Potential Recovery Assessment of the Embodied Resources in Qatar’s Wastewater
Abstract
1. Introduction
2. Aims of the Study and General Information
2.1. Research Objectives and Overview
- Mass flows of solids (dissolved, suspended and volatile);
- Mass flows of COD (Chemical Oxygen Demand), BOD (Biochemical Oxygen Demand), and Oil and Grease;
- Mass flows of nutrients (phosphorous and nitrogen);
- Mass flows of alkalinity, chloride, and sulfide.
2.2. Background Information
2.2.1. Solids in Wastewater
2.2.2. Organic Matter (COD, BOD, Oil and Grease)
2.2.3. Nutrients in Wastewater (Phosphorous, Nitrogen)
2.2.4. Other Constituents (Alkalinity, Chloride, Sulfide)
3. Materials and Methods
3.1. Study Area and Data Sources
3.2. Type of Information
3.3. Information Processing
4. Results and Discussion
4.1. Wastewater Characterization in Qatar
- The ratio of Volatile Suspended Solids (VSS) to the Total Suspended Solids (TSS) is lower than the typical value, around 80%.
- The ratio of Biochemical Oxygen Demand (BOD) to the Total Suspended Solids (TSS) is 1.33, which is higher than the typical range between 0.75 and 0.85.
- The phosphorus content is 5 mg/L, which is at the lowest end of the typical value ranges.
- The alkalinity of CaCO3 is quite high in comparison with the typical low and medium values between 50–200 mg/L.
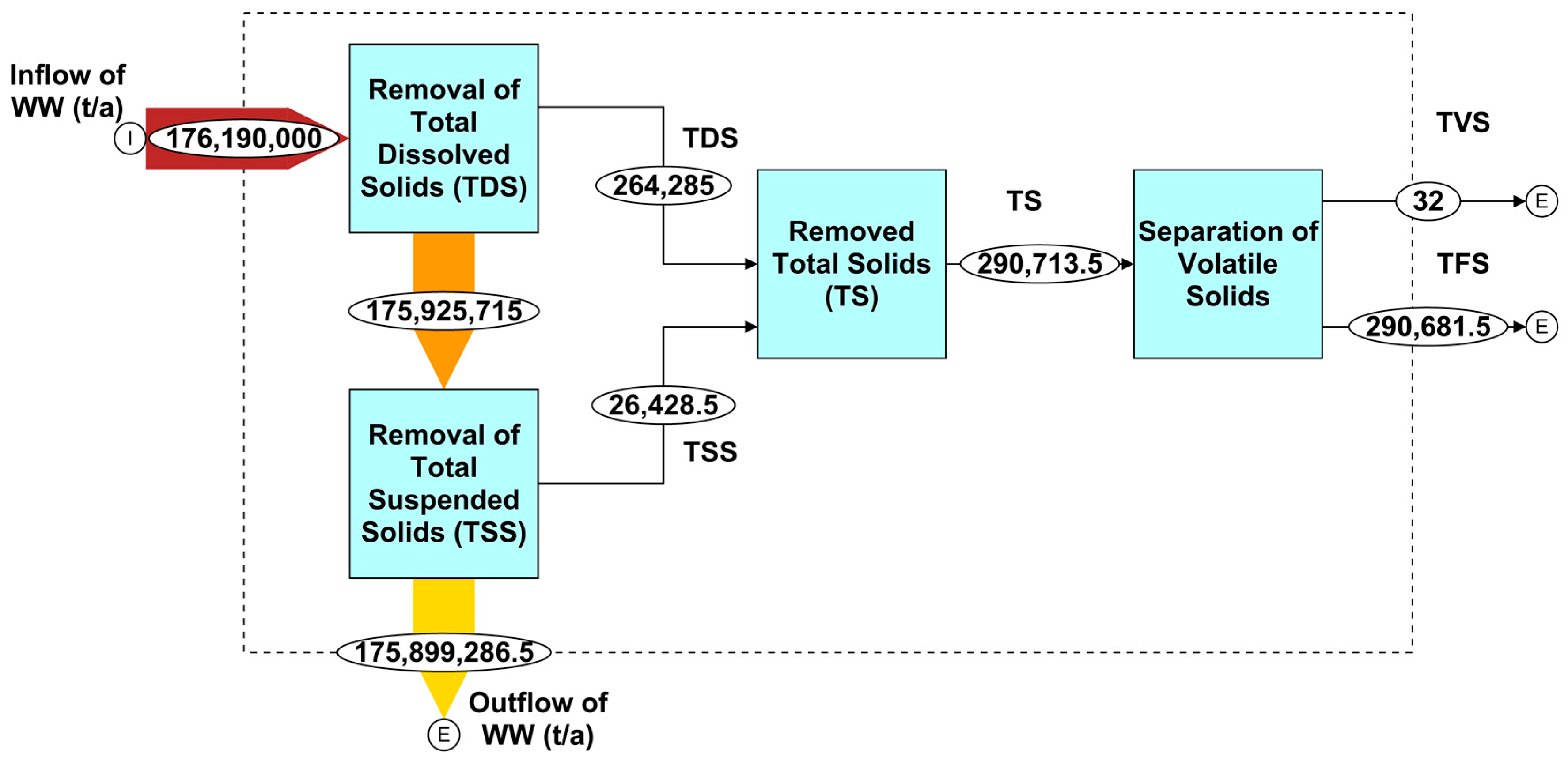
4.2. Mass Flow Analysis of Solids (Dissolved, Suspended, and Volatile) in Qatar’s Wastewater
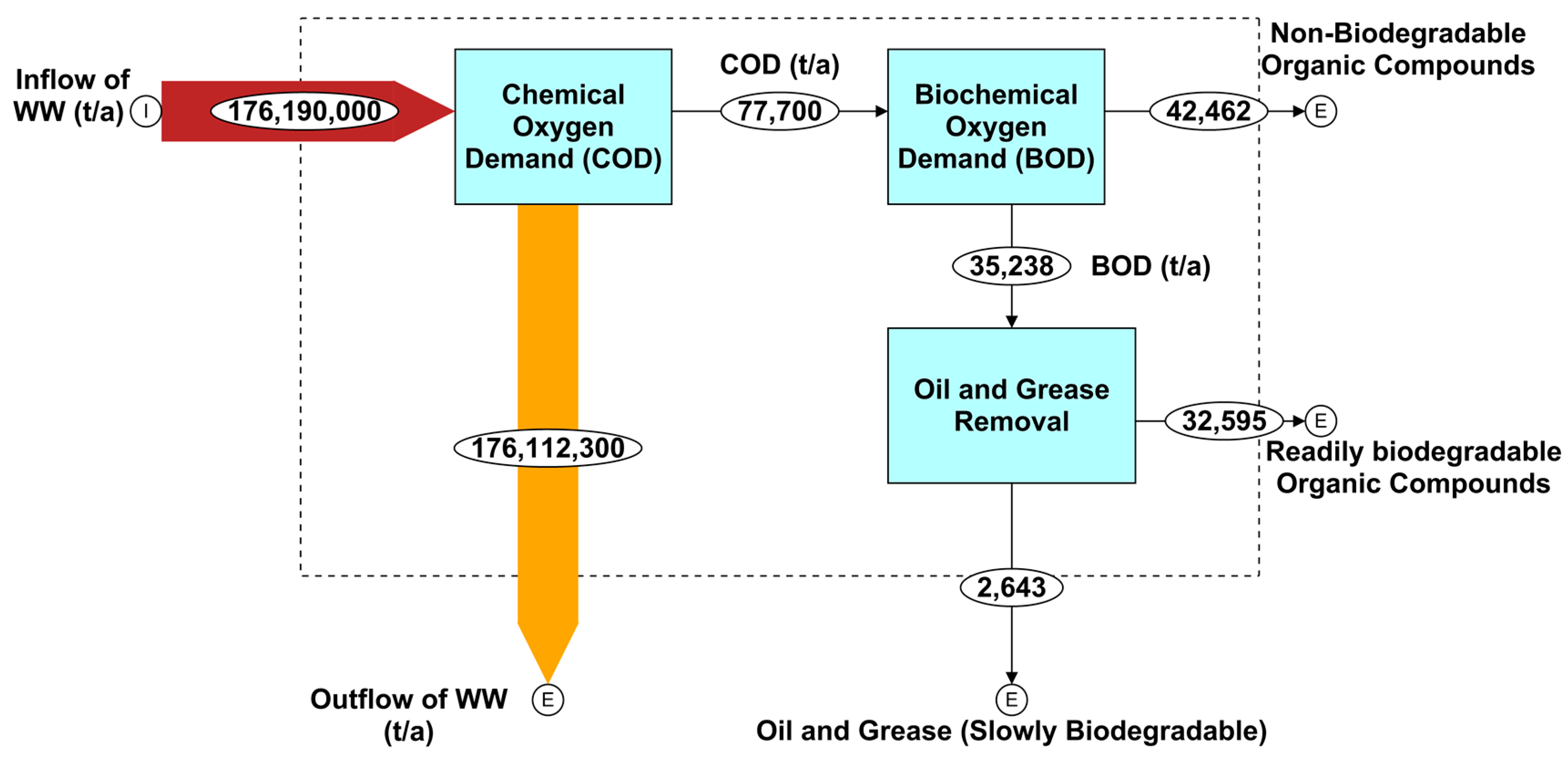
4.3. Mass Flow Analysis of COD and BOD in Qatar’s Wastewater
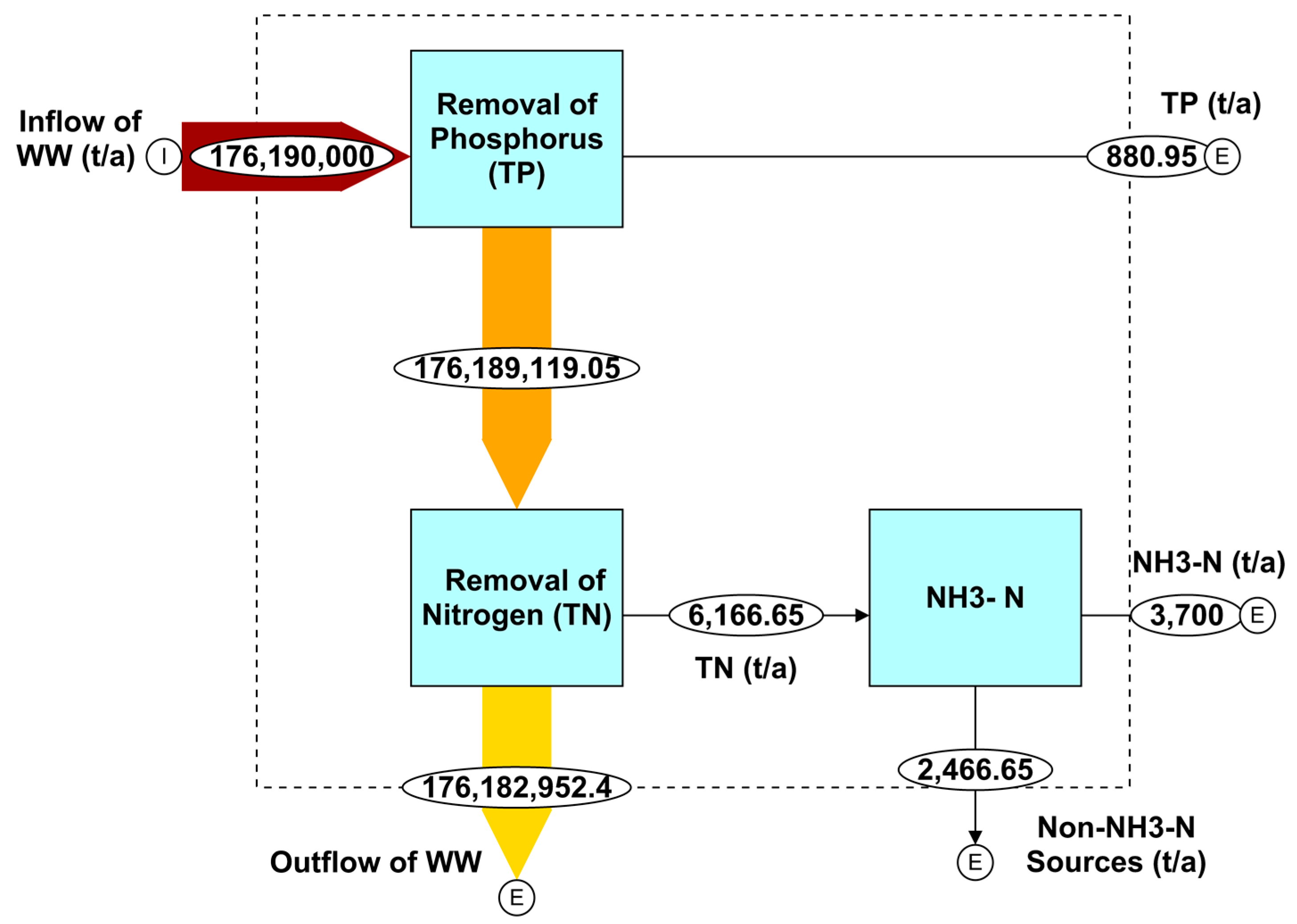
4.4. Mass Flow Analysis of Nutrients in Qatar’s Wastewater
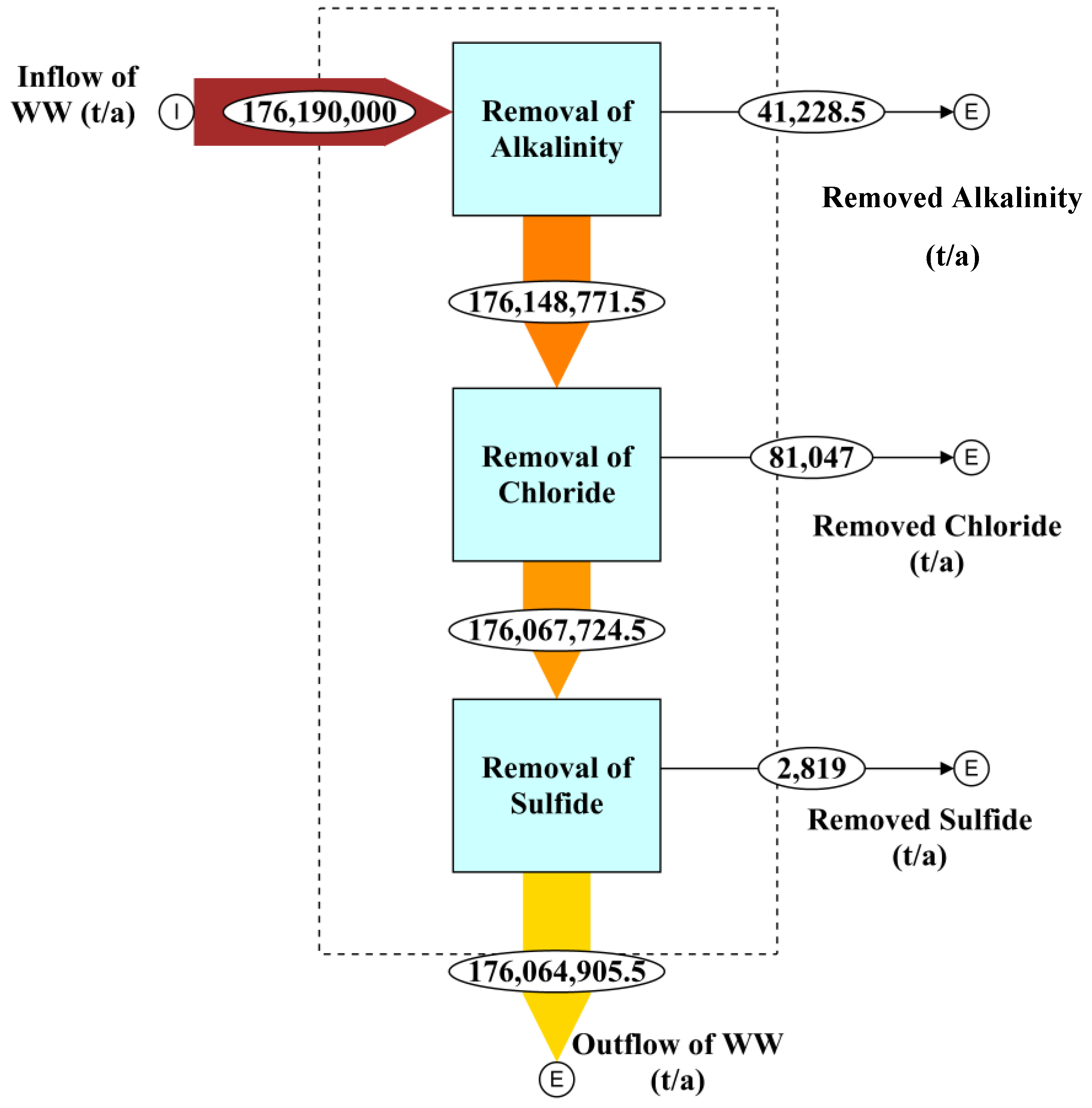
4.5. Mass Flow Analysis of Alkalinity, Chloride, and Sulfide
4.6. Relevance and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Metcalf and Eddy, Inc. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2013; ISBN 9781259250934. [Google Scholar]
- Tomar, M. Quality Assessment of Water and Wastewater; Lewis Publishers: Boca Raton, FL, USA, 1999; ISBN 9781566703826. [Google Scholar]
- Ellis, T.G. Chemistry of wastewater. In Environmental and Ecological Chemistry; Sabljic, A., Ed.; EOLSS Publishers: Oxford, UK, 2009; Volume 2, pp. 327–350. ISBN 978-1-84826-693-3. [Google Scholar]
- Almeida, M.C.; Butler, D.; Friedler, E. At-source domestic wastewater quality. Urban Water 1999, 1, 49–55. [Google Scholar] [CrossRef]
- Molden, D. (Ed.) Water for Food, Water for Life: A Comprehensive Assessment of Water Management in Agriculture; Earthscan: London, UK; IWMI International Water Management Institute: Colombo, NC, USA, 2007; ISBN 978-1-84407-396-2. [Google Scholar]
- Capodaglio, A.G. Integrated, Decentralized Wastewater Management for Resource Recovery in Rural and Peri-Urban Areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef]
- Steflova, M.; Koop, S.; Elelman, R.; Vinyoles, J.; Van Leeuwen, K. Governing non-potable water-reuse to alleviate water stress: The case of Sabadell, Spain. Water 2018, 10, 739. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abdulrahim, H.K.; Mohieldeen, Y. Qatar and GCC water security. Desalination Water Treat. 2015, 55, 2302–2325. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Coping with Water Scarcity: An Action Framework for Agriculture and Food Security; FAO Water Report 38; FAO: Rome, Italy, 2012; ISBN 978-92-5-107304-9. [Google Scholar]
- Jaramillo, M.F.; Restrepo, I. Wastewater reuse in agriculture: A review about its limitations and benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef]
- Mo, W.; Zhang, Q. Energy-nutrients-water nexus: Integrated resource recovery in municipal wastewater treatment plants. J. Environ. Manag. 2013, 127, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Qatar Ministry of Development Planning and Statistics. Water Statistics in the State of Qatar 2015; Qatar Ministry of Development Planning and Statistics: Doha, Qatar, 2017. [Google Scholar]
- Manawi, Y.; Kayvani Fard, A.; Hussien, M.A.; Benamor, A.; Kochkodan, V. Evaluation of the current state and perspective of wastewater treatment and reuse in Qatar. Desalination Water Treat. 2017, 71, 1–11. [Google Scholar] [CrossRef]
- Qatar General Secretariat for Development Planning. Qatar National Development Strategy 2011–2016, Towards Qatar National Vision 2030; Qatar General Secretariat for Development Planning: Doha, Qatar, 2011. [Google Scholar]
- Eckard, R. Global Markets and Technologies for Water Recycling and Reuse; BCC Research: Wellesly, MA, USA, 2017. [Google Scholar]
- De Boer, M.A.; Romeo-Hall, A.G.; Rooimans, T.M.; Slootweg, J.C. An assessment of the drivers and barriers for the deployment of urban phosphorus recovery technologies: A case study of The Netherlands. Sustainability 2018, 10, 1790. [Google Scholar] [CrossRef]
- Römer, W.; Steingrobe, B. Fertilizer Effect of phosphorus recycling products. Sustainability 2018, 10, 1166. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The potential phosphorus crisis: Resource conservation and possible escape technologies: A review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Lin, Y.; Guo, M.; Shah, N.; Stuckey, D.C. Economic and environmental evaluation of nitrogen removal and recovery methods from wastewater. Bioresour. Technol. 2016, 215, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H. Energy recovery from wastewater treatment plants through sludge anaerobic digestion: Effect of low-organic-content sludge. Environ. Sci. Pollut. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- El-Shafai, S.A.; El-Gohary, F.A.; Naser, F.A.; Van der Steen, P.; Gijzen, H.J. Nitrogen recovery in an integrated system for wastewater treatment and tilapia production. Environmentalist 2007, 27, 287–302. [Google Scholar] [CrossRef]
- Luo, H.; Qin, B.; Liu, G.; Zhang, R.; Tang, Y.; Hou, Y. Selective recovery of Cu2+ and Ni2+ from wastewater using bioelectrochemical system. Front. Environ. Sci. Eng. 2015, 9, 522–527. [Google Scholar] [CrossRef]
- Jiang, K.; Zhou, K. Recovery of cryolite with high molar ratio from high fluorine-containing wastewater. Euro-Mediterr. J. Environ. Integr. 2017, 2, 22. [Google Scholar] [CrossRef]
- Li, Q.; Liu, T.; Deng, P. Recovery of mercury and lead from wastewater by sulfide precipitation-flotation. In Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2015; pp. 667–674. [Google Scholar]
- Lu, B.; Xu, J.; Zhang, M.; Pang, W.; Xie, L. Phosphorus removal and recovery from wastewater by highly efficient struvite crystallization in an improved fluidized bed reactor. Korean J. Chem. Eng. 2007, 34, 2879–2885. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wu, S.; Yang, Z.H.; Zhao, F. Nitrogen recovery from wastewater using microbial fuel cells. Front. Environ. Sci. Eng. 2016, 10, 185–191. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q.; Rittmann, B.E. Chemistry: Reuse water pollutants. Nature 2015, 528, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.C.; Xue, X.; González-Mejía, A.; Garland, J.; Cashdollar, J. Sustainable water systems for the city of tomorrow—A conceptual framework. Sustainability 2015, 7, 12071–12105. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Henze, M.; Comeau, Y. Wastewater characterization. In Biological Wastewater Treatment: Principles, Modelling and Design; Henze, M., Van Loosdrecht, M.C.M., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2008; Chapter 3; pp. 33–52. ISBN 9781843391883. [Google Scholar]
- Henze, M.; Ledin, A. Types, characteristics and quantities of classic, combined domestic wastewaters. In Decentralised Sanitation and Reuse: Concepts, Systems and Implementation; Lens, P., Zeeman, G., Lettinga, G., Eds.; IWA Publishing: London, UK, 2001; Chapter 4; pp. 59–72. ISBN 1900222477. [Google Scholar]
- Westcot, D.W. Quality Control of Wastewater for Irrigated Crop Production; FAO Water Report 10; FAO: Rome, Italy, 1997; ISBN 92-5-103994-1. [Google Scholar]
- Dökmen, F. Temporal variation of biological oxygen demand (BOD), chemical oxygen demand (COD), and pH values in surface waters of Gölcük-Kocaeli, Turkey. In Plants, Pollutants and Remediation; Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 341–347. ISBN 978-94-017-7193-1. [Google Scholar]
- Orhon, D.; Cokgör, E.U. COD fractionation in wastewater characterization—The state of the art. J. Chem. Technol. Biotechnol. 1997, 68, 283–293. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Kelly, W.R.; Panno, S.V.; Hackley, K. The Sources, Distribution, and Trends of Chloride in the Waters of Illinois; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2012. [Google Scholar]
- Ashghal Public Works Authority Qatar, Schlumberger Water Services. Shallow Groundwater Monitoring in Greater Doha, Wakrah and Al Khor; Final Hydrogeological Report of Consultancy Project Implemented by Schlumberger Water Services (project cp761); Ashghal Public Works Authority Qatar: Doha, Qatar, 2013. [Google Scholar]
- Ashghal Public Works Authority Qatar. Qatar Integrated Drainage Master Plan, 2013; Final Master Plan Report, Document No: QAT/D110001/13/019/01; Ashghal: Doha, Qatar, 2013; Volume 1. [Google Scholar]
- Cencic, O.; Rechberger, H. Material flow analysis with software STAN. J. Environ. Eng. Manag. 2008, 18, 3–7. [Google Scholar]
- Cencic, O. Nonlinear data reconciliation in material flow analysis with software STAN. Sustain. Environ. Res. 2016, 26, 291–298. [Google Scholar] [CrossRef]
- Firmansyah, I.; Spiller, M.; de Ruijter, F.J.; Carsjens, G.J.; Zeeman, G. Assessment of nitrogen and phosphorus flows in agricultural and urban systems in a small island under limited data availability. Sci. Total Environ. 2017, 574, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Jakrawatana, N.; Ngammuangtueng, P.; Gheewala, S.H. Linking substance flow analysis and soil and water assessment tool for nutrient management. J. Clean. Prod. 2017, 142, 1158–1168. [Google Scholar] [CrossRef]
- Theobald, T.F.H.; Schipper, M.; Kern, J. Phosphorus flows in Berlin-Brandenburg, a regional flow analysis. Resour. Conserv. Recycl. 2016, 112, 1–14. [Google Scholar] [CrossRef]
- Fisher, R.M.; Alvarez-Gaitan, J.P.; Stuetz, R.M.; Moore, S.J. Sulfur flows and biosolids processing: Using material flux analysis (MFA) principles at wastewater treatment plants. J. Environ. Manag. 2017, 198, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 14, 573–586. [Google Scholar] [CrossRef]
- Kwietniewska, E.; Tys, J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Ghasimi, D.S.M.; Tao, Y.; De Kreuk, M.; Zandvoort, M.H.; Van Lier, J.B. Microbial population dynamics during long-term sludge adaptation of thermophilic and mesophilic sequencing batch digesters treating sewage fine sieved fraction at varying organic loading rates. Biotechnol. Biofuels 2015, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Fachagentur Nachwachsende Rohstoffe (FNR). Handreichung Biogasgewinnung und -nutzung, 3rd ed.; FNR: Gülzow, Germany, 2006; ISBN 3-00-014335-5. (In German) [Google Scholar]
- Ambulkar, A.R. Nutrient pollution and wastewater treatment systems. In Oxford Research Encyclopedia of Environmental Science; Oxford University Press: Oxford, UK and New York, USA, 2017. [Google Scholar] [CrossRef]
- Bartone, C.R. Reuse of wastewater at the San Juan de Miraflores stabilization ponds: Public health, environmental, and socio-economic implications. PAHO Bull. 1985, 19, 147–164. [Google Scholar]
- Shende, G.B. Status of wastewater treatment and agricultural reuse with special reference to Indian experience and research and development needs. In Proceedings of the FAO Regional Seminar on the Treatment and Use of Sewage Effluent for Irrigation, Nicosia, Cyprus, 7–9 October 1985; Pescod, M.S., Arar, A., Eds.; Butterworths: London, UK, 1985; pp. 185–209. [Google Scholar]
- Helmer, R.; Hespanhol, I. (Eds.) Water Pollution Control—A Guide to the Use of Water Quality Management Principles; Published on Behalf of UNEP and WHO; E&FN Spon: London, UK, 1997; ISBN 0-419-22910-8. [Google Scholar]
- Darwish, M.; Abdulrahim, H.; Nasser Mabrouk, A.; Hassan, A.; Shomar, B. Reclaimed wastewater for agriculture irrigation in Qatar. Glob. J. Agric. Res. Rev. 2015, 3, 106–120. [Google Scholar]
- Krishnakumar, B.; Majumdar, S.; Manilal, V.B.; Haridas, A. Treatment of sulphide containing wastewater with sulphur recovery in a novel reverse fluidized loop reactor (RFLR). Water Res. 2005, 39, 639–647. [Google Scholar] [CrossRef] [PubMed]
- IWA Resource Recovery Cluster. State of the Art Compendium Report on Resource Recovery from Water; IWA International Water Association: London, UK, 2015. [Google Scholar]
- Cai, J.; Zheng, P.; Qaisar, M.; Zhang, J. Elemental sulfur recovery of biological sulfide removal: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2079–2099. [Google Scholar] [CrossRef]
- Tang, H.; Erzat, A.; Liu, Y. Recovery of soluble chloride salts from the wastewater generated during the washing process of municipal solid wastes incineration fly ash. Environ. Technol. 2014, 35, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; He, J.; Wei, M.; Evans, D.G.; Duan, X. Uptake of chloride ion from aqueous solution by calcined layered double hydroxides: Equilibrium and kinetic studies. Water Res. 2006, 40, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Neff, J.; Lee, K.; DeBlois, E.M. Produced water: Overview of composition, fates, and effects. In Produced Water; Lee, K., Neff, J., Eds.; Springer: New York, NY, USA, 2011; pp. 3–54. ISBN 978-1-4614-0046-2. [Google Scholar]
- Mustafa, S.F. Removal of Chloride from Wastewater by Advanced Softening Process Using Electrochemically Generated Aluminum Hydroxide. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2014. [Google Scholar]
| Analysis parameter | Unit | Concentrated | Moderate | Diluted | Very Diluted |
|---|---|---|---|---|---|
| Biochemical Oxygen Demand, BOD5 * | mg/L | 350 | 250 | 150 | 100 |
| Chemical Oxygen Demand, COD * | mg/L | 740 | 530 | 320 | 210 |
| Total Organic Compounds, TOC * | mg/L | 250 | 180 | 110 | 70 |
| Total Suspended Solids, TSS * | mg/L | 450 | 300 | 190 | 120 |
| Volatile Suspended Solids, VSS * | mg/L | 320 | 210 | 140 | 80 |
| Conductivity * | μS/cm | 1200 | 1000 | 800 | 700 |
| Total Phosphorous, TP * | mg/L | 23 | 16 | 10 | 6 |
| Fats; Oil and Grease * | mg/L | 100 | 70 | 40 | 30 |
| Total Nitrogen, TN * | mg/L | 80 | 50 | 30 | 20 |
| Ammonia-Nitrogen, NH3-N ** | mg/L | 75 | 45 | 20 | N/A |
| pH ** | - | 8 | 7.5 | 7 | N/A |
| Alkalinity ** | mg/L | 350 | 200 | 50 | N/A |
| Chloride ** | mg/L | 600 | 400 | 200 | N/A |
| Sulfide ** | mg/L | 10 | 0.5 | 0.1 | N/A |
| Total Solids (TS) | Total Suspended Solids (TSS) | Total Dissolved Solids (TDS) |
| Total Volatile Solids (TVS) | Volatile Suspended Solids (VSS) | Volatile Dissolved Solids (VDS) |
| Total Fixed Solids (TFS) | Fixed Suspended Solids (FSS) | Fixed Dissolved Solids (FDS) |
| 2010 | 2011 | 2012 | 2013 | |
|---|---|---|---|---|
| Total urban wastewater generated | 121.73 | 140.31 | 164.24 | 176.19 |
| Of which treated | 101.65 | 123.89 | 142.34 | 158.79 |
| Undergoing secondary treatment only (biological treatment) | 0.20 | 0.20 | 0.25 | 0.27 |
| Undergoing secondary and tertiary treatment (nutrient removal, disinfection) | 101.45 | 123.69 | 142.09 | 157.89 |
| Of which discharged without treatment | 20.08 | 16.43 | 21.90 | 18.04 |
| Parameter | Unit | Value | Pollution Strength * |
|---|---|---|---|
| pH | - | 7.3 | Moderate |
| Conductivity | μS/cm | 2400 | Concentrated |
| Total Suspended Solids | mg/L | 150 | Diluted |
| Total Volatile Solids | mg/L | 110 | Diluted |
| Total Dissolved Solids | mg/L | 1500 | - |
| BOD5 (5 days at 20 °C) | mg/L | 200 | Moderate |
| COD | mg/L | 441 | Moderate |
| NH3-N | mg/L | 21 | Moderate |
| Total Nitrogen | mg/L | 30–40 | Moderate |
| Total Phosphorus | mg/L | 5 | Very diluted |
| Alkalinity (as CaCO3) | mg/L | 234 | Moderate to Concentrated |
| Chloride | mg/L | 460 | Concentrated |
| Oil & Grease | mg/L | 15 | Very diluted |
| Sulfide | mg/L | 16 | Concentrated |
| Total Coliform | MPN/100 mL | 107–108 | - |
| Fecal Coliform | MPN/100 mL | 104–107 | - |
| Wheat | Moong Beans | Rice | Potato | Cotton | |
|---|---|---|---|---|---|
| 8 yrs 1 | 5 yrs 1 | 7 yrs 1 | 4 yrs 1 | 3 yrs 1 | |
| Raw wastewater 2 | 3.34 | 0.9 | 2.97 | 23.11 | 2.56 |
| Settled wastewater 2 | 3.35 | 0.87 | 2.94 | 20.78 | 2.3 |
| Stabilized pond effluent 2 | 3.35 | 0.78 | 2.98 | 22.31 | 2.41 |
| Freshwater + NPK 2,3 | 2.7 | 0.72 | 2.03 | 17.16 | 1.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsheyab, M.A.T.; Kusch-Brandt, S. Potential Recovery Assessment of the Embodied Resources in Qatar’s Wastewater. Sustainability 2018, 10, 3055. https://doi.org/10.3390/su10093055
Alsheyab MAT, Kusch-Brandt S. Potential Recovery Assessment of the Embodied Resources in Qatar’s Wastewater. Sustainability. 2018; 10(9):3055. https://doi.org/10.3390/su10093055
Chicago/Turabian StyleAlsheyab, Mohammad A. T., and Sigrid Kusch-Brandt. 2018. "Potential Recovery Assessment of the Embodied Resources in Qatar’s Wastewater" Sustainability 10, no. 9: 3055. https://doi.org/10.3390/su10093055
APA StyleAlsheyab, M. A. T., & Kusch-Brandt, S. (2018). Potential Recovery Assessment of the Embodied Resources in Qatar’s Wastewater. Sustainability, 10(9), 3055. https://doi.org/10.3390/su10093055






