Theoretical Study on the Production of Environment-Friendly Recycled Cement Using Inorganic Construction Wastes as Secondary Materials in South Korea
Abstract
1. Introduction
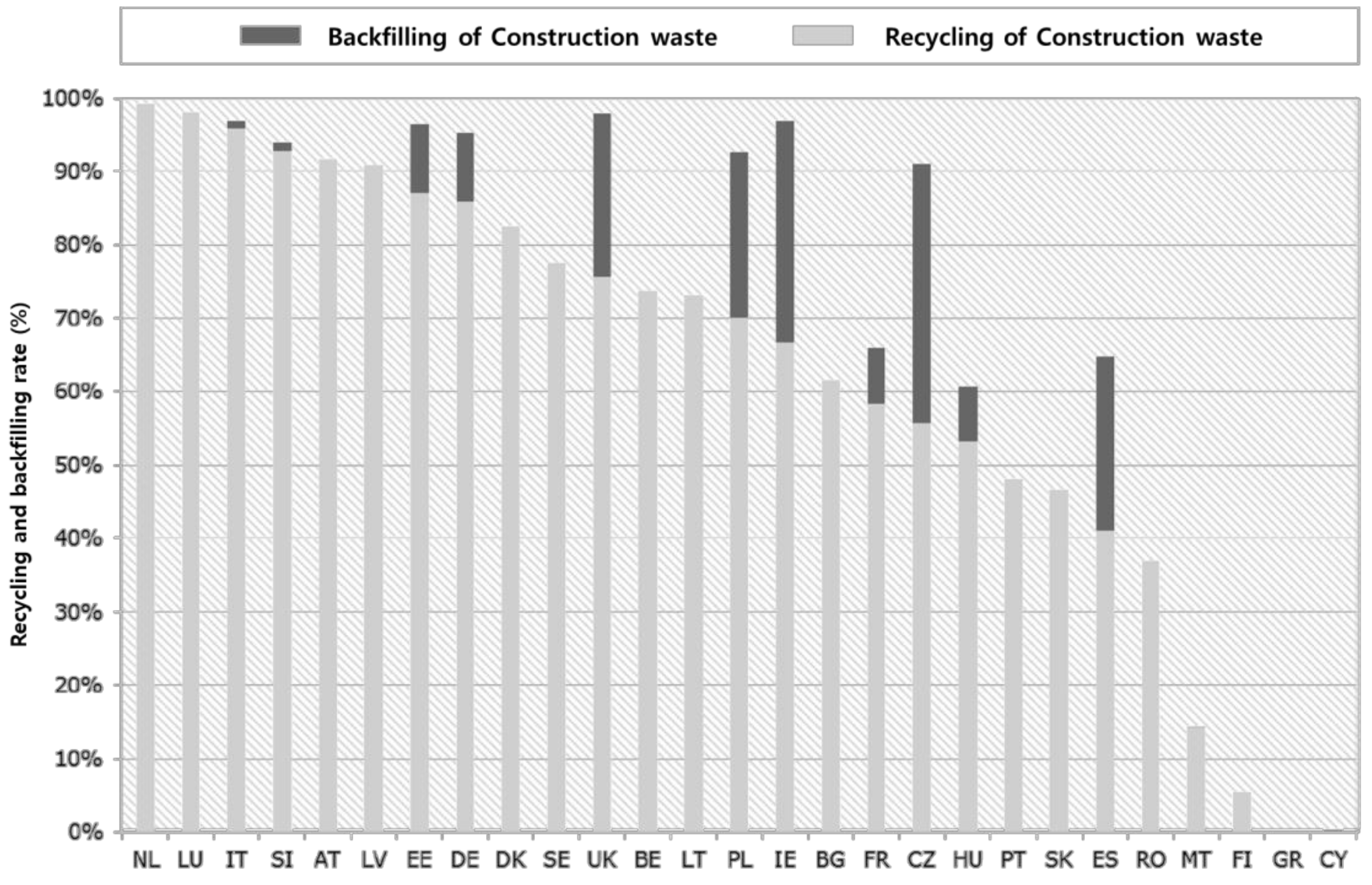
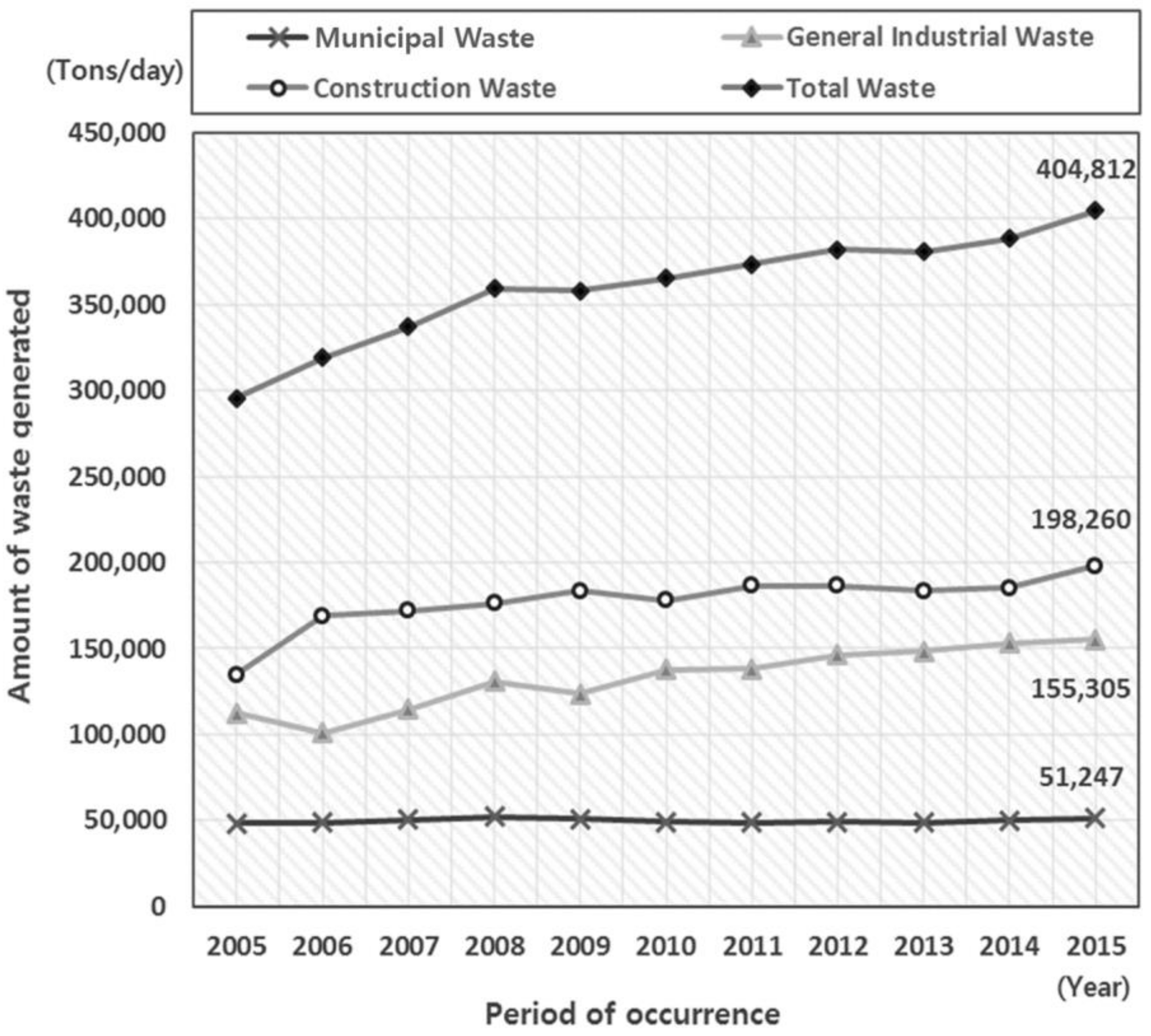
2. Types and Trends of Construction Wastes
3. Analysis of Inorganic Construction Wastes
3.1. Chemical Composition
3.2. Theoretical Consideration of Chemical Components
3.2.1. Bogue Formula
[1.430 × Fe2O3 (wt %)] − [2.852 × SO3 (wt %)]
[2.859 × Fe2O3 (wt %)] − [2.852 × SO3 (wt %)]
3.2.2. Other Prediction Formulas and Chemical Factors
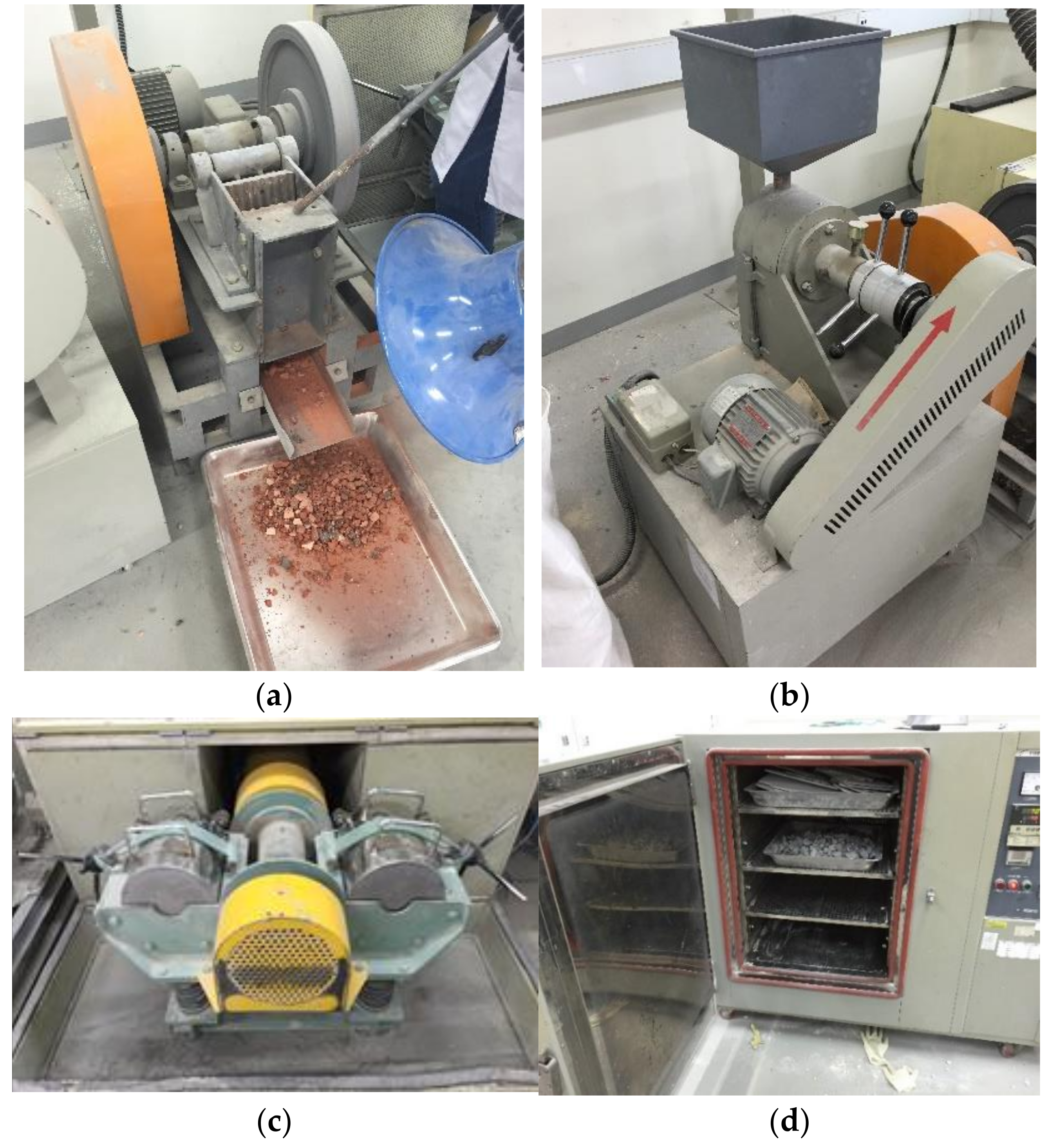
3.3. Collection and Chemical Analysis of Inorganic Construction Wastes
3.4. Analyses of Inorganic Construction Wastes and Theoretical Combinations
4. Predictive Analysis of Clinker Calcination
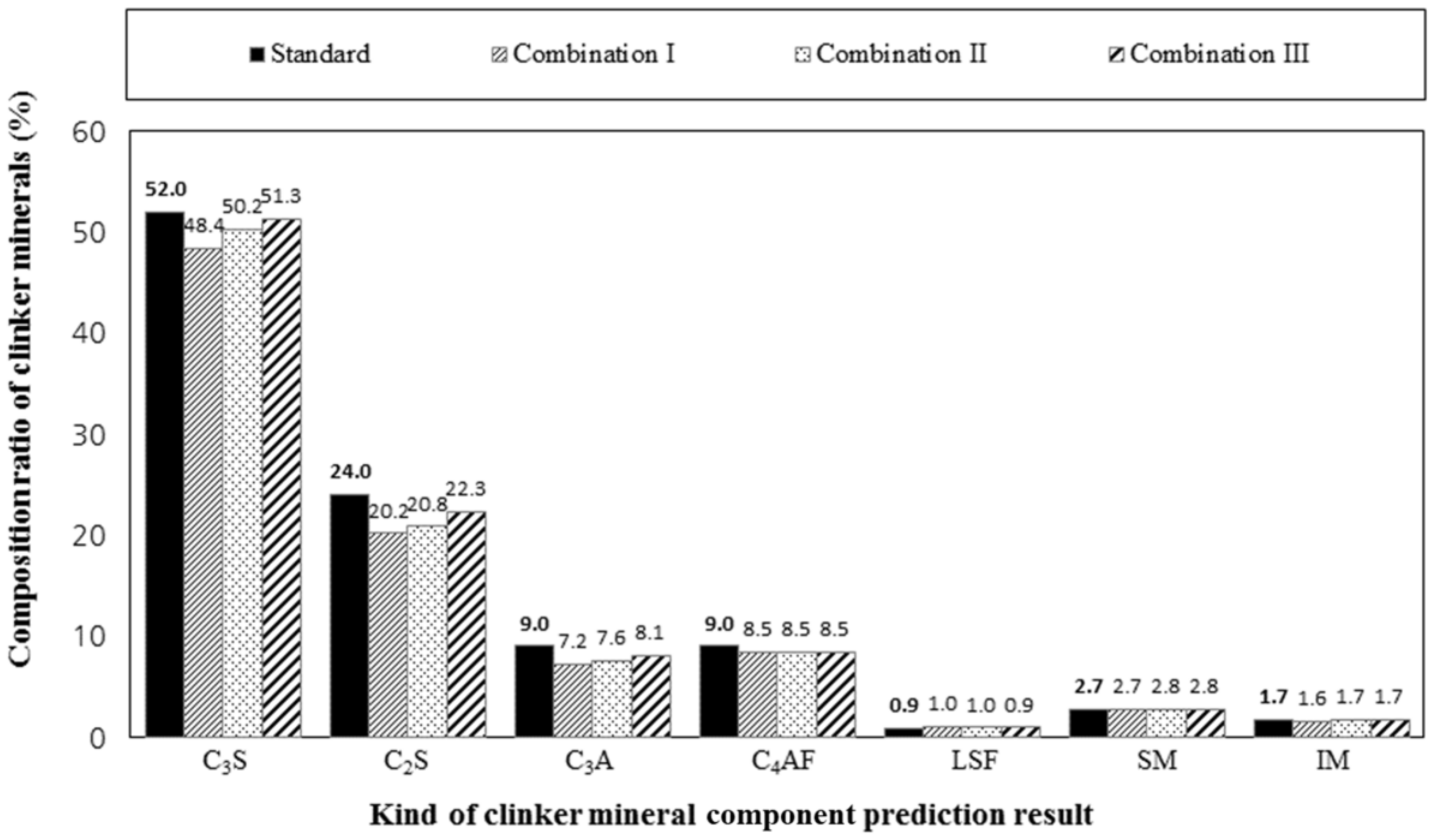
4.1. Analysis of Clinker Minerals Using the Bogue Formula
4.2. Analysis and Discussion of the Mineral Composition Predictions
4.3. Effects Expected by Developing Recycled Cement
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Passer, A.; Lasvaux, S.; Allacker, K.; De Lathauwer, D.; Spirinckx, C.; Wittstock, B.; Kellenberger, D.; Gschösser, F.; Wall, J.; Wallbaum, H. Environmental product declarations entering the building sector: Critical reflections based on 5 to 10 years experience in different European countries. Int. J. Life Cycle Assess. 2015, 20, 1199–1212. [Google Scholar] [CrossRef]
- Zabalza Bribián, I.; Valero Capilla, A.; Aranda Usón, A. Life cycle assessment of building materials: Comparative analysis of energy and environmental impacts and evaluation of the eco-efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Giesekam, J.; Barrett, J.R.; Taylor, P. Construction sector views on low carbon building materials. Build. Res. Inf. 2016, 44, 423–444. [Google Scholar] [CrossRef]
- Taylor, M.; Tam, C.; Gielen, D. Energy efficiency and CO2 emissions from the global cement industry. Korea 2006, 50, 61–67. [Google Scholar]
- Kim, T.; Tae, S.; Chae, C.U.; Lee, K. Proposal for the evaluation of eco-efficient concrete. Sustainability 2016, 8, 705. [Google Scholar] [CrossRef]
- Brown, M.T.; Buranakarn, V. Emergy indices and ratios for sustainable material cycles and recycle options. Resour. Conserv. Recycl. 2003, 38, 1–22. [Google Scholar] [CrossRef]
- Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2018.
- Tae, S.; Kim, R. A study on improvement of durability of reinforced concrete structures mixed with Natural Inorganic Minerals. Constr. Build. Mater. 2011, 25, 4263–4270. [Google Scholar] [CrossRef]
- Tae, S. Corrosion inhibition of steel in concrete with natural inorganic minerals in corrosive environments due to chloride attack. Constr. Build. Mater. 2012, 35, 270–280. [Google Scholar] [CrossRef]
- Bamaga, S.; Ismail, M.A.; Lee, H.-S.; Budiea, A.M.A. Effect of Incorporation of Waste Ash on the Behavior of Concrete and Mortar. Int. J. Sustain. Build. Technol. Urban Dev. 2010, 1, 74–79. [Google Scholar] [CrossRef]
- Ortega, J.; Esteban, M.; Rodríguez, R.; Pastor, J.; Ibanco, F.; Sánchez, I.; Climent, M. Influence of Silica Fume Addition in the Long-Term Performance of Sustainable Cement Grouts for Micropiles Exposed to a Sulphate Aggressive Medium. Materials 2017, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Pastor, J.; Albaladejo, A.; Sánchez, I.; Climent, M. Durability and compressive strength of blast furnace slag-based cement grout for special geotechnical applications. Mater. Constr. 2014, 64, e003. [Google Scholar] [CrossRef]
- Joshaghani, A.; Balapour, M.; Ramezanianpour, A. Effect of controlled environmental conditions on mechanical, microstructural and durability properties of cement mortar. Constr. Build. Mater. 2018, 164, 134–149. [Google Scholar] [CrossRef]
- Ramezanianpour, A.; Malhotra, V. Effect of curing on the compressive strength, resistance to chloride-ion penetration and porosity of concretes incorporating slag, fly ash or silica fume. Cem. Concr. Compos. 1995, 17, 125–133. [Google Scholar] [CrossRef]
- A Research of Consolidation Plan for Recyclability of Construction Wastes Resource; Korea Institute of Civil Engineering and Building Technology: Seoul, Korea, 2014.
- Ishak, S.A.; Hashim, H.; Ting, T.S. Eco innovation strategies for promoting cleaner cement manufacturing. J. Clean. Prod. 2016, 136, 133–149. [Google Scholar] [CrossRef]
- Alaka, H.A.; Oyedele, L.O.; Toriola-Coker, O.L. Effect of excess dosages of superplasticizer on the properties of highly sustainable high-volume fly ash concrete. Int. J. Sustain. Build. Technol. Urban Dev. 2016, 7, 73–86. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lee, H.-S. The Evaluation of Carbonation Depth of Concrete Incorporating Industrial By-Product Materials. Int. J. Sustain. Build. Technol. Urban Dev. 2011, 2, 253–258. [Google Scholar] [CrossRef]
- Kim, R.; Tae, S.; Roh, S. A study on the deduction of major construction wastes in construction phase through environmental impact assessment. Adv. Mater. Res. 2014, 1025, 1025–1026. [Google Scholar] [CrossRef]
- Kynclova, M.; Fiala, C.; Hajek, P. High Performance Concrete as a Sustainable Material. Int. J. Sustain. Build. Technol. Urban Dev. 2011, 2, 63–68. [Google Scholar] [CrossRef]
- Resource-Efficient Use of Mixed Waste–Main Findings and Outlook; European Commission DG Environment: Brussels, Belgium, 2016.
- Oshiro, A.; Positieri, M.; Cáceres, G.; Lopez, A.; Di Gioia, C.; Fernández, M. Contribution to the recycling of waste products in construction: Concrete made with non-traditional aggregates. Struct. Concr. Time Proc. Fib Symp. 2005, 2, 793–800. [Google Scholar]
- Aguilar-Penagos, A.; Gómez-Soberón, J.M.; Rojas-Valencia, M.N. Physicochemical, mineralogical and microscopic evaluation of sustainable bricks manufactured with construction wastes. Appl. Sci. 2017, 7, 1012. [Google Scholar] [CrossRef]
- Puertas, F.; Santos, R.; Alonso, M.M.; Del Río, M. Alkali-activated cement mortars containing recycled clay-based construction and demolition waste. Ceram. Silik. 2015, 59, 202–210. [Google Scholar]
- Cabrera-Covarrubias, F.G.; Gomez-Soberon, J.M.; Almaral-Sanchez, J.L.; Arredondo-Rea, S.P.; Mendivil-Escalante, J.M. Mechanical and Basic Deformation Properties of Mortar with Recycled Glass as a Fine Aggregate Replacement. Int. J. Civ. Eng. 2018, 16, 107–121. [Google Scholar] [CrossRef]
- Xuan, D.X.; Houben, L.J.M.; Molenaar, A.A.A.; Shui, Z.H. Mixture optimization of cement treated demolition waste with recycled masonry and concrete. Mater. Struct. Mater. Constr. 2012, 45, 143–151. [Google Scholar] [CrossRef]
- Srinivas, P.; Kumar, K.S. Utilization of incinerated municipal solid waste ash in the manufacture of cement hollow bricks. Nat. Environ. Pollut. Technol. 2009, 8, 329–334. [Google Scholar]
- Cabrera-Covarrubias, F.G.; Gómez-Soberón, J.M.; Almaral-Sánchez, J.L.; Arredondo-Rea, S.P.; Gómez-Soberón, M.C.; Corral-Higuera, R. An experimental study of mortars with recycled ceramic aggregates: Deduction and prediction of the stress-strain. Materials 2016, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Waste Generation and Processing Status of the Country; Ministry of Environment: Sejong-Si, Korea, 2015.
- Kim, J. Assessment and Estimation of Particulate Matter Formation Potential and Respiratory Effects from Air Emission Matters in Industrial Sectors and Cities/Regions. Korean Soc. Eng. Educ. 2017, 39, 220–228. [Google Scholar] [CrossRef]
- Bok, Y.; Tae, S.; Kim, R. Analysis of CO2 emission in the waste disposal process based on computation of construction waste. Adv. Mater. Res. 2014, 1025–1026. [Google Scholar] [CrossRef]
- Kim, R.H.; Tae, S.H. A study on the construction waste forecasting method considering material loss rate in South Korea. Adv. Mater. Res. 2014, 905, 187–190. [Google Scholar] [CrossRef]
- Bovea, M.D.; Powell, J.C. Developments in life cycle assessment applied to evaluate the environmental performance of construction and demolition wastes. Waste Manag. 2016, 50, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, C.A.; Worrell, E.; De Jager, D.; Blok, K.; Riemer, P. Emission reduction of greenhouse gases from the cement industry. World 2002, 7. [Google Scholar] [CrossRef]
- Lee, J.; Tae, S.; Kim, R. A Study on the analysis of CO2 emissions of apartment housing in the construction process. Sustainability 2018, 10, 365. [Google Scholar] [CrossRef]
- Shi, C.; Fernández Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Cement in Korea 2016; Korea Cement Association: Seoul, Korea, 2017.
- Oh, D.-Y.; Noguchi, T.; Kitagaki, R.; Park, W.-J. CO2 emission reduction by reuse of building material waste in the Japanese cement industry. Renew. Sustain. Energy Rev. 2014, 38, 796–810. [Google Scholar] [CrossRef]
- Kang, T.H.; Jung, M.S.; Ahn, J.C.; Kang, B.H. Recovering hydration performance of cementitious powder by concrete waste according to burning temperature. Korea Inst. Build. Constr. 2003, 3, 81–87. [Google Scholar]
- Lee, J.S.; Yoo, H.M.; Yang, W.S.; Park, J.K.; Jo, S.J.; Kim, B.S.; Seo, Y.C. A study on clay brick Manufacturing with powders of CRT glass waste. J. Korea Soc. Waste Manag. 2013, 30, 86–93. [Google Scholar] [CrossRef]
- Suárez, S.; Roca, X.; Gasso, S. Product-specific life cycle assessment of recycled gypsum as a replacement for natural gypsum in ordinary Portland cement: Application to the Spanish context. J. Clean. Prod. 2016, 117, 150–159. [Google Scholar] [CrossRef]
- Singh, M.; Mridul, G. Making of anhydrite cement from waste gypsum. Cem. Concr. Res. 2000, 30, 571–577. [Google Scholar] [CrossRef]
- Thomas, M.; Lothenbach, B.; Glasser, F.P. Thermodynamic properties of Portland cement hydrates in the system CaO–Al2O3–SiO2–CaSO4–CaCO3–H2O. Cem. Concr. Res. 2007, 37, 1379–1410. [Google Scholar] [CrossRef]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Ay, N.; Ünal, M. The use of waste ceramic tile in cement production. Cem. Concr. Res. 2000, 30, 497–499. [Google Scholar] [CrossRef]
- Walenta, G.; Füllmann, T. Advances in quantitative XRD analysis for clinker, cements, and cementitious additions. Powder Diffr. 2004, 19, 40–44. [Google Scholar] [CrossRef]
- Sungshin Cement & Ready-Mixed Concrete Material Safety Data. Sungshin Cement: Seoul, Korea, 2011.
- Zimbili, O.; Salim, W.; Ndambuki, M. A review on the usage of ceramic wastes in concrete production. Int. J. Civ. Arch. Struct. Constr. Eng. 2014, 8, 91–95. [Google Scholar]
- Park, C.W.; An, J.C.; Gang, B.H. A study on the technique to manufacture recycled cement from cementitious powders for complete recycling of concrete structures. J. Korea Inst. Build. Constr. 2004, 4, 143–151. [Google Scholar] [CrossRef]
- Han, C.; Yang, X.; Zhou, H.; Tang, Y. Steel slag and its application in cement industries. Mater. Rev. 2010, 24, 440–443. [Google Scholar]
- Cheng, X.; Shan, L.; Zhou, Z.; Liu, F. Reconstructing steel slag according to composition of cement clinker. J. Wuhan Univ. Technol. 2009, 4, 31. [Google Scholar]
- Chen, D.; Tan, K. Study on mineral admixture of concrete prepared with electric furnace slag. Bull. Chin. Ceram. Soc. 2006, 6, 45. [Google Scholar]


| Major Countries | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|
| China | 2210 | 2420 | 2480 | 2350 | 2410 | 2400 |
| India | 270 | 280 | 260 | 270 | 290 | 280 |
| United States | 74.9 | 77.4 | 83.2 | 83.4 | 85.9 | 86.3 |
| Vietnam | 60 | 58 | 60.5 | 61 | 70 | 78 |
| Turkey | 63.9 | 71.3 | 75 | 77 | 77 | 77 |
| Indonesia | 32 | 56 | 65 | 65 | 63 | 66 |
| Saudi Arabia | 50 | 57 | 55 | 55 | 61 | 63 |
| South Korea | 48 | 47.3 | 63.2 | 63 | 55 | 59 |
| Egypt | 46.1 | 50 | 50 | 55 | 55 | 58 |
| Russia | 61.5 | 66.4 | 68.4 | 69 | 56 | 58 |
| Iran | 70 | 72 | 65 | 65 | 53 | 56 |
| Brazil | 68.8 | 70 | 72 | 72 | 60 | 54 |
| Japan | 51.3 | 57.4 | 53.8 | 55 | 56 | 53 |
| Waste Classification | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |
|---|---|---|---|---|---|---|---|
| Construction waste material (tons/day) | Concrete | 114,302 | 121,181 | 117,754 | 111,653 | 114,908 | 124,451 |
| Asphalt concrete | 32,535 | 35,245 | 35,738 | 35,398 | 33,725 | 35,509 | |
| Other (1) | 2132 | 2339 | 2957 | 3280 | 2393 | 3230 | |
| Subtotal | 148,969 | 158,765 | 156,448 | 150,331 | 151,026 | 163,190 | |
| Combustible waste (tons/day) | Wood | 636 | 592 | 683 | 704 | 866 | 923 |
| Synthetic resin | 839 | 1096 | 1261 | 1695 | 1586 | 1654 | |
| Other (2) | 98 | 20 | 21 | 19 | 67 | 11 | |
| Subtotal | 1573 | 1708 | 1964 | 2418 | 2519 | 2588 | |
| Non-combustible waste (tons/day) | Construction sludge | 645 | 1403 | 644 | 1052 | 707 | 995 |
| Other (3) | 9 | 4 | 7 | 6 | 170 | 41 | |
| Subtotal | 654 | 1407 | 651 | 1058 | 877 | 1036 | |
| Construction soil debris (tons/day) | 5347 | 4838 | 5094 | 5067 | 5863 | 7659 | |
| Mixed construction waste (4) (tons/day) | 21,577 | 19,699 | 22,471 | 24,664 | 25,097 | 23,787 | |
| Total (tons/day) | 178,120 | 186,417 | 186,629 | 183,538 | 185,382 | 198,260 | |
| Portland | CaO (wt %) | SiO2 (wt %) | Al2O3 (wt %) | Fe2O3 (wt %) |
|---|---|---|---|---|
| Type I | 63.8 | 22.1 | 5.0 | 3.0 |
| Type II | 63.6 | 23.3 | 3.9 | 3.9 |
| Type III | 64.9 | 20.8 | 4.5 | 2.8 |
| Type IV | 63.0 | 25.9 | 3.0 | 2.8 |
| Type V | 65.0 | 22.4 | 3.4 | 4.4 |
| No. | Construction Waste | SiO2 (wt %) | Al2O3 (wt %) | Fe2O3 (wt %) | CaO (wt %) | MgO (wt %) | Na2O (wt %) | K2O (wt %) | SO3 (wt %) | TiO2 (wt %) | L.O.I * (wt %) | Total (wt %) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Waste tiles | 61.40 | 17.43 | 1.73 | 8.80 | 1.13 | 0.68 | 1.27 | - | 0.36 | 6.61 | 99.41 |
| 2 | Waste glass | 71.00 | 1.47 | 0.07 | 8.91 | 4.04 | 13.10 | 0.83 | 0.24 | - | - | 99.66 |
| 3 | Waste bricks | 64.34 | 24.10 | 4.81 | 0.57 | 1.13 | 0.78 | 2.89 | - | 1.09 | - | 99.71 |
| 4 | Waste autoclaved lightweight concrete | 48.30 | 3.69 | 1.88 | 28.10 | 1.59 | 0.26 | 0.62 | 1.66 | - | 13.30 | 99.40 |
| 5 | Waste gypsum boards | 1.60 | 0.69 | 0.22 | 54.32 | 0.1 | 0.46 | 0.23 | 41.47 | 0.49 | - | 99.58 |
| 6 | Waste concrete powder | 45.50 | 11.90 | 1.90 | 29.80 | 1.90 | - | 3.00 | 1.40 | - | 2.30 | 97.70 |
| Portland | C3S (wt %) | C2S (wt %) | C3A (wt %) | C4AF (wt %) |
|---|---|---|---|---|
| Type I | 52 | 24 | 9 | 9 |
| Type II | 47 | 32 | 4 | 11 |
| Type III | 62 | 14 | 9 | 8 |
| No. | Construction Waste | SiO2 (wt %) | Al2O3 (wt %) | Fe2O3 (wt %) | CaO (wt %) | MgO (wt %) | Na2O (wt %) | K2O (wt %) | SO3 (wt %) | TiO2 (wt %) | Other (wt %) | L.O.I * (wt %) | Total (wt %) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Waste ceiling material | 5.84 | 1.10 | 1.06 | 39.21 | 0.54 | 0.00 | 0.32 | 24.62 | 0.41 | 0.52 | 26.39 | 100.00 |
| 2 | Waste gypsum board | 0.91 | 0.32 | 0.30 | 36.42 | 0.22 | 0.30 | 0.12 | 39.29 | 0.03 | 0.13 | 21.96 | 100.00 |
| 3 | Waste cement block | 50.69 | 10.01 | 3.93 | 19.56 | 1.26 | 0.67 | 3.69 | 0.99 | 0.46 | 0.63 | 8.12 | 100.00 |
| 4 | Waste concrete powder | 55.46 | 10.28 | 3.42 | 22.35 | 1.16 | 0.92 | 3.77 | 1.20 | 0.37 | 0.71 | 0.37 | 100.00 |
| 5 | Waste tile | 57.46 | 15.95 | 2.62 | 9.55 | 0.48 | 1.02 | 2.23 | 0.44 | 0.72 | 1.32 | 8.22 | 100.00 |
| 6 | Waste brick | 63.05 | 21.00 | 5.80 | 2.13 | 0.71 | 1.33 | 3.49 | 0.02 | 0.74 | 0.50 | 1.24 | 100.00 |
| 7 | Electric furnace slag | 16.19 | 11.22 | 36.32 | 23.10 | 2.71 | 0.00 | 0.07 | 0.21 | 0.84 | 9.36 | 0.00 | 100.01 |
| 8 | Limestone | 12.21 | 2.40 | 0.77 | 44.75 | 2.15 | 0.01 | 0.64 | 0.00 | 0.00 | 0.00 | 36.55 | 99.48 |
| No. | Construction Waste | Combination I | Combination II | Combination III |
|---|---|---|---|---|
| 1 | Waste tile (wt %) | 4.1 | 4.4 | 4.8 |
| 2 | Waste cement block (wt %) | 0.7 | 0.5 | 0.3 |
| 3 | Waste ceiling material (wt %) | 17.5 | 12.4 | 7.2 |
| 4 | Limestone (wt %) | 75.0 | 80.0 | 85.0 |
| 5 | Electric furnace slag (wt %) | 2.7 | 2.7 | 2.7 |
| Total (wt %) | 100.0 | 100.0 | 100.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Tae, S.; Kim, R. Theoretical Study on the Production of Environment-Friendly Recycled Cement Using Inorganic Construction Wastes as Secondary Materials in South Korea. Sustainability 2018, 10, 4449. https://doi.org/10.3390/su10124449
Kim J, Tae S, Kim R. Theoretical Study on the Production of Environment-Friendly Recycled Cement Using Inorganic Construction Wastes as Secondary Materials in South Korea. Sustainability. 2018; 10(12):4449. https://doi.org/10.3390/su10124449
Chicago/Turabian StyleKim, Jihoon, Sungho Tae, and Rakhyun Kim. 2018. "Theoretical Study on the Production of Environment-Friendly Recycled Cement Using Inorganic Construction Wastes as Secondary Materials in South Korea" Sustainability 10, no. 12: 4449. https://doi.org/10.3390/su10124449
APA StyleKim, J., Tae, S., & Kim, R. (2018). Theoretical Study on the Production of Environment-Friendly Recycled Cement Using Inorganic Construction Wastes as Secondary Materials in South Korea. Sustainability, 10(12), 4449. https://doi.org/10.3390/su10124449





