1. Introduction
The management of agricultural waste, animal manure (bovine, swine, and poultry), and food residues by means of anaerobic digestion (AD) is becoming more important [
1,
2]. In anaerobic digestion (AD), the microorganisms in the absence of oxygen break down the biodegradable matter and produce a valuable biogas consisting of mostly methane [
3]. The biogas can either be used directly or it can be converted into electrical energy. The other residue of anaerobic digestion is digestate [
4]. The digestate represents the AD effluent (i.e., the digested substrate), which is removed from the reactor. The nature and composition of treated substrate and the operating parameters of the AD process have a significant influence on the physical and chemical characteristics of digestate [
3]. The ammonia content of digestate depends on the total nitrogen content of feedstock. Cereal grains, poultry, and pig manures show high ammonia content. However, the bovine manure and corn silage present a lower ammonia content [
5].
The main issues concerning the landspreading of digestate on agricultural soils that represents the main route are related to the high content of nutrients especially nitrogen [
6] and represent the increasing number of AD plants especially in the region with intensive livestock farming [
7]. Therefore, in order to comply with the European Nitrates Directive (91/676/EEC) [
8], suitable treatments for nitrogen reduction in digestate must be applied. Moreover, the recent Italian legislation has restricted the landspreading of digestate derived from AD of different materials and/or residues [
9].
The first step of digestate treatments usually carried out at farm scale concerns the solid/liquid separation. The solid fraction with high content of organic nitrogen can be spread on agricultural soils directly or after a composting process [
10]. In the liquid phase, the high contents of nitrogen generally with high ammonia percentage, phosphorus, and other macro and micronutrients [
11] represent a serious problem for its recovery/disposal.
Furthermore, some authors [
12] have studied the feasibility to increase the methane productions in AD up to 104% [
13] by using the pretreatment of animal manure fibers with aqueous ammonia soaking. Despite this treatment being very interesting, the proposed solution could increase the ammonia content in digestate involving, especially in Italy, additional issues for its recovery on agricultural soils.
As concerns grow regarding the ammonia removal from liquid digestate, several treatments are proposed in the scientific literature. The methods based on physical-chemical processes include struvite precipitation: the ammonia removal yields from the digestate of poultry manure reached 86.4% (with Mg:N:P molar ratio of 1:1:1) and 97.4% (adjusting Mg:N:P molar ratio to 1.5:1:1) [
14]. However, the contents of heavy metals precipitated together with struvite [
15] must be controlled.
The ammonia stripping on livestock manure digestates allowed to obtain ammonia removal yields up to 85%, which operates at 40 °C with a pH adjustment [
16]. However, Serna-Maza et al. [
17] showed that the ammonia removal from fresh digestate was more difficult than digestate stored for long periods.
Moreover, the evaporation is an interesting solution to remove nitrogen from pig slurry with a previous anaerobic digestion to increase the economic feasibility [
18]. Guercini et al. [
19] proposed to use the cogeneration heat to support both the AD plant and the subsequent evaporation of the digestate.
The membrane technology for ammonia removal from digestate could be applied. The use of hollow fiber membranes, submerged into the digestate from the anaerobic reactor that treats slaughterhouse wastes, led to a reduction of free ammonia by 70% [
20]. However, the membrane parameters (such as bubble point, breakthrough pressure, airflow, pore size, contact angle, thickness, etc.) must be evaluated to predict the performance process [
21]. As concerns the membrane processes, an innovative solution based on a thermophilic aerobic membrane reactor for the treatment of sewage sludge and high strength aqueous waste [
22] is able to increase the ammonia content in permeate and the precipitation of nutrients (nitrogen and phosphorus) in the form of salts. Moreover, the proposed solution can be conveniently integrated in traditional wastewater treatment plants (based on a conventional activated sludge process) with the aim to reduce the content of nutrients in the discharged effluent and to recover the heat from the thermophilic aerobic reactor [
23].
The choice of suitable technology and the required pretreatments depend on different aspects mainly related to the qualitative characteristics of digestate. The ammonia stripping and chemical precipitation require simple pretreatments (usually screw press or belt filter press) in order to reduce the content of suspended solid. The other proposed technologies instead require pretreatments with ultrafiltration (UF) followed by cleaning/regeneration cycles [
24]. The farmers prefer to adopt simple and robust treatment plants with low investment and maintenance costs.
The ammonia stripping technology is a process where the nitrogen, in the form of ammonia, is removed from a liquid by gas flow through the liquid. The volatility of ammonia in an aqueous solution can be enhanced by increasing the temperature and pH (usually obtained through chemical addition).
The ammonia stripping process includes the following operations [
25]: (i) conversion of ammonium ions (NH
4+) to ammonia gas (NH
3) (ammonia dissociation equilibrium), (ii) diffusion of NH
3 to the air-water interface (water-side mass transfer), (iii) release of NH
3 to the air at the interface (volatilization), and (iv) diffusion of NH
3 from the air-water interface into the air above (air-side mass transfer). The whole process depends on pH, temperature, and mass transfer area.
Ammonia gas (NH3) is thereafter absorbed in an acid solution (sulphuric or nitric acid) in order to obtain fertilizer for agricultural use (ammonium sulphate or nitrate respectively) with low organic contamination. The cleaned gas can be reused in the stripping column.
The efficiency of air stripping mainly depends on the following parameters: feed pH, feed temperature, ratio of air to feed, and feed characteristics.
The high thermal energy requirement has often restricted the applicability of ammonia stripping. Therefore, this technology is used when there is heat available, which results from the production of electrical power by endothermic motors in excess of the intrinsic needs arising from the need to control the temperature of the plant for the biogas production.
Concerning the stripping reactor, the design of the contacting system between the digestate and the gas used to strip out the NH3 is very important. The aim is to maximize the extent of contact (maximum rate of mixing and highest specific surface area) while minimizing energy costs due to the equipment design. The most common mass transfer design for air stripping systems uses packed towers: columns filled with packing material in order to increase the surface area available for the ammonia mass transfer.
Despite the fact that most common reactors used for ammonia stripping are based on packed columns, the suspended solids can clog the column. This aspect is called fouling. The high content of suspended solids reduces the stripping performance. This reduction is due both to the fouling that involves problems on stripping management and to high content of organic acids and colloidal compounds that reduce the process efficiency [
26]. As a consequence, efficient solid–liquid separation is necessary beforehand. In addition, a high maintenance and cleaning effort may be necessary.
Regarding the nitrogen partition in livestock manure, Fabbri and Piccinini (2012) [
27] show that, in swine, manure ammonia represents about 60% of total nitrogen, which increases by 10% due to AD. Even in the case of bovine manure, the AD involves an increase of ammonia content from 43% to 52%. Furthermore, concerning the solid/liquid separation process, they show that the ammonia nitrogen with respect to total nitrogen increases: (i) in the case of swine manure, from 70% to 90% and (ii) in the case of bovine manure, from 50% to 70%.
In the present study, the ammonia recovery from digestate derived from the AD of swine, bovine, poultry manure, and corn silage was analyzed. Experimental activities were carried out on two different air stripping full-scale plants: the first is a packed column reactor and the second is an air bubble reactor without filling material in order to reduce the issues concerning fouling. In this study, the advantages and disadvantages of the alternative solutions are reported.
2. Material and Methods
In this section, the characteristics of the ammonia stripping plants at full-scale are reported. The main characteristics of liquid digestate (treated in these plants) are also shown. Moreover, the operative conditions of the tests carried out during the experimental activities are reported.
2.1. Description of Plants and Digestate Characteristics
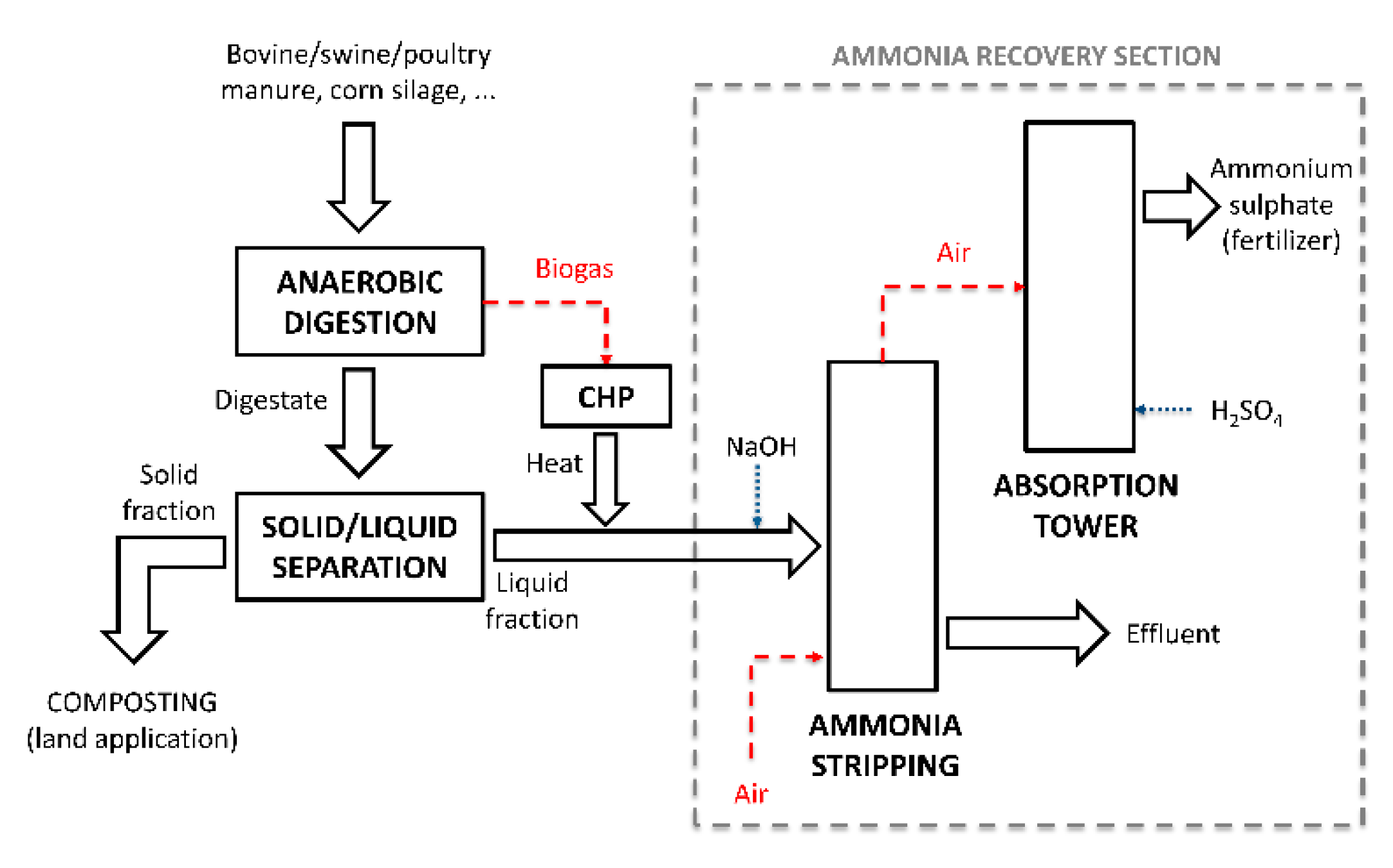
The
Figure 1 reports a schematic diagram of the process for the anaerobic treatment of livestock manure and agricultural residues in order to recover ammonia as fertilizer. The treatment consists of an anaerobic digestion where bovine, swine, poultry manure, and corn silage are fed as well as a solid/liquid separation for the obtaining of liquid digestate and the subsequent ammonia recovery section. Three different flows come from the treatment including: (i) the solid fraction, which is sent to a composting process, (ii) the liquid fraction, which is sent to ammonia stripping, and (iii) the air contaminated by ammonia, which is sent to an absorption tower for fertilizer production.
During the experimental activities, two different kinds of plants were used. The stripping reactor of plant #1 consists of a packed column that receives the liquid digestate obtained from a rotary drum with a mesh of 350 µm followed in a second phase of experimentation by a vibrating screen with a mesh of 150 µm. The ammonia stripping section of plant #2 is an air bubble reactor that treats the liquid digestate derived from a screw press separator with a mesh of 5 mm. Both plants are equipped with a similar packing absorption tower for ammonium sulphate production (see
Table 1).
Regarding plant #1 (
Figure 2a), the ammonia stripping reactor is a vertical column filled with packing material. The specific surface is 200 m
2 m
−3. The flow treated is 2.5 m
3 h
−1 and the air flowrate is about 4500 m
3 h
−1. In the first part of the experiment, plant #1 operated continuously while, in a second period in a semi-batch mode (i.e., liquid phase in batch with air flow). In semi-batch conditions, the volume of liquid digestate (5 m
3 and 13.2 m
3 for plant#1 and #2, respectively) was maintained into the stripping reactor for a suitable time (2 h for plant #1 and 6 h for plant #2). The air flowrate is supplied continuously.
In the stripping column of plant #2 (
Figure 2b), the air is supplied with medium/fine bubble diffusers. The plant worked both in continuous (with complete mixing) and in a semi-batch mode.
In both plants, the ammonia recovery is obtained in an acid scrubber with a pH of 4.3. In these conditions, even if the concentration of ammonia in the absorbent solution reaches the 10% w/w (weight to weight), the concentration of NH3 in the flu-gas is maintained around 3 to 5 mgN m−3 of dry air. For the absorption process, a 50% v/v (volume/volume) sulphuric acid (H2SO4) solution is used, which allows the production of di-ammonium sulphate solution with a nitrogen content of 8% to 10%.
The geometrical characteristics of the plants used in the experimental activities are reported in
Table 1. In
Table 2, the qualitative characteristics of the liquid fraction of digestate fed to the different plants are shown. It can be observed that plant #2 is able to treat liquid fraction with suspended solids content up to 5%. This value cannot be reached in the case of plant #1 due to the issue concerning the fouling of packing material. In fact, the configuration of plant #1 does not allow the treatment of the substrate with a suspended solid content higher than 2%.
2.2. Operative Conditions of Experimental Tests
The operative conditions of the tests carried out during the experiment are shown in
Table 3. Concerning plant #1, at the beginning of the experiment, it worked in a continuous mode (phase I) with pH and temperature ranging from 9.5 to 10 pH and from 35 to 40 °C, respectively. Then the plant worked in semi-batch conditions (phase II) with pH ranges from 9.2 to 9.5. In this case, the alkaline reagent (NaOH) consumption was significantly lower than in phase I.
The semi-batch conditions, due to the high rates of ammonia removal, reached the same performance obtained in the continuous mode, but with pH values significantly lower, which indicates an important reduction of alkaline reagent consumption.
The vibrating screen with a mesh of 150 µm works at the end of phase I.
At the beginning of experimental activities with plant #2, a preliminary test was performed in semi-batch conditions using an aqueous solution of ammonium sulphate with ammonia concentration equal to 2500 mgN L−1. Then plant #2 worked in a continuous mode with a temperature of 48 °C and pH ranging from 9.5 to 10.1 (obtained with NaOH dosage). This test showed a foaming issue due to the dosage of an alkaline reagent and the air supply with medium/fine bubble diffusers. Thus, 6 additional tests, always in a continuous mode, were performed without the use of an alkaline reagent (4 tests) and with the presence of NaOH and a defoamer agent (2 tests).
In order to evaluate the influence of operating parameters on the ammonia removal in plant #2, a deeper study based on a two-level factorial experiment is developed. The development of the factorial experimentation was focused on three operating parameters: temperature, digestate flowrate, and air flowrate. The pH was not modified with an alkaline reagent dosage. In this case, the stabilization of pH (at 9.2 to 9.3) is due to the equilibrium obtained from CO2 and ammonia removal. This choice is due to the high concentration of bicarbonate ion in the solution fed to the plants. In fact, the high concentration of bicarbonate ion (0.32 N) involves that the increase (with NaOH solution) of digestate pH more than 9.2 is too expensive and the stabilization of pH (without a reagent dosage) to a pH of 9.2 to 9.3 (from 7.7 to 7.8), due to the CO2 removal obtained from the bicarbonate decomposition, is favored.
The variation of operating parameters in the factorial experimentation is reported in
Table 4. All tests were carried out in a continuous mode with an HRT (Hydraulic Retention Time) equal to 6 h for a higher digestate flowrate (2.2 m
3 h
−1) and 9.5 h for a lower flowrate (1.4 m
3 h
−1).
With regard to the deepening study based on a two-level factorial experiment, the correlation between the real removal yields of ammonia (RR) and the values estimated (RS) with two linear models is considered.
The aim of this study is to evaluate the effects of the operating parameters tested (temperature, digestate flowrate, and air flowrate) on ammoniacal nitrogen removal yields by the use of a simple linear model in which no constant is present: the hypothesis is RS = RR for RR = 0.
The following linear model was applied below.
where T is the temperature [°C], Q
a is the air flowrate [Nm
3 h
−1], Q
d is the digestate flowrate [m
3 h
−1], and a, b, and c are the weights of the operating parameters reported above.
In addition, a more complex linear model is proposed. In this case, the correlation between the operating parameters at two and three levels is taken into account.
3. Results
The results of the tests carried out on plant #1 and plant #2 are reported in this section. In particular, the effects of different operative conditions on the ammonia removal yields are shown. Moreover, the results of a two-level factorial experiment (carried out on plant #2) are reported in order to study the influence of operating parameters on the ammonia removal in plant #2.
3.1. Plant #1 Results
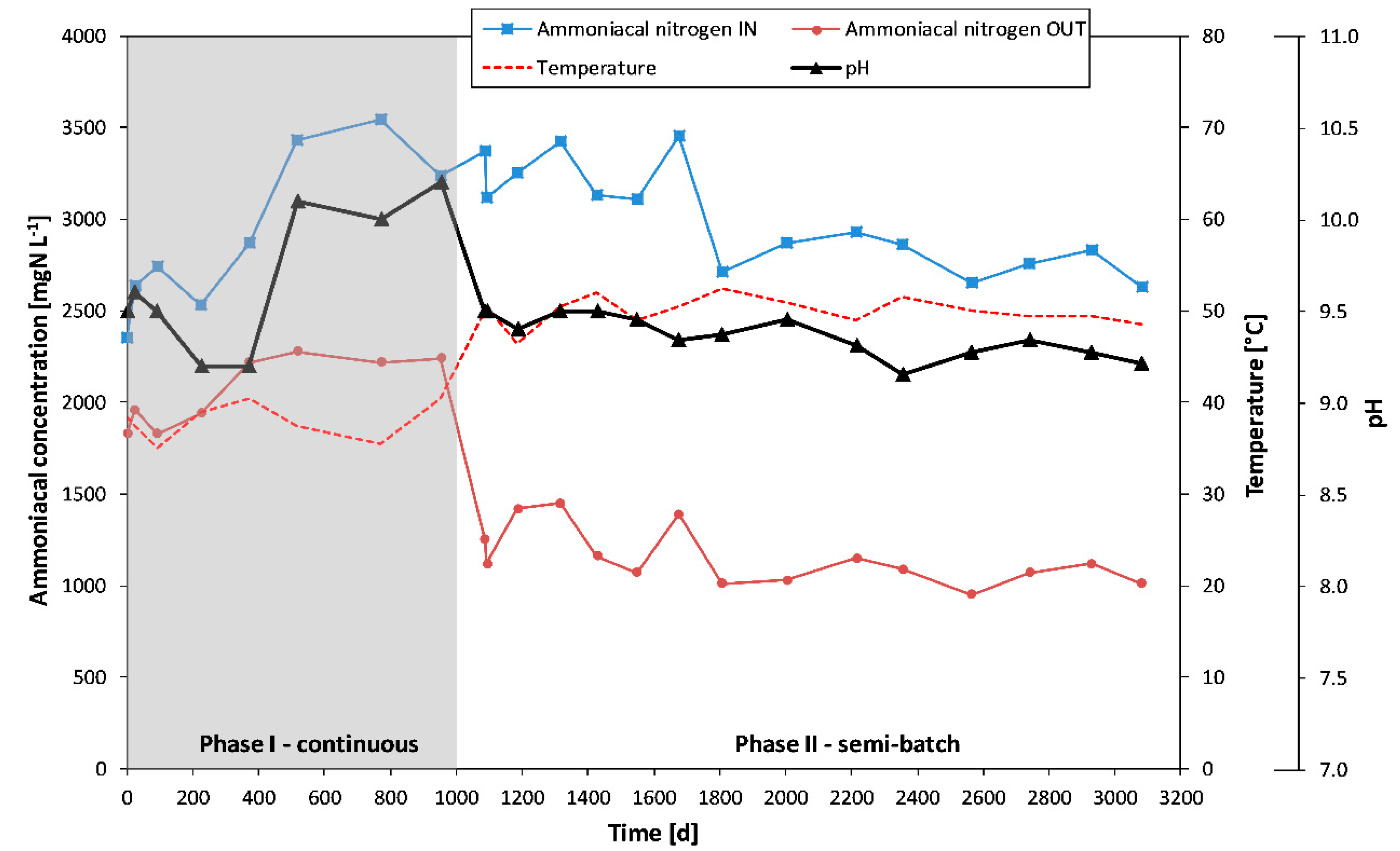
Figure 3 reports pH and temperature in the stripping reactor and the trend of both ammoniacal nitrogen input and output concentrations during the working of plant #1 (both in a continuous and a semi-batch mode). Moreover, in
Figure 4, the ammonia removal yields are reported.
As shown in these figures, the removal yields on plant #1 that works in a continuous mode (like a rear-mixed plug-flow column) varied from 22% to 37%. In these conditions (phase I), the system has a low efficiency. Low enhancement of ammonia removal yields (mean values from 25% up to 34%) can be obtained only by increasing the dosages of an alkaline reagent by 2 to 2.5 times (as shown by tests #6, #7, and #8 in which the pH rises from mean values of 9.4—in tests #1 to #5—up to 10.1).
When plant #1 worked in semi-batch conditions (phase II) and a pH similar to the first period of phase I (mean value of 9.4) performance increased, the ammonia removal yields raised from 25% (mean value of the first period of phase I) to 66% (tests #14). Moreover, in these conditions, the operative pH has been obtained with a lower dosage of an alkaline reagent (less than about 30%) with respect to a continuous mode (phase I).
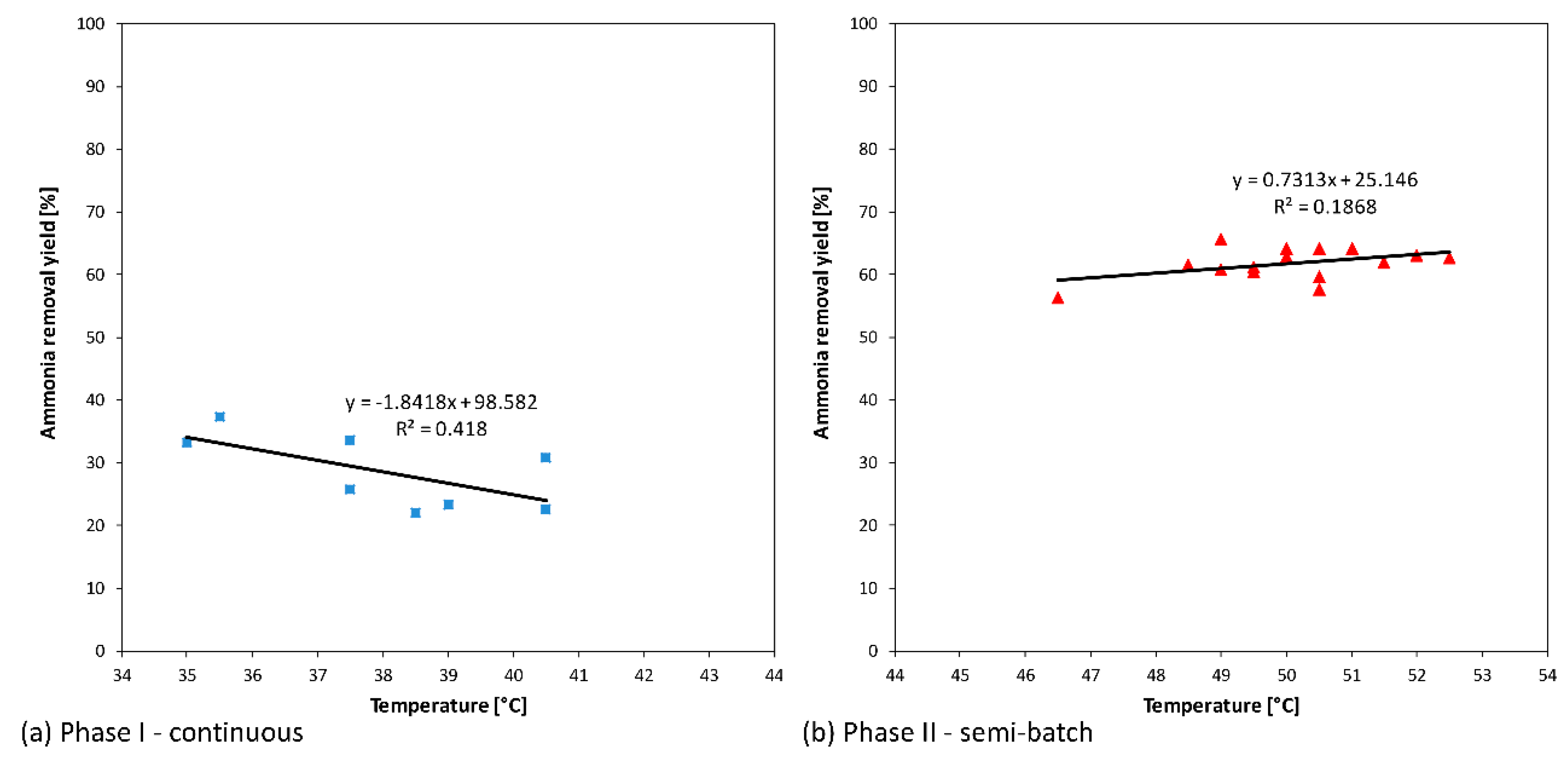
According to the Henry law on gas/liquid equilibrium of ammonia/water system, in the same conditions, the ammonia removal yields increase with a rising temperature. As expected, in
Figure 4b, the performance enhancement of the system vs. temperature can be observed. The low value of the correlation index (R
2) equal to 0.19 point out that, in addition to the temperature, several operative parameters (that are controlled such as pH or uncontrolled such as fouling) have an important effect on the results.
The influence of these parameters is more evident in
Figure 4a in which the decrease of ammonia removal yields seems be due to the temperature increasing.
The results reported in
Figure 4 show that an increase in the ammonia removal yields from 25% to 62% (mean values) can be observed when the temperature increases from the mean values of 38 °C (
Figure 4a) to 50 °C (
Figure 4b). These results are consistent with the Henry law and with the work conditions that changed from a continuous to a semi-batch mode.
3.2. Plant #2 Results
Regarding plant #2,
Table 5 shows the trend of the input and output ammoniacal nitrogen concentrations and the removal yields obtained during continuous and semi-batch (for preliminary test only) conditions. The operating temperature and pH are also reported.
In the preliminary test (in the semi-batch mode), the ammonia removal yield is equal to 79%.
The lower ammonia removal yields (from 23% to 39%) are obtained in a continuous mode with a temperature ranging from 45 to 55 °C and with pH ranging from 9.2 to 9.5 (obtained without an alkaline reagent dosage). Moreover, the increase of temperature and pH improves the performance. An ammonia removal yield of 66% was reached when operating at 58 °C and a pH equal to 10.5.
It can be noted that the high bicarbonate concentrations in the digestate fed to the stripping reactor with the temperature increasing by 45 to 55 °C allow us to work with a pH from 9.2 to 9.5 without the dosage of a strong alkaline reagent. This behavior is due to the thermal decomposition of bicarbonates in carbonates with the development of CO2, which is subsequently removed from the aqueous phase.
When the pH value is 9.3 to 9.5, the concentration of un-dissociated ammonia (also known as free ammonia nitrogen) is about 40% while the ammonium ion content (NH4+) is 60%. The increase of pH up to the value of 10.5 significantly enhances the performance by raising the free ammonia nitrogen percentage up to values close to 100%.
Regarding the study based on a two-level factorial experimentation, the results (in terms of real removal yields of ammonia—R
R) are reported in
Table 6.
The results of the simple linear model (Equation (1)), obtained by the minimization of the squared error sum (RR − RS)2, are the following: a = 0.711, b = 0.0228, and c = −7.88.
Regarding the more complex linear model (Equation (2)), the results are: a = 1.898, b = −0.2387, c = −8.4630, d = 0.00253, e = −0.6221, f = 0.1297, and g = −0.0012.
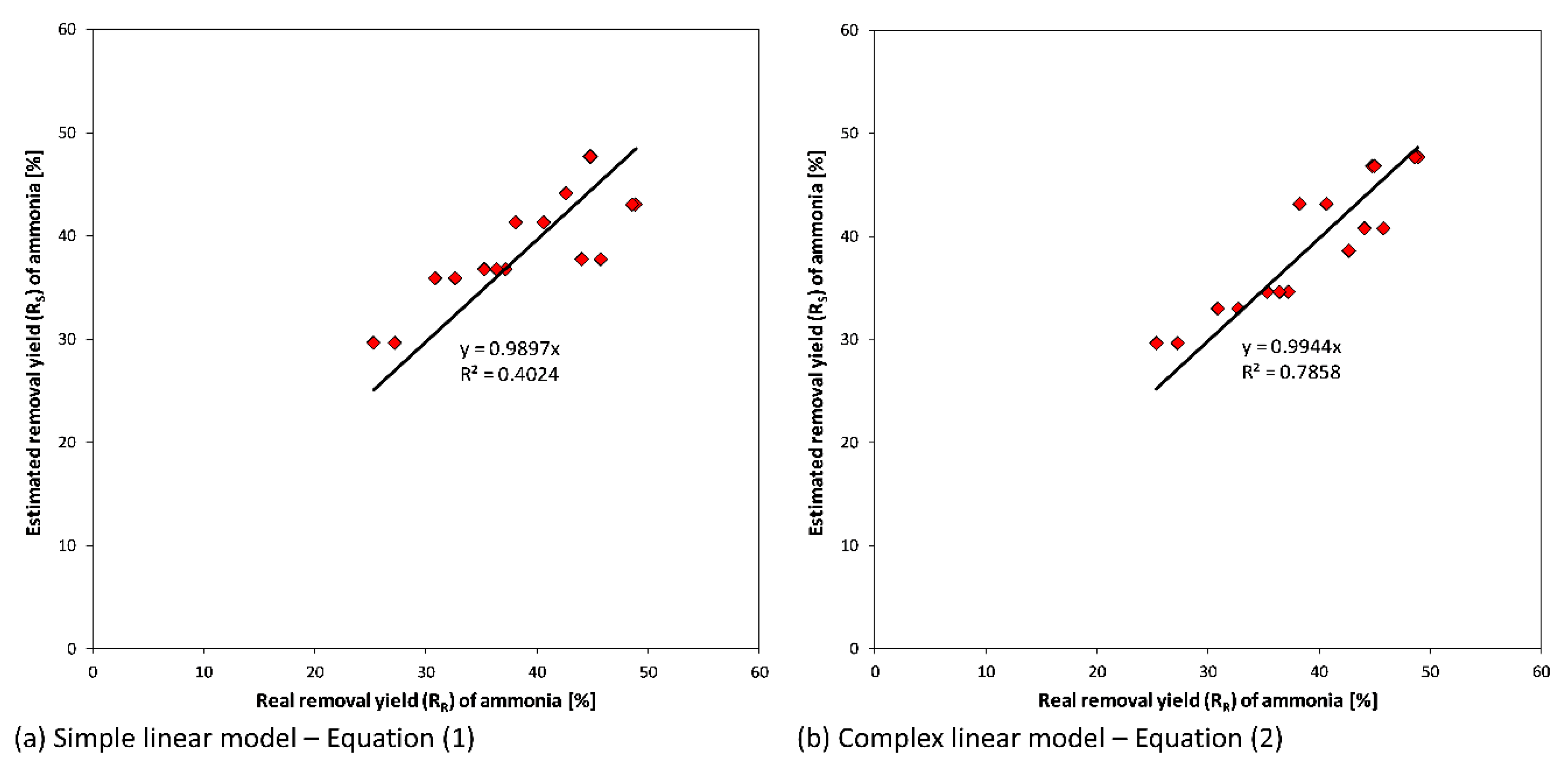
In
Figure 5, the correlations between R
R and R
S for both models are reported. Concerning the linear model described with Equation (1), the slope of the interpolation line is near 0.99. This result suggests that the operating parameters taken into account play an important role in process performance (
Figure 5a). Despite the fact that the correlation coefficient (R
2) is not very high due to the variation of digestate characteristics during the experimentation, the model is able to obtain a suitable estimation of ammoniacal nitrogen removal yields.
Regarding the more complex model (Equation (2)), the results obtained (
Figure 5b) in terms of a correlation coefficient are better than the first model. However, the physical meaning of the calculated weights is very difficult to explain. Therefore, the simple linear model (Equation (1)) is recommended. The calculated values of weights (a, b, and c) revealed the positive effects of temperature and air flowrate on the ammonia removal yields while it is observed that the increase of digestate flowrate (with a reduction of the mean HRT in the stripping reactor) involves a performance reduction.
Since the weights are inversely related to the values of respective operating parameters (T, Qa, and Qd) in order to define the real weight of the variables on the ammoniacal nitrogen removal yield, which are the coefficients obtained by using the square error sum minimization reported above, these have been normalized. The coefficients a, b, and c have been multiplied by the 10% of the average value of the corresponding parameters. The results obtained are: anormalized = 3.91, bnormalized = 1.37, and cnormalized = −1.42. Therefore, the normalization (to the range 0–100) of the mean values for each parameter (T = 55 °C, Qa = 600 Nm3 h−1, Qd = 1.8 m3 h−1) was carried out.
4. Discussion
Concerning plant #1, the experimental results show that the main parameters that play an important role on ammonia removal are work conditions (i.e., semi-batch or continuous mode), pH, and temperature.
Concerning pH, an increase from 9.4 to 10 involves the enhancement of ammonia removal yields from 25% to 34%. As confirmed by Guštin and Marinšek-Logar [
28], the increase of pH values promote the ammonia removal but only up to a pH of 10.
The effects of temperature and work conditions are not evaluable individually because the change from continuous to semi-batch conditions falls with the main increasing of the temperature (from 38 to 50 °C).
The results point out that the work conditions of the plant (i.e., semi-batch or continuous mode) should be the main parameters that affect the performance of a stripping reactor. We have observed that, in semi-batch conditions, the ammonia removal yields are higher than 50% with respect to a continuous mode operation.
The management of plant #1 highlighted the following main issues: high-energy costs and fouling. The high-energy costs are mainly due to the electrical power required from the radial fan (12 to 14 kW) and the thermal power (120 kW in winter conditions) for the preheated air supply in the stripping reactor (4000 to 4500 Nm3 h−1 at 45 °C) by using an air/water exchanger. Furthermore, regarding the fouling issue, a more efficient digestate pretreatment (with a mesh of 150 µm) with respect to the conventional solid/liquid separators (screw press or rotary drum) must be applied. Therefore, a better removal of suspended solids especially with a fibrous matrix can be obtained. The concentration of suspended solids in the pretreated digestate inlet to stripping column is lower than 1% and the fouling of packing material is reduced with a significant performance improvement.
Plant #2 worked only in a continuous mode (with the exception of the preliminary test—see
Table 3). The results obtained during the nine months of management (HRT equal to 6 h) with a temperature variation from 48 to 58 °C (that depends on external weather conditions) showed that the pH seems to be the main parameter that affects the performance. Therefore, the dosage of NaOH is the key factor regarding (i) the ammonia removal yields and (ii) the management costs of the plant.
An important result obtained from this work concerns the equilibrium pH, which, as already pointed out, at a temperature between 45 to 55 °C, autonomously (without the dosage of alkaline reagent) reaches values from 9.2 to 9.5, i.e., in a suitable range to obtain an efficient ammonia removal from the aqueous phase. These results are confirmed by previous experimental works [
29,
30]. However, this aspect is strictly related to the characteristics of the substrate treated with AD especially to its alkalinity that depends both on the different feeding substrate and on the anaerobic degradation process of the same substrate.
In any case, in order to verify this operational feasibility on the digestate treated during this work, the two-levels of factorial experimentation are based on three parameters (temperature, air flowrate, and digestate flowrate) was developed. The two-level factorial test has proven that the temperature is a main parameter when no alkaline reagent is added. In this case, despite the fact that no statistical analyses have been carried out, the temperature seems to significantly affect the process performance. In fact, the temperature influences the reaction kinetics and the equilibrium of phase transfer by an exponential law. However, the other two parameters (air and digestate flowrates) must be taken into account in order to obtain ammonia removal yields that allow the correct management of nitrogen content in the digestate. The experiment results suggest the need for a proper management of available thermal energy with the aim to operate always in optimal conditions with respect to the process temperature.
The study of plant #2 allows us to confirm the feasibility to work on digestate with high concentrations of suspended solids without the occurrence of a fouling problem. Moreover, it was observed that, despite the fact that the high concentration of suspended solids (values higher than 1%) has a negative effect on the rheology, this aspect does not affect the alkaline reagent dosage to obtain pH values from 10.0 to 10.5. The amount of alkaline reagent significantly influences the digestate alkalinity.
Concerning economic aspects, the operating cost, in terms of euro per m3 of digestate treated, related to plant #2 (without the dosage of alkaline reagent) is about one-third of that in plant #1. The avoided dosage of the alkaline reagent involves a significant cost saving (estimated in 2–3 € per m3 of digestate). Moreover, regarding the air supply, the electrical power of the rotary lobe compressor in plant #2 (7–8 kW) is lower than the value required for the radial fan of plant #1 (12–14 kW). Lastly, regarding plant #1, the semi-batch operative mode seems to be more efficient than the continuous mode with complete mixing.
Future developments regarding the study of a stripping reactor allowed to treat (with a lower thermal energy input) livestock manure (or other substrate) with low energy content cannot suitable for biogas production with anaerobic digestion. In this case, the thermal energy required for the process could be obtained from the protonation/dissolution reaction of ammonia gas in sulphuric acid.
5. Conclusions
In this work, two different ammonia stripping full-scale plants are studied. The substrate tested is liquid digestate derived from the anaerobic digestion of livestock manure and corn silage.
Plant #1, based on a packed column, shows low ammonia removal yields (from 22% to 37%) when it works in a continuous mode. Instead, the semi-batch conditions increase the performance (up to 66%) and reduce the alkaline reagent dosage due to the application of pH significantly lower than the values maintained in the continuous mode tests (9.2–9.5 with respect to 10–10.2). Despite these results, the main issues are related to high-energy costs due to the preheated air supply and to the significant digestate pretreatment required to avoid fouling.
Therefore, plant #2, based on an air bubble reactor without filling material, was studied. The lower performance (ammonia removal yields from 23% to 39%) were obtained in a continuous mode with temperatures ranging from 45 to 55 °C and, without an alkaline reagent dosage, pH autonomously raised from 9.2 to 9.5. When the temperature and pH increase, the performance enhances and reaches an ammonia removal yield of 66%.
The use of plant #2 allows us to obtain ammonia removal yields up to 50% (with HRT equal to 9.5 h) in a continuous mode. This value is higher than the performance obtained with the use of plant #1 in a continuous operation. Moreover, the fouling issue is avoided, the air flowrate required is one order of magnitude lower, and no dosage of NaOH is provided. This aspect is very important because the digestate with low sodium content can be recovered on agricultural soil avoiding issues concerning a salinity increase.