The Evaluation of Hazards to Man and the Environment during the Composting of Sewage Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composting Substrates and System
2.2. Analysis of Abiotic Parameters
2.3. Compost Stability
2.4. Microbiological Analysis
2.5. Antibiotic Resistance
3. Results
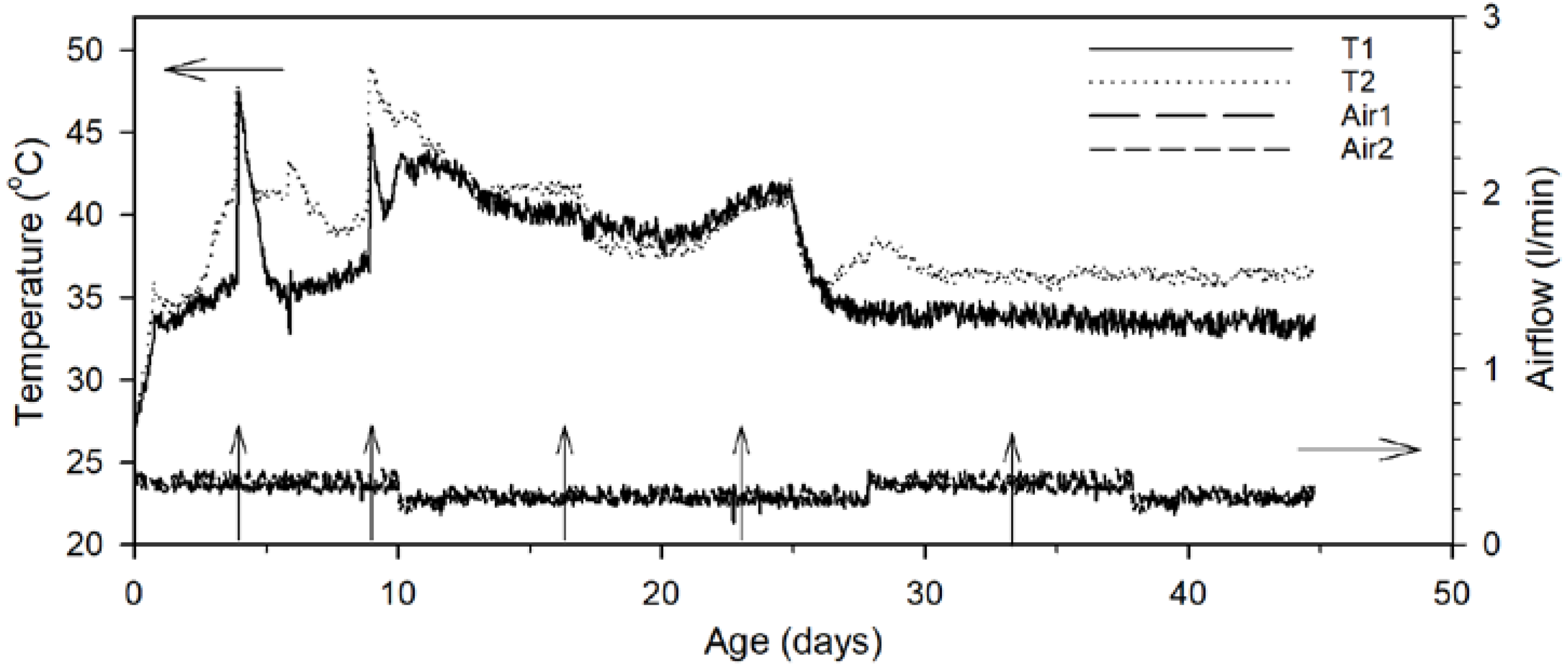
3.1. Abiotic Factors
3.2. Heavy Metals
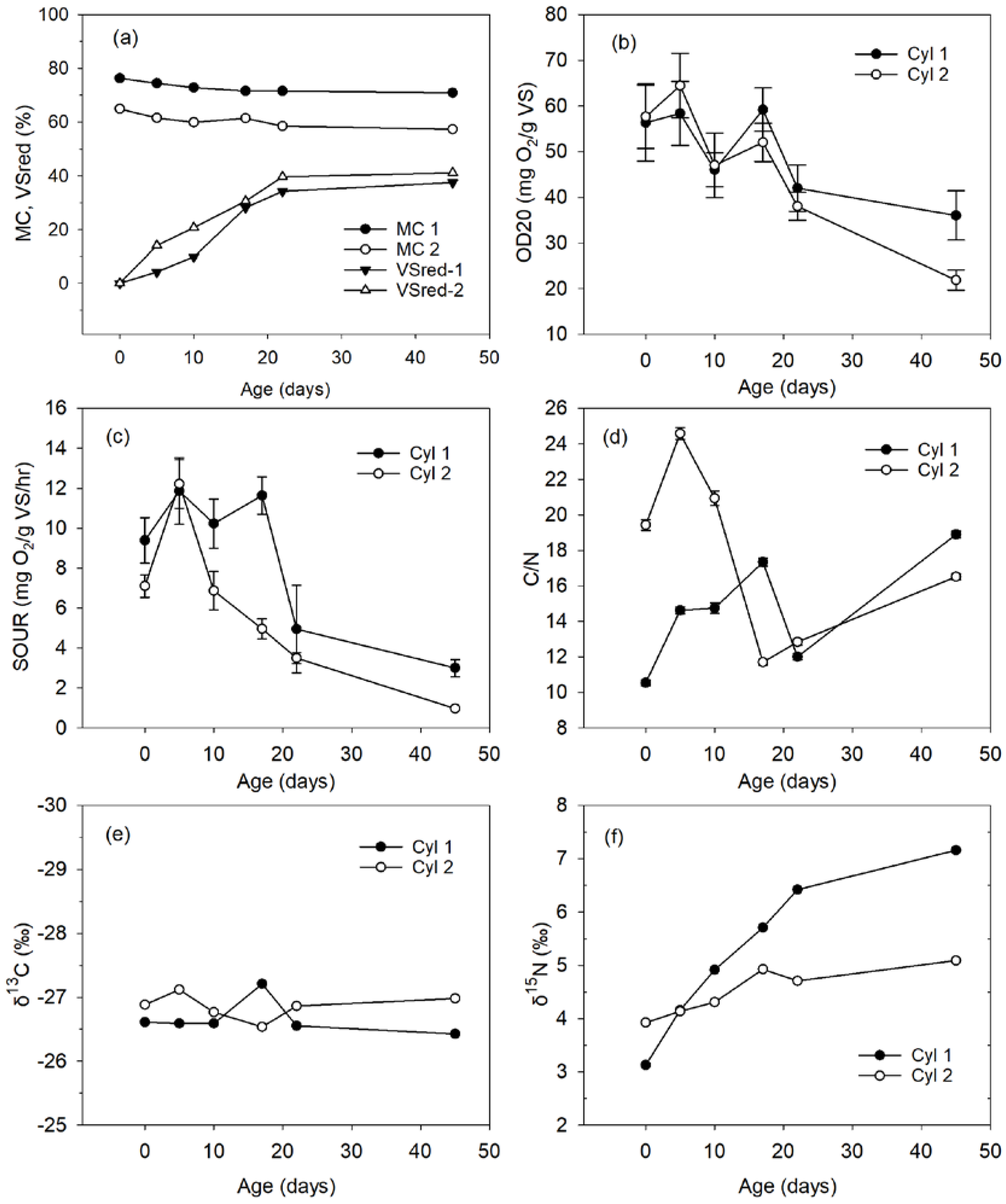
3.3. Compost Stability
3.4. Compost Sanitisation
3.5. Antibiotic Resistance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, D.; Luo, W.; Li, Y.; Wang, G.; Li, G. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions. Bioresour. Technol. 2018, 250, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, W.; Wang, K.; Li, Y. Usage of pumice as bulking agent in sewage sludge composting. Bioresour. Technol. 2015, 190, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Asses, N.; Farhat, A.; Cherif, S.; Hamdi, M.; Bouallagui, H. Comparative study of sewage sludge co-composting with olive mill wastes or green residues: Process monitoring and agriculture value of the resulting composts. Process Saf. Environ. Prot. 2018, 114, 25–35. [Google Scholar] [CrossRef]
- Dumontet, S.; Dinel, H.; Baloda, S.B. Pathogen reduction in sewage sludge by composting and other biological treatment: A review. Biol. Agric. Hortic. 1999, 16, 409–430. [Google Scholar] [CrossRef]
- Lasaridi, K.E.; Stentiford, E.I.; Evans, T. Windrow composting of wastewater sewage sludge: Process performance and product stability assessment. Water Sci. Technol. 2000, 42, 217–226. [Google Scholar] [CrossRef]
- Peigne, J.; Girardin, P. Environmental impacts of farm-scale composting practices. Water Air Soil Pollut. 2004, 153, 45–68. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Selvam, A. Speciation of heavy metals during co-composting of sewage sludge with lime. Chemosphere 2006, 63, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Kastner, M.; Mahro, B. Microbial degradation of polycyclic aromatic hydrocarbons in soil affected by the organic matrix of compost. Appl. Microbiol. Biotechnol. 1996, 44, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.L.; Tsang, Y.Y.; Chiu, S.W. Use of spent mushroom compost to bioremediate PAH-contaminated samples. Chemosphere 2003, 52, 1539–1546. [Google Scholar] [CrossRef]
- Moretti, S.M.L.; Bertoncini, E.I.; Abreu-Junior, C.H. Composting sewage sludge with green waste from tree pruning. Sci. Agric. 2015, 72, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Aubain, P.; Gazzo, A.; Moux, J.L.; Mugnier, E.; Brunet, H.; Landrea, B. Disposal and Recycling Routes for Sewage Sludge; European Comission DG Environment: Brussels, Belgium, 2002. [Google Scholar]
- Deportes, I.; Benoid-Guyod, J.; Zmirou, D. Hazards to man and environment posed by the use of urban waste compost: A review. Sci. Total Environ. 1995, 172, 197–222. [Google Scholar] [CrossRef]
- European Commission. Disposal and Recycling Routes for Sewage Sludge; Prepared by SEDE and Arthur Andersen for the European Commission, DG Environment; European Commission: Brussels, Belgium, 2001; ISBN 92-894-1798-6.
- Mohee, R.; Driver, M.-F.; Sobratee, N. Transformation of spent broiler litter from exogenous matter to compost in a sub-tropical context. Bioresour. Technol. 2008, 99, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Domestic waste composting facilities: A review of human health risks. Environ. Int. 2009, 35, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lasaridi, K.; Chroni, C.; Abeliotis, K.; Kyriacou, A.; Zorpas, A.A. Environmental assessment of composting in the context of sustainable waste management. In Sustainability behind Sustainability; Nova Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 229–242, ISBN 978-1-63-321595-5, 978-1-63-321573-3. [Google Scholar]
- Hogg, D.; Favoino, E.; Centemero, M.; Caimi, V.; Amlinger, F.; Devliegher, W.; Brinton, W.; Antler, S. Comparison of Compost Standards within the EU, North America and Australia; WRAP: Oxon, UK, 2002; ISBN 1-84405-003-3. [Google Scholar]
- Manios, T.; Stentiford, E.I. Heavy metals fractionation during the thermophilic phase of sewage sludge composting in aerated static piles. J. Environ. Sci. Health Part A 2006, 41, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, M.; Makridis, L.; Pirounaki, E.K.; Chroni, C.; Kyriacou, A.; Lasaridi, K.; Manios, T. Fate and effect of linuron and metribuzin on the co-composting of green waste and sewage sludge. Waste Manag. 2010, 30, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Briancesco, R.; Coccia, A.M.; Chiaretti, G.; Della Libera, S.; Semproni, M.; Bonadonna, L. Assessment of microbiological and parasitological quality of composted wastes: Health implications and hygienic measures. Waste Manag. Res. 2008, 26, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Fourti, O.; Jedidi, N.; Hassen, A. Behaviour of main microbiological parameters and of enteric microorganisms during the composting of municipal solid wastes and sewage sludge in a semi-industrial composting plant. Am. J. Environ. Sci. 2008, 4, 103–110. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Standards for the Use and Disposal of Sewage Sludge; 40 CFR Part 503. Federal Register; USEPA: Washington, DC, USA, 1993; Volume 58, pp. 9248–9415.
- D’Imporzano, G.; Crivelli, F.; Adani, F. Biological compost stability influences odor molecules production measured by electronic nose during food-waste high-rate composting. Sci. Total Environ. 2008, 402, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasaridi, K.; Protopapa, I.; Kotsou, M.; Pilidis, G.; Manios, T.; Kyriacou, A. Quality assessment of composts in the Greek market: The need for standards and quality assurance. J. Environ. Manag. 2006, 80, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Blanch, A.R.; Caplin, J.L.; Iversen, A.; Kuhn, I.; Manero, A.; Taylor, H.D.; Vilanova, X. Comparison of enterococcal populations related to urban and hospital wastewater in various climatic and geographic European regions. J. Appl. Microbiol. 2003, 94, 994–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardabassi, L.; Dalsgaard, A. Occurrence, structure and mobility of Tn1546-like elements in environmental isolates of vancomycin-resistant enterococci. Appl. Environ. Microbiol. 2004, 70, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.; Kuhn, I.; Franklin, A.; Mollby, R. High prevalence of vancomycin-resistant enterococci in Swedish sewage. Appl. Environ. Microbiol. 2002, 68, 2838–2842. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, A.; Mitsou, E.; Abeliotis, K.; Chroni, C.; Lasaridi, K.-E. Mapping of antibiotic resistant enteroococci in wastewater treatment plants in Greece. Desalin. Water Treat. 2018, 112, 250–257. [Google Scholar] [CrossRef]
- Mezrioui, N.; Baleux, B. Resistance patterns of E. coli strains isolated from domestic sewage before and after treatment in both aerobic lagoon and activated sludge. Water Res. 1994, 28, 2394–2406. [Google Scholar] [CrossRef]
- Vilanova, X.; Manero, A.; Cerda-Cuellar, M.; Blanch, A.R. The composition and persistence of faecal coliforms and eneterococcal populations in sewage treatment plants. J. Appl. Microbiol. 2004, 96, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Linden, P.K.; Miller, C.B. Vancomycin-resistant enterococci: The clinical effect of a common nosocomial pathogen. Diagn. Microbiol. Infect. Dis. 1999, 33, 113–120. [Google Scholar] [CrossRef]
- Portillo, A.; Ruiz-Larrea, F.; Zarazaga, M.; Alonso, A.; Martinez, J.L.; Torres, C. Macrolide resistance genes in Enterococcus spp. Antimicrob. Agents Chemother. 2000, 44, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Special Secretariat of Water. Database for the Operation, Monitoring of Wastewater Treatment Plants in Greece. 2018. Available online: http://astikalimata.ypeka.gr/Services/Pages/Browse.aspx (accessed on 5 June 2018).
- Federal Compost Quality Assurance Organization (FCQAO). Methods Book for the Analysis of Compost; Kompost–Information No. 230; Budesgutegemeinschaft Kompost e.V.: Köln, Germany, 1994. [Google Scholar]
- Lasaridi, K.E.; Stentiford, E.I. A simple respirometric technique for assessing compost stability. Water Res. 1998, 32, 3717–3723. [Google Scholar] [CrossRef]
- Chroni, C.; Kyriacou, A.; Manios, T.; Lasaridi, K.E. Investigation of the microbial community structure and activity as indicators of compost stability and composting process evolution. Bioresour. Technol. 2009, 100, 3745–3750. [Google Scholar] [CrossRef] [PubMed]
- Collee, J.G. Mackie & McCartney Practical Medical Microbiology, 14th ed.; Churchill Livingstone: New York, NY, USA, 1996. [Google Scholar]
- Forbes, B.A.; Sahm, D.F.; Weissfeld, A.S. Bailey & Scott Diagnostic Microbiology; Elsevier Applied Science: New York, NY, USA, 2002. [Google Scholar]
- Bezirtzoglou, E.; Romond, C. Rapid identification and enumeration of Clostridium perfringens in the human faecal flora. Microb. Ecol. Health Dis. 1990, 3, 159–163. [Google Scholar] [CrossRef]
- Garrec, N.; Picard-Bonnauad, F.; Pourcher, A.M. Occurrence of Listeria spp. and Listeria monocytogenes in sewage sludge used for land application: Effect of dewatering, limiting and storage in tank on survival of Listeria species. FEMS Immunol. Med. Microbiol. 2003, 35, 275–283. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (NCCLS). Approved Standard M2-A8, Performance Standards for Antimicrobial Disk Susceptibility Tests; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- NCCLS. M100-S13 (M2), Disk Diffusion Supplemented Tables; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- Kulikowska, D.; Gusiatin, Z. Sewage sludge composting in a two-stage system: Carbon and nitrogen transformations and potential ecological risk assessment. Waste Manag. 2015, 38, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Ponsa, S.; Pagans, E.; Sanchez, A. Composting of dewatered wastewater sludge with various ratios of pruning waste used as a bulking agent and monitored by respirometer. Biosyst. Eng. 2009, 102, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Changa, C.M.; Wang, P.; Watsom, M.E.; Hoitink, H.A.J.; Mitchel, F.C., Jr. Assessment of the reliability of a commercial maturity test kit for composted manures. Compos. Sci. Util. 2003, 11, 125–143. [Google Scholar] [CrossRef]
- Sesay, A.A.; Lasaridi, K.E.; Stentiford, E.I. Aerated static pile composting of municipal solid waste (MSW): A comparison of positive pressure aeration with hybrid positive and negative aeration. Waste Manag. Res. 1998, 16, 264–272. [Google Scholar] [CrossRef]
- Lasaridi, K.E.; Papadimitriou, E.K.; Balis, C. Development and demonstration of a thermogradient respirometer. Compos. Sci. Util. 1996, 4, 53–61. [Google Scholar] [CrossRef]
- Haney, R.L.; Brinton, W.F.; Evans, E. Soil CO2 respiration: Comparison of chemical titration, CO2 IRGA analysis and the Solvita gel system. Renew. Agric. Food Syst. 2008, 23, 171–176. [Google Scholar] [CrossRef]
- Kalamdhad, A.S.; Pasha, M.; Kazmi, A.A. Stability evaluation of compost by respiration techniques in a rotary drum composter. Resour. Conserv. Recycl. 2008, 52, 829–834. [Google Scholar] [CrossRef]
- Haug, R.T. The Practical Handbook of Compost Engineering; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Hogberg, P. Tansley review No. 95 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Balestra, U.; Fischer, C.M.; Aragno, M.; Hunziker, J.; Sharp, Z. Stable Carbon Isotopes as a Diagnosis Tool of Bacterial Activity in Landfills and Composting Systems. In Proceedings of the 3rd International Symposium on Subsurface Microbiology, Davos, Switzerland, 15–21 September 1996; p. 120. [Google Scholar]
- Chroni, C.; Kyriacou, A.; Stamatiadis, S.; Lasaridi, K.-E. Stable Isotope Fractionation and Respirometry for Composting Monitoring. In Proceedings of the 7th International ORBIT 2010 Conference, Heraklion, Crete, Greece, 29 June–3 July 2010; pp. 903–910. [Google Scholar]
- Favoino, E. The EU Legislation and Resulting Requirements Following for National Organic Waste Management Strategies and Policies. 2nd Baltic Biowaste Conference, European Compost Network. 2008. Available online: http://www.compostnetwork.info/bbc/download/02_Favoino.pdf (assessed on 11 November 2008).
- Federal Government of Germany. Ordinance on Environmentally Compatible Storage of Waste from Human Settlements and on Biological Waste-Treatment Facilities of 20 February 2001; Federal Government of Germany: Berlin, Germany, 2001.
- Godley, A.; Muller, W.; Frederickson, J.; Barker, H. Comparison of the SRI and DR4 Biodegradation Test Methods for Assessing the Biodegradability of Untreated and MBT Treated Municipal Solid Waste. In International Symposiun MBT 2005; Kühle-Weidemeier, M., Ed.; Cuvillier Verlag: Göttingen, Germany, 2005; pp. 548–559. [Google Scholar]
- Adani, F.; Gigliotti, G.; Valentini, F.; Laraia, R. Respiration index determination: A comparative study of different methods. Compos. Sci. Util. 2003, 11, 144–151. [Google Scholar] [CrossRef]
- Beuchat, L.R. Surface Decontamination of Fruits and Vegetables Eaten Raw: A Review; Food Safety Unit, WHO/FSF/FOS/98.2; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Michino, H.; Otsuki, K. Risk factors in causing outbreaks of food-borne illness originating in school lunch facilities in Japan. J. Vet. Med. Sci. 2000, 62, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Nguz, K.; Shindano, J.; Samapundo, S.; Huyghebaert, A. Microbiological evaluation of fresh-cut organic vegetables produced in Zambia. Food Control 2005, 16, 623–628. [Google Scholar] [CrossRef]
- Reinthaler, E.F.; Posch, J.; Feierl, G.; Wust, G.; Haas, D.; Ruckenbauer, G.; Mascher, F.; Marth, E. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 2003, 37, 1685–1690. [Google Scholar] [CrossRef]
- Niemi, M.; Sibakov, M.; Niemela, S. Anibiotic resistance among different species of faecal coliforms isolated from water samples. Appl. Environ. Microbiol. 1983, 45, 79–83. [Google Scholar] [PubMed]
- Wilcks, A.; Andersen, S.; Licht, T. Characterization of transferable tetracycline resistance genes in Enterococcus faecalis isolated from raw food. FEMS Microbiol. Lett. 2005, 243, 15–19. [Google Scholar] [CrossRef] [PubMed]
| Heavy Metals Concentrations (mg/kg dw) | ||||||
|---|---|---|---|---|---|---|
| Cd | Cu | Ni | Pb | Zn | ||
| Sewage Sludge | Initial | 1.2 | 332 | 27 | 151 | 816 |
| Cylinder 1 | Initial | 0.9 | 244 | 23 | 114 | 703 |
| Final | 1.2 | 292 | 32 | 158 | 896 | |
| Cylinder 2 | Initial | 0.7 | 180 | 18 | 85 | 519 |
| Final | 1.1 | 276 | 27 | 134 | 752 | |
| Indicative limits | Sludge Directive 1 & Greek limits for SS 2 | 20–40 | 10–1750 | 300–400 | 750–1200 | 2500–4000 |
| Greek limits for MSW compost 2 | 10 | 500 | 200 | 500 | 2000 | |
| Eco-label 3 | 1 | 100 | 50 | 100 | 300 | |
| Austrian limits, A+ | 0.7 | 70 | 25 | 45 | 200 | |
| A (+50%) | 1 | 150 | 60 | 120 | 500 | |
| B 4 | 3 | 500 | 100 | 200 | 1800 | |
| French limits 5 | 3 | 300 | 60 | 180 | 600 | |
| US EPA 6 | 39 | 1500 | 420 | 300 | 2800 | |
| US Washington State, grade A 7 | 10 | 750 | 210 | 150 | 1400 | |
| Microbial Population | Sewage Sludge | Cylinder 1 | Cylinder 2 | ||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| Total mesophilic (×107) | 5.3 ± 2.5 | 4.2 ± 1.8 | 4.6 ± 3.6 | 6.1 ± 3.8 | 3.3 ± 0.3 |
| Fungi (×105) | 0.3 ± 0.1 | 0.5 ± 0.2 | 1.5 ± 0.7 | 1.1 ± 0.7 | 28 ± 12 |
| Total coliforms (×105) | 9.3 ± 0.9 | 8.4 ± 0.6 | 0.9 ± 0.4 | 9.2 ± 0.9 | 13 ± 7 |
| Faecal streptococci (×106) | 1.7 ± 0.4 | 1.4 ± 0.3 | 0.2 ± 0.1 | 1.8 ± 0.6 | 1.1 ± 0.4 |
| E. coli (×105) | 2.5 ± 0.7 | 2.1 ± 0.7 | ND † | 2.8 ± 0.8 | ND |
| Clostridium perfringens (×102) | 320 ± 110 | 240 ± 110 | 8 ± 2 | 310 ± 100 | 6 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasaridi, K.-E.; Manios, T.; Stamatiadis, S.; Chroni, C.; Kyriacou, A. The Evaluation of Hazards to Man and the Environment during the Composting of Sewage Sludge. Sustainability 2018, 10, 2618. https://doi.org/10.3390/su10082618
Lasaridi K-E, Manios T, Stamatiadis S, Chroni C, Kyriacou A. The Evaluation of Hazards to Man and the Environment during the Composting of Sewage Sludge. Sustainability. 2018; 10(8):2618. https://doi.org/10.3390/su10082618
Chicago/Turabian StyleLasaridi, Konstantia-Ekaterini, Thrassyvoulos Manios, Stamatis Stamatiadis, Christina Chroni, and Adamantini Kyriacou. 2018. "The Evaluation of Hazards to Man and the Environment during the Composting of Sewage Sludge" Sustainability 10, no. 8: 2618. https://doi.org/10.3390/su10082618
APA StyleLasaridi, K.-E., Manios, T., Stamatiadis, S., Chroni, C., & Kyriacou, A. (2018). The Evaluation of Hazards to Man and the Environment during the Composting of Sewage Sludge. Sustainability, 10(8), 2618. https://doi.org/10.3390/su10082618






