, E.; Holysz, L.; Terpilowski, K.; Wiacek, A.E. Influence of ionic surfactants and lecithin on stability of titanium dioxide in aqueous electrolyte solution. Croat. Chem. Acta 2007, 80, 395–403. [Google Scholar]
- Saleh, N.; Kim, H.-J.; Phenrat, T.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Ionic strength and composition affect the mobility of surface-modified Fe0 nanoparticles in water-saturated sand columns. Environ. Sci. Technol. 2008, 42, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- Nevskaia, D.M.; Guerrero-Ruız, A.; de D. López-González, J. Adsorption of polyoxyethylenic nonionic and anionic surfactants from aqueous solution: Effects induced by the addition of NaCl and CaCl2. J. Colloid Interface Sci. 1998, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Jiang, Z.; Wang, X. SDBS Modified XC-72 Carbon for the Removal of Pb (II) from Aqueous Solutions. Plasma Process. Polym. 2010, 7, 552–560. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q. Critical review in adsorption kinetic models. J. Zhejiang Univ. A 2009, 10, 716–724. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef]
- Hu, J.-D.; Zevi, Y.; Kou, X.-M.; Xiao, J.; Wang, X.-J.; Jin, Y. Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci. Total Environ. 2010, 408, 3477–3489. [Google Scholar] [CrossRef]
- Filius, J.D.; Meeussen, J.C.L.; Lumsdon, D.G.; Hiemstra, T.; van Riemsdijk, W.H. Modeling the binding of fulvic acid by goethite: The speciation of adsorbed FA molecules. Geochim. Cosmochim. Acta 2003, 67, 1463–1474. [Google Scholar] [CrossRef]
- Piccolo, A.; Conte, P.; Spaccini, R.; Chiarella, M. Effects of some dicarboxylic acids on the association of dissolved humic substances. Biol. Fertil. Soils 2003, 37, 255–259. [Google Scholar]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef] [PubMed]
- Filius, J.D.; Lumsdon, D.G.; Meeussen, J.C.L.; Hiemstra, T.; Van Riemsdijk, W.H. Adsorption of fulvic acid on goethite. Geochim. Cosmochim. Acta 2000, 64, 51–60. [Google Scholar] [CrossRef]
- Romero-Cano, M.; Martín-Rodríguez, A.; de las Nieves, F. Electrokinetic behaviour of polymer colloids with adsorbed Triton X-100. Colloid Polym. Sci. 2002, 280, 526–532. [Google Scholar]
- Sharma, K.P.; Aswal, V.K.; Kumaraswamy, G. Adsorption of nonionic surfactant on silica nanoparticles: Structure and resultant interparticle interactions. J. Phys. Chem. B 2010, 114, 10986–10994. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Hankins, N.P.; Hey, M.J.; Kingman, S.W. In situ mechanistic study of SDS adsorption on hematite for optimized froth flotation. Ind. Eng. Chem. Res. 2004, 43, 5326–5338. [Google Scholar] [CrossRef]
- Dobson, K.D.; Roddick-Lanzilotta, A.D.; McQuillan, A.J. In situ infrared spectroscopic investigation of adsorption of sodium dodecylsulfate and of cetyltrimethylammonium bromide surfactants to TiO2, ZrO2, Al2O3, and Ta2O5 particle films from aqueous solutions. Vib. Spectrosc. 2000, 24, 287–295. [Google Scholar] [CrossRef]
- Gao, X.; Chorover, J. Adsorption of sodium dodecyl sulfate (SDS) at ZnSe and α-Fe2O3 surfaces: Combining infrared spectroscopy and batch uptake studies. J. Colloid Interface Sci. 2010, 348, 167–176. [Google Scholar] [CrossRef]
- Hug, S.J. In situ fourier transform infrared measurements of sulfate adsorption on hematite in aqueous solutions. J. Colloid Interface Sci. 1997, 188, 415–422. [Google Scholar] [CrossRef]
- Somasundaran, P. Reagents in Mineral Technology; Routledge: Abingdon-on-Thames, UK, 2018; ISBN 1351419625. [Google Scholar]
- Bhagat, R.P. Kinetics of sodium dodecyl benzene sulfonate adsorption on hematite and its interaction with polyacrylamide. Colloid Polym. Sci. 2001, 279, 33–38. [Google Scholar] [CrossRef]
- Tan, G.; Ford, C.; John, V.T.; He, J.; McPherson, G.L.; Bose, A. Surfactant solubilization and the direct encapsulation of interfacially active Phenols in mesoporous silicas. Langmuir 2008, 24, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, A.; Pojana, G.; Callegaro, S.; Marcomini, A. Agglomeration and sedimentation of titanium dioxide nanoparticles (n-TiO2) in synthetic and real waters. J. Nanopart. Res. 2013, 15, 1684. [Google Scholar] [CrossRef]
- Omar, F.M.; Aziz, H.A.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 2014, 468, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Walker, S.L.; Mylon, S.E. Aggregate morphology of nano-TiO2: Role of primary particle size, solution chemistry, and organic matter. Environ. Sci. Process. Impacts 2013, 15, 275–282. [Google Scholar] [CrossRef]
- Han, Y.; Kim, D.; Hwang, G.; Lee, B.; Eom, I.; Kim, P.J.; Tong, M.; Kim, H. Aggregation and dissolution of ZnO nanoparticles synthesized by different methods: Influence of ionic strength and humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2014, 451, 7–15. [Google Scholar] [CrossRef]
- Gelabert, A.; Sivry, Y.; Ferrari, R.; Akrout, A.; Cordier, L.; Nowak, S.; Menguy, N.; Benedetti, M.F. Uncoated and coated ZnO nanoparticle life cycle in synthetic seawater. Environ. Toxicol. Chem. 2014, 33, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lombi, E.; Donner, E.; Tavakkoli, E.; Turney, T.W.; Naidu, R.; Miller, B.W.; Scheckel, K.G. Fate of zinc oxide nanoparticles during anaerobic digestion of wastewater and post-treatment processing of sewage sludge. Environ. Sci. Technol. 2012, 46, 9089–9096. [Google Scholar] [CrossRef]
- Ma, R.; Levard, C.; Judy, J.D.; Unrine, J.M.; Durenkamp, M.; Martin, B.; Jefferson, B.; Lowry, G.V. Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids. Environ. Sci. Technol. 2013, 48, 104–112. [Google Scholar] [CrossRef]
- Rasool, K.; Lee, D.S. Effects of sulfidation on ZnO nanoparticle dissolution and Aggregation in sulfate-containing suspensions. J. Nanosci. Nanotechnol. 2015, 15, 7334–7340. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Solera-Hernández, C. Anionic surfactants removal by natural coagulant/flocculant products. Ind. Eng. Chem. Res. 2009, 48, 5085–5092. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100, 475–502. [Google Scholar] [CrossRef]
- Sousa, V.S.; Corniciuc, C.; Teixeira, M.R. The effect of TiO2 nanoparticles removal on drinking water quality produced by conventional treatment C/F/S. Water Res. 2017, 109, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, B.; Zhao, S.; Li, R.; Yue, Q.; Wang, Y.; Liu, S. Simultaneous removal of nano-ZnO and Zn2+ based on transportation character of nano-ZnO by coagulation: Enteromorpha polysaccharide compound polyaluminum chloride. Environ. Sci. Pollut. Res. 2017, 24, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Czech, B. Surfactants removal from water and wastewater using Co modified TiO2/Al2O3 photocatalysts. Ann. UMCS Chem. 2011, 66, 81–93. [Google Scholar] [CrossRef]
- Inam, M.; Khan, R.; Park, D.; Lee, Y.-W.; Yeom, I. Removal of Sb(III) and Sb(V) by Ferric Chloride Coagulation: Implications of Fe Solubility. Water 2018. [Google Scholar] [CrossRef]
- Inam, M.A.; Khan, R.; Park, D.R.; Ali, B.A.; Uddin, A.; Yeom, I.T. Influence of pH and Contaminant Redox Form on the Competitive Removal of Arsenic and Antimony from Aqueous Media by Coagulation. Minerals 2018, 8, 574. [Google Scholar] [CrossRef]
- Paton-Morales, P.; Talens-Alesson, F.I. Effect of ionic strength and competitive adsorption of Na+ on the flocculation of lauryl sulfate micelles with Al3+. Langmuir 2001, 17, 6059–6064. [Google Scholar] [CrossRef]




| Nanomaterials Parameter | Unit | Technique | Value |
|---|---|---|---|
| Manufacturer-reported size | nm | TEM | <50 |
| Bulk Density | g(cm3)−1 | - | 5.60 |
| Solubility | High | ||
| BET specific surface area | m2g−1 | BET | 12.2 ± 0.4 |
| Iso-electric point (pH iep, see Figure S2B) | - | Zetasizer | 9.2 |
| Zeta potential in pure water (at pH7) | mV | +14 ± 2.1 | |
| HDD measured in pure water (n = 50) | nm | DLS | 280 ± 35 |
| Purity/moisture content (see Figure S1D) | wt.% | TGA/ICP-OES | 96.52/1.85 |
| Crystalline structure (Figure 1C) | - | XRD | Hexagonal |
| Shape | Polyhedral roughly round | ||
| Hamaker Constant | J | - | 1.9 × 10−20 |
| Net energy barrier in pure water (IS 5 × 10−6 M) | kT (a) | - | 42.8 |
| Surfactant Type | Formula | Structure | Molecular Weight (g/mol) |
|---|---|---|---|
| SDS (Anionic) | CH3(CH2)11OSO3Na | 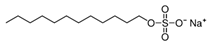 | 288.38 |
| NPEO (Nonionic) | C9H19C6H4(OCH2CH2)9OH |  | 616.82 |
| Surfactant Type | Concentration (w:v) % | Water Code | Released Zn2+ (mg/L) | ZnO NPs (mg/L) | ζ-potential (mV) | HDD (nm) | pH | |
|---|---|---|---|---|---|---|---|---|
| Control | 0 | - | Control | 0.893 ± 0.10 | 8.56 ± 0.41 | 18.0 ± 1.3 | 458 ± 56 | 7.06 ± 0.02 |
| Anionic | 0.030 | SDS | W1 | 1.524 ± 0.04 | 6.78 ± 0.50 | −16.4 ± 0.5 | 280 ± 35 | 6.94 ± 0.04 |
| 0.050 | W2 | 1.680 ± 0.16 | 6.12 ± 0.31 | −28.3 ± 0.8 | 205 ± 70 | 6.76 ± 0.17 | ||
| Nonionic | 0.030 | NPEO | W3 | 0.684 ± 0.28 | 8.05 ± 0.14 | 16.4 ± 0.6 | 520 ± 60 | 7.02 ± 0.01 |
| 0.050 | W4 | 0.921 ± 0.33 | 7.57 ± 0.24 | 12.1 ± 0.4 | 485 ± 48 | 6.85 ± 0.13 | ||
| Anionic | 0.050 | SDS | (WW+SDS) | 3.587 ± 0.02 | 5.027 ± 0.08 | −25.6 ± 0.1 | 190 ± 35 | 7.87 ± 0.21 |
| (TW+SDS) | 2.423 ± 0.01 | 7.41 ± 0.10 | −14.0 ± 0.3 | 235 ± 62 | 6.95 ± 0.34 | |||
| Nonionic | 0.050 | NPEO | (WW+NPEO) | 2.719 ± 0.02 | 6.09 ± 0.23 | −17.8 ± 0.5 | 265 ± 79 | 7.56 ± 0.15 |
| (TW+NPEO) | 0.769 ± 0.01 | 8.26 ± 0.08 | 8.6 ± 0.2 | 584 ± 98 | 7.02 ± 0.01 | |||
| Pseudo-First-Order | Pseudo-Second-Order | ||||||
|---|---|---|---|---|---|---|---|
| Surfactant | pH | qe (mg/g) | k1 (1/h) | R2 | qe | k2 (g/mg/h) | R2 |
| SDS | 5 | 11.58 | 0.745 | 0.914 | 12.76 | 0.0354 | 0.974 |
| 7 | 24.77 | 1.881 | 0.928 | 26.33 | 0.0471 | 0.993 | |
| 9 | 63.27 | 6.582 | 0.858 | 64.72 | 0.1105 | 0.971 | |
| NPEO | 5 | 8.134 | 0.806 | 0.950 | 8.956 | 0.0535 | 0.987 |
| 7 | 23.76 | 2.099 | 0.966 | 25.09 | 0.0573 | 0.984 | |
| 9 | 47.66 | 4.740 | 0.896 | 49.09 | 0.0887 | 0.974 | |
| Langmuir Fitting | Freundlich Fitting | ||||||
|---|---|---|---|---|---|---|---|
| Surfactant | pH | KL | qmax | R2 | KF | n | R2 |
| SDS | 5.0 | 0.343 ± 0.06 | 92.67 ± 4.15 | 0.984 | 32.753 ± 4.43 | 3.569 ± 0.57 | 0.947 |
| 7.0 | 0.413 ± 0.07 | 128.52 ± 5.26 | 0.985 | 46.528 ± 6.98 | 3.55 ± 0.60 | 0.930 | |
| 9.0 | 0.827 ± 1.05 | 130.11 ± 26.58 | 0.527 | 85.073 ± 9.46 | 8.89 ± 3.10 | 0.891 | |
| NPEO | 5.0 | 0.516 ± 0.01 | 76.40 ± 0.62 | 0.999 | 31.206 ± 4.39 | 4.05 ± 0.77 | 0.921 |
| 7.0 | 0.569 ± 0.09 | 88.91 ± 3.28 | 0.984 | 36.49 ± 5.75 | 4.00 ± 0.85 | 0.904 | |
| 9.0 | 9.731 ± 2.02 | 87.47 ± 1.96 | 0.987 | 70.52 ± 2.46 | 12.34 ± 2.07 | 0.981 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, R.; Inam, M.A.; Iqbal, M.M.; Shoaib, M.; Park, D.R.; Lee, K.H.; Shin, S.; Khan, S.; Yeom, I.T. Removal of ZnO Nanoparticles from Natural Waters by Coagulation-Flocculation Process: Influence of Surfactant Type on Aggregation, Dissolution and Colloidal Stability. Sustainability 2019, 11, 17. https://doi.org/10.3390/su11010017
Khan R, Inam MA, Iqbal MM, Shoaib M, Park DR, Lee KH, Shin S, Khan S, Yeom IT. Removal of ZnO Nanoparticles from Natural Waters by Coagulation-Flocculation Process: Influence of Surfactant Type on Aggregation, Dissolution and Colloidal Stability. Sustainability. 2019; 11(1):17. https://doi.org/10.3390/su11010017
Chicago/Turabian StyleKhan, Rizwan, Muhammad Ali Inam, Muhammad Mazhar Iqbal, Muhammad Shoaib, Du Ri Park, Kang Hoon Lee, Sookyo Shin, Sarfaraz Khan, and Ick Tae Yeom. 2019. "Removal of ZnO Nanoparticles from Natural Waters by Coagulation-Flocculation Process: Influence of Surfactant Type on Aggregation, Dissolution and Colloidal Stability" Sustainability 11, no. 1: 17. https://doi.org/10.3390/su11010017
APA StyleKhan, R., Inam, M. A., Iqbal, M. M., Shoaib, M., Park, D. R., Lee, K. H., Shin, S., Khan, S., & Yeom, I. T. (2019). Removal of ZnO Nanoparticles from Natural Waters by Coagulation-Flocculation Process: Influence of Surfactant Type on Aggregation, Dissolution and Colloidal Stability. Sustainability, 11(1), 17. https://doi.org/10.3390/su11010017