Leaf Temperature and Vapour Pressure Deficit (VPD) Driving Stomatal Conductance and Biochemical Processes of Leaf Photosynthetic Rate in a Subtropical Evergreen Coniferous Plantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. CO2 Response Curve
2.3. Statistical Analysis
3. Results
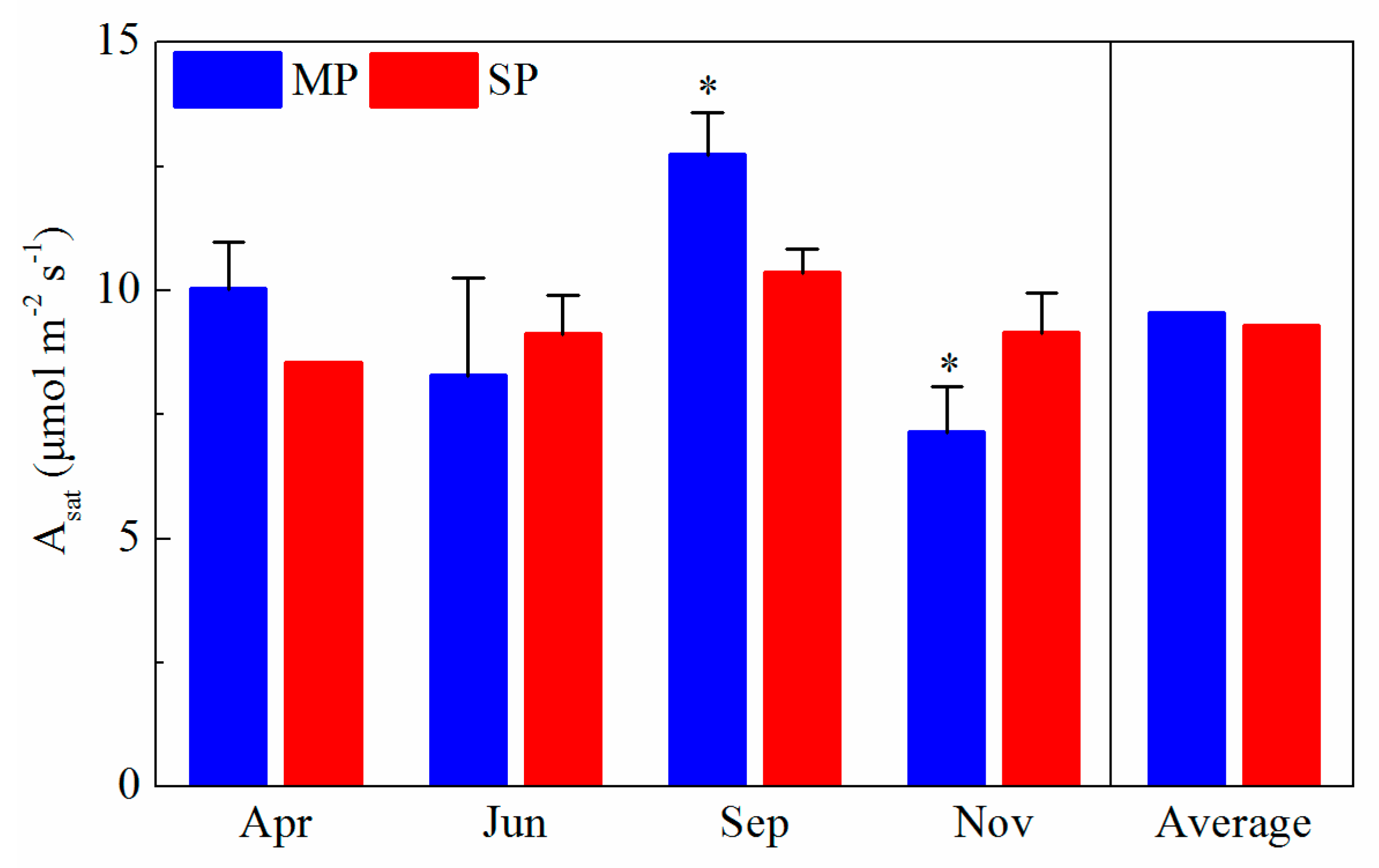
3.1. Seasonal Variations in Environmental Condition, Leaf Traits, and Leaf Photosynthetic Rates
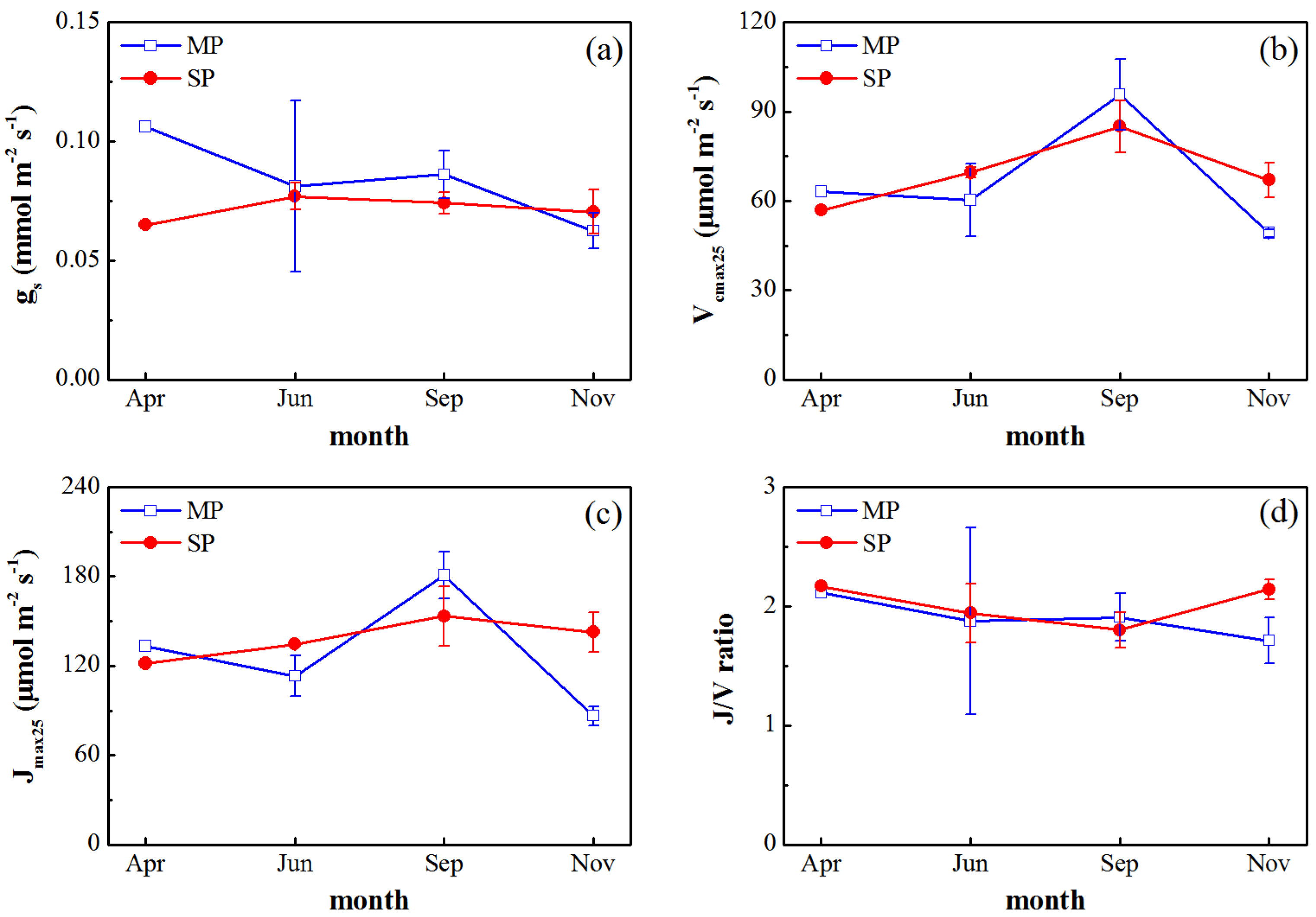
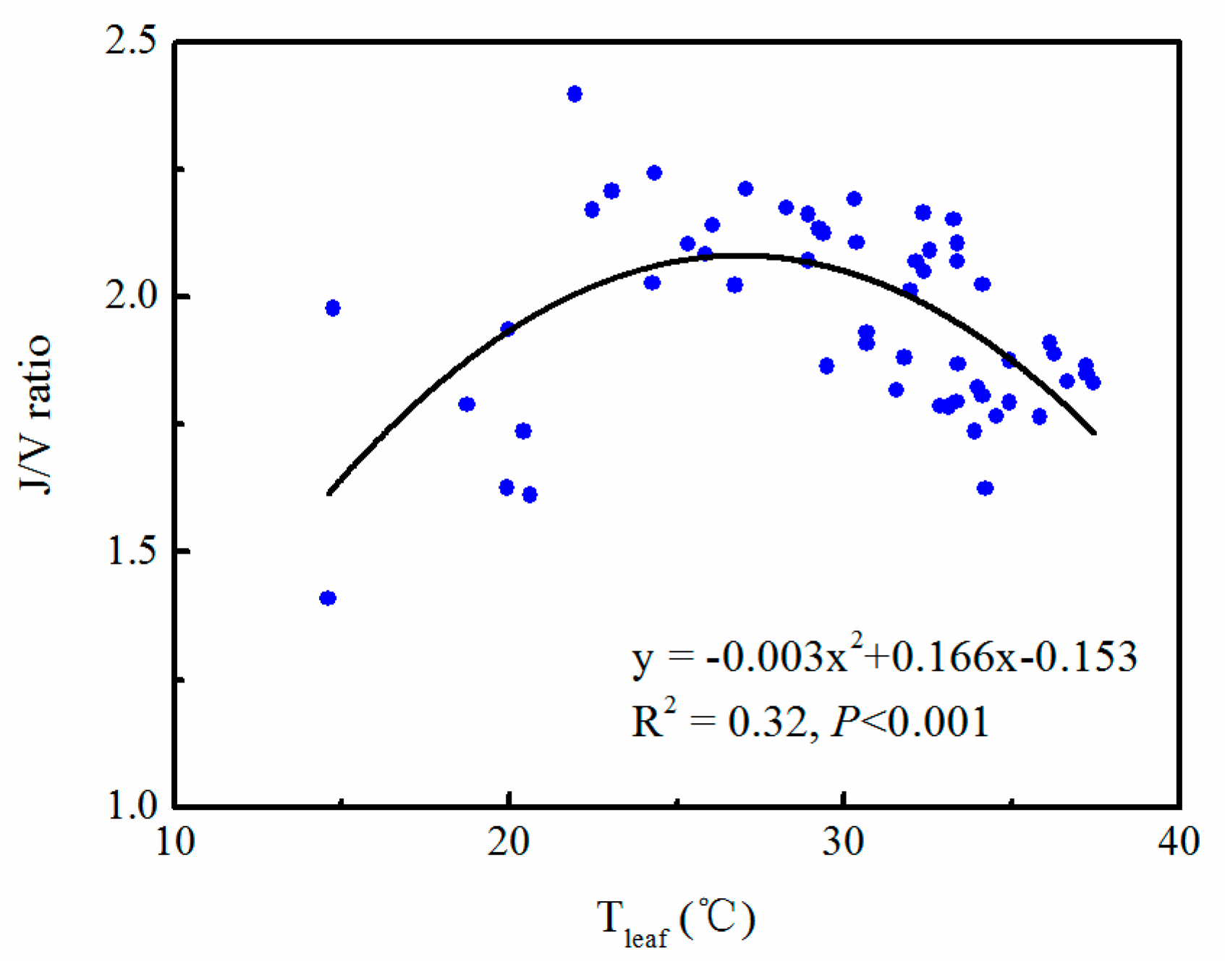
3.2. Seasonal Variations in Gas Exchange Parameters
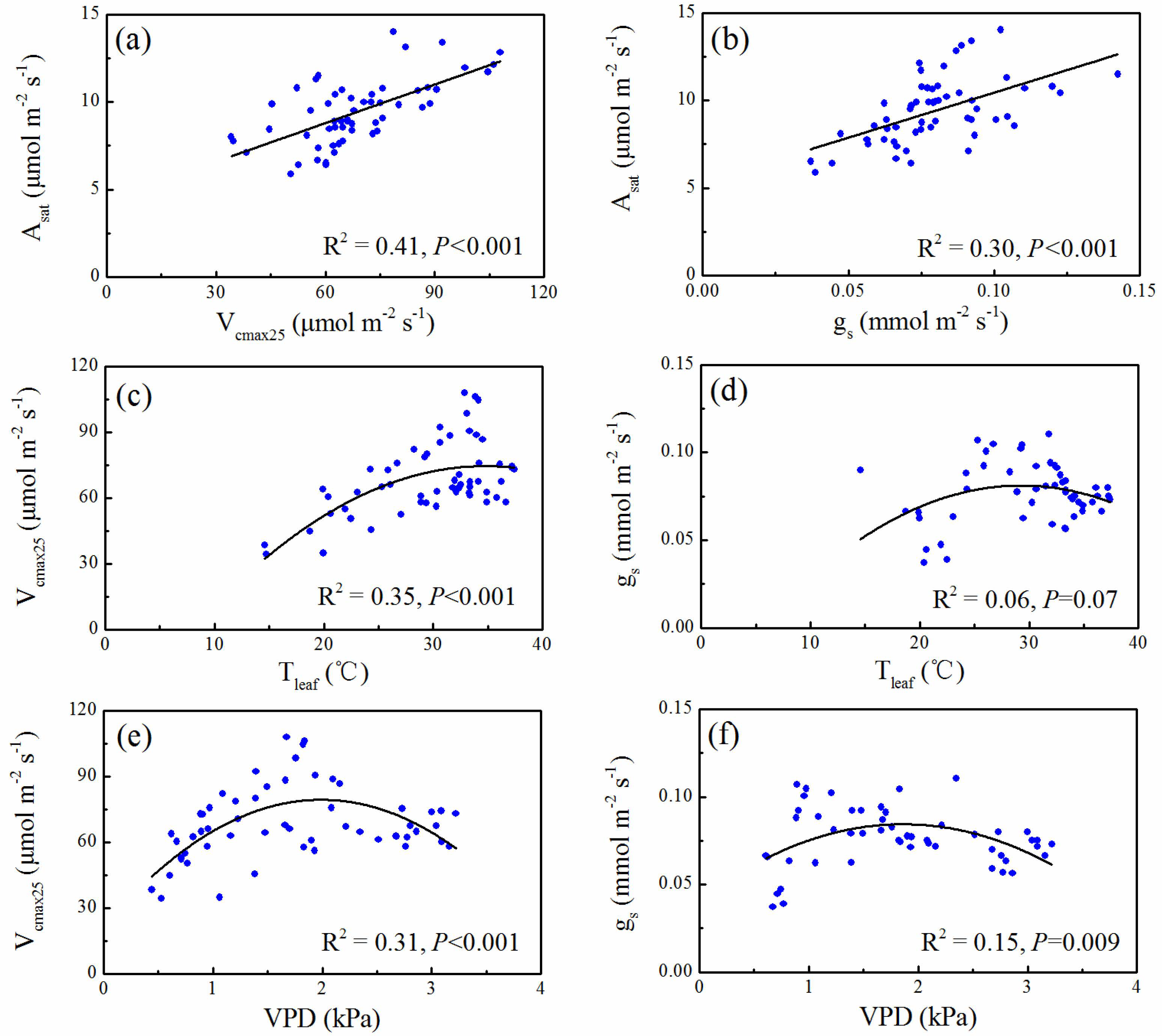
3.3. Correlation between Gas Exchange Parameters and Leaf Temperature and VPD
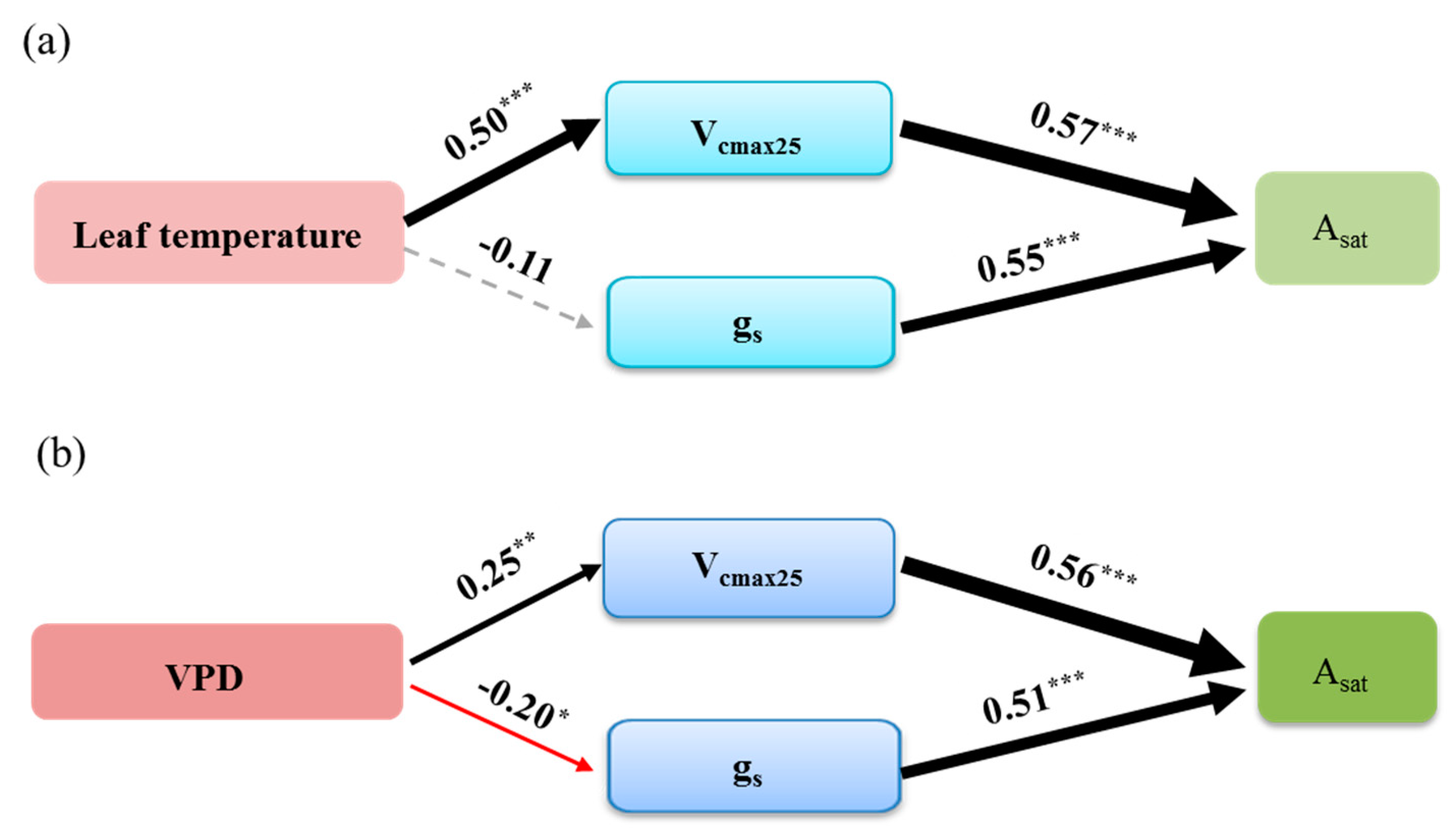
3.4. The Effect of Stomatal and Biochemical Parameters on Asat in Response to Leaf Temperature and VPD
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.R.; Chen, Z.; Piao, S.L.; Peng, C.H.; Ciais, P.; Wang, Q.F.; Li, X.R.; Zhu, X.J. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [PubMed]
- Dodet, M.; Collet, C. When should exotic forest plantation tree species be considered as an invasive threat and how should we treat them? Biol. Invasions 2012, 14, 1765–1778. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Xu, M.; Ma, Z.; Wang, Z.-L. Soil organic carbon stocks and CO2 effluxes of native and exotic pine plantations in subtropical China. Catena 2015, 128, 167–173. [Google Scholar] [CrossRef]
- Ma, Z.; Hartmann, H.; Wang, H.; Li, Q.; Wang, Y.; Li, S. Carbon dynamics and stability between native Masson pine and exotic slash pine plantations in subtropical China. Eur. J. For. Res. 2013, 133, 307–321. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, Y.W.; Lu, T.G.; Zhang, Z.G.; Han, X. Proteomics analysis reveals a dynamic diurnal pattern of photosynthesis-related pathways in maize leaves. PLoS ONE 2017, 12, e0180670. [Google Scholar] [CrossRef] [PubMed]
- Haase, P.; Pugnaire, F.I.; Clark, S.C.; Incoll, L.D. Environmental control of canopy dynamics and photosynthetic rate in the evergreen tussock grass Stipa tenacissima. Plant Ecol. 1999, 145, 327–339. [Google Scholar] [CrossRef]
- Hoyaux, J.; Moureaux, C.; Tourneur, D.; Bodson, B.; Aubinet, M. Extrapolating gross primary productivity from leaf to canopy scale in a winter wheat crop. Agric. For. Meteorol. 2008, 148, 668–679. [Google Scholar] [CrossRef]
- Xu, Q.Z.; Huang, B.R. Effects of differential air and soil temperature on carbohydrate metabolism in creeping bentgrass. Crop Sci. 2000, 40, 1368–1374. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhou, G.; Huang, W.; Duan, H. Warming effects on photosynthesis of subtropical tree species: A translocation experiment along an altitudinal gradient. Sci. Rep. 2016, 6, 24895. [Google Scholar] [CrossRef] [PubMed]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C-3, C-4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Reddy, V.R. Methods of mesophyll conductance estimation: Its impact on key biochemical parameters and photosynthetic limitations in phosphorus-stressed soybean across CO2. Physiol. Plant 2016, 157, 234–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, S.Q.; Chi, Y.G.; Li, Q.K.; Huang, K.; Yu, Q.Z. Responses of photosynthetic parameters to drought in subtropical forest ecosystem of China. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Munjonji, L.; Ayisi, K.K.; Boeckx, P.; Haesaert, G. Stomatal Behavior of Cowpea Genotypes Grown Under Varying Moisture Levels. Sustainability 2018, 10, 12. [Google Scholar] [CrossRef]
- Craparo, A.C.W.; Steppe, K.; Van Asten, P.J.A.; Laderach, P.; Jassogne, L.T.P.; Grab, S.W. Application of thermography for monitoring stomatal conductance of Coffea arabica under different shading systems. Sci. Total Environ. 2017, 609, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Egea, G.; Verhoef, A.; Vidale, P.L. Towards an improved and more flexible representation of water stress in coupled photosynthesis-stomatal conductance models. Agric. For. Meteorol. 2011, 151, 1370–1384. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Caemmerer, S.V.; Berry, J.A. A Biochemical-Model of Photosynthetic CO2 Assimilation in Leaves of C-3 Species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C-3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.G.; Chun, J.A.; Fleisher, D.; Reddy, V.; Timlin, D.; Resop, J. Parameter Estimation of the Farquhar-von Caemmerer-Berry Biochemical Model from Photosynthetic Carbon Dioxide Response Curves. Sustainability 2017, 9, 1288. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.J.; Zhang, H.L.; Fu, P.L.; Fan, Z.X. Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 2017, 31, 2202–2211. [Google Scholar] [CrossRef]
- Damour, G.; Simonneau, T.; Cochard, H.; Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 2010, 33, 1419–1438. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Vicinelli, E.; Ponti, F.; Cantoni, L.; Magnani, F. Seasonal and interannual variability of photosynthetic capacity in relation to leaf nitrogen in a deciduous forest plantation in northern Italy. Tree Physiol. 2005, 25, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Xu, M.; Pan, H.L.; Yu, X.B. Leaf-age effects on temperature responses of photosynthesis and respiration of an alpine oak, Quercus aquifolioides, in southwestern China. Tree Physiol. 2015, 35, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Alton, P.B. Retrieval of seasonal Rubisco-limited photosynthetic capacity at global FLUXNET sites from hyperspectral satellite remote sensing: Impact on carbon modelling. Agric. For. Meteorol. 2017, 232, 74–88. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Zaehle, S.; De Kauwe, M.G.; Walker, A.P.; Dietze, M.C.; Hanson, P.J.; Hickler, T.; Jain, A.K.; Luo, Y.Q.; Parton, W.; et al. Using ecosystem experiments to improve vegetation models. Nat. Clim. Chang. 2015, 5, 528–534. [Google Scholar] [CrossRef]
- Zaehle, S.; Medlyn, B.E.; De Kauwe, M.G.; Walker, A.P.; Dietze, M.C.; Hickler, T.; Luo, Y.Q.; Wang, Y.P.; El-Masri, B.; Thornton, P.; et al. Evaluation of 11 terrestrial carbon-nitrogen cycle models against observations from two temperate Free-Air CO2 Enrichment studies. New Phytol. 2014, 202, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Guanter, L.; Berry, J.A.; Joiner, J.; van der Tol, C.; Huete, A.; Gitelson, A.; Voigt, M.; Kohler, P. Estimation of vegetation photosynthetic capacity from space-based measurements of chlorophyll fluorescence for terrestrial biosphere models. Glob. Chang. Biol. 2014, 20, 3727–3742. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.G.; Malyshev, S.L.; Shevliakova, E.; Kattge, J.; Dukes, J.S. Foliar temperature acclimation reduces simulated carbon sensitivity to climate. Nat. Clim. Chang. 2016, 6, 407. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Cheng, S.-L.; Yu, G.-R.; Fang, H.-J.; Mo, J.-M.; Xu, M.-J.; Gao, W.-L. Response of carbon utilization and enzymatic activities to nitrogen deposition in three forests of subtropical China. Can. J. For. Res. 2015, 45, 394–401. [Google Scholar] [CrossRef]
- Sharkey, T.D. What gas exchange data can tell us about photosynthesis. Plant Cell Environ. 2016, 39, 1161–1163. [Google Scholar] [CrossRef] [PubMed]
- Gower, S.T.; Kucharik, C.J.; Norman, J.M. Direct and indirect estimation of leaf area index, f(APAR), and net primary production of terrestrial ecosystems. Remote Sens. Environ. 1999, 70, 29–51. [Google Scholar] [CrossRef]
- Chen, J.M.; Rich, P.M.; Gower, S.T.; Norman, J.M.; Plummer, S. Leaf area index of boreal forests: Theory, techniques, and measurements. J. Geophys. Res. Atmos. 1997, 102, 29429–29443. [Google Scholar] [CrossRef]
- Li, X.R.; Liu, Q.J.; Cai, Z.; Ze Qing, M.A. Leaf area index measurement of Pinus elliotii plantation. Acta Ecol. Sin. 2006, 26, 4099–4105. [Google Scholar]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher-Plants. Annu. Rev. Plant Phys. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Li, R.S.; Yang, Q.P.; Zhang, W.D.; Zheng, W.H.; Chi, Y.G.; Xu, M.; Fang, Y.T.; Gessler, A.; Li, M.H.; Wang, S.L. Thinning effect on photosynthesis depends on needle ages in a Chinese fir (Cunninghamia lanceolata) plantation. Sci. Total Environ. 2017, 580, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Q.; Hui, D.F.; Wallace, L.; Luo, Y.Q. Direct and indirect effects of experimental warming on ecosystem carbon processes in a tallgrass prairie. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Sage, R.F. Temperature and humidity effects on branchlet gas-exchange in white spruce: An explanation for the increase in transpiration with branchlet temperature. Trees Struct. Funct. 1999, 14, 161–168. [Google Scholar] [CrossRef]
- Mott, K.A.; Peak, D. Stomatal responses to humidity and temperature in darkness. Plant Cell Environ. 2010, 33, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.A.; Rees, D.; Sayre, K.D.; Lu, Z.M.; Condon, A.G.; Saavedra, A.L. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 1998, 38, 1467–1475. [Google Scholar]
- Lu, Z.M.; Radin, J.W.; Turcotte, E.L.; Percy, R.; Zeiger, E. High Yields in Advanced Lines of Pima Cotton Are Associated with Higher Stomatal Conductance, Reduced Leaf-Area and Lower Leaf Temperature. Physiol. Plantarum 1994, 92, 266–272. [Google Scholar] [CrossRef]
- Farquhar, G.D. Feedforward Responses of Stomata to Humidity. Aust. J. Plant Physiol. 1978, 5, 787–800. [Google Scholar] [CrossRef]
- Shirke, P.A.; Pathre, U.V. Influence of leaf-to-air vapour pressure deficit (VPD) on the biochemistry and physiology of photosynthesis in Prosopis juliflora. J. Exp. Bot. 2004, 55, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.S.; Eamus, D.; Bell, D. Optimization theory of stomatal behaviour—II. Stomatal responses of several tree species of north Australia to changes in light, soil and atmospheric water content and temperature. J. Exp. Bot. 1999, 50, 393–400. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.H.; Li, X.N.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Kuang, S.B.; Long, G.Q.; Meng, Z.G.; Li, L.G.; Chen, Z.J.; Zhang, G.H.; Yang, S.C. Steady-state and dynamic photosynthetic performance and nitrogen partitioning in the shade-demanding plant Panax notoginseng under different levels of growth irradiance. Acta Physiol. Plant 2014, 36, 2409–2420. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.H.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits—V-cmax and J(max)—To leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Reich, P.B.; Sun, S.C.; Atkin, O.K. Contrasting leaf trait scaling relationships in tropical and temperate wet forest species. Funct. Ecol. 2013, 27, 522–534. [Google Scholar] [CrossRef]
- Chi, Y.G.; Xu, M.; Shen, R.C.; Yang, Q.P.; Huang, B.R.; Wan, S.Q. Acclimation of Foliar Respiration and Photosynthesis in Response to Experimental Warming in a Temperate Steppe in Northern China. PLoS ONE 2013, 8, e0056482. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum. J. Exp. Bot. 2005, 56, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Hikosaka, K.; Hirose, T. The balance between RuBP carboxylation and RuBP regeneration: A mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Funct. Plant Biol. 2005, 32, 903–910. [Google Scholar] [CrossRef]
- Xu, C.G.; Fisher, R.; Wullschleger, S.D.; Wilson, C.J.; Cai, M.; McDowell, N.G. Toward a Mechanistic Modeling of Nitrogen Limitation on Vegetation Dynamics. PLoS ONE 2012, 7, e0037914. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K. Nitrogen partitioning in the photosynthetic apparatus of Plantago asiatica leaves grown under different temperature and light conditions: Similarities and differences between temperature and light acclimation. Plant Cell Physiol. 2005, 46, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis: Mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, S.; Gonzalez-Zurdo, P.; Babiano, J.; Escudero, A. Responses of photosynthetic parameters to differences in winter temperatures throughout a temperature gradient in two evergreen tree species. Eur. J. For. Res. 2016, 135, 871–883. [Google Scholar] [CrossRef]
- Kenzo, T.; Ichie, T.; Watanabe, Y.; Yoneda, R.; Ninomiya, I.; Koike, T. Changes in photosynthesis and leaf characteristics with tree height in five dipterocarp species in a tropical rain forest. Tree Physiol. 2006, 26, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Mcalpine, K.G.; Jesson, L.K.; Kubien, D.S. Photosynthesis and water-use efficiency: A comparison between invasive (exotic) and non-invasive (native) species. Aust. Ecol. 2008, 33, 10–19. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, W.; Zou, B.; Neher, D.A.; Fu, S.; Xia, H.; Li, Z. Seedling growth and soil nutrient availability in exotic and native tree species: Implications for afforestation in southern China. Plant Soil 2012, 364, 207–218. [Google Scholar] [CrossRef]
- Xu, Z.L.; Peng, H.H.; Feng, Z.D.; Abdulsalih, N. Predicting Current and Future Invasion of Solidago Canadensis: A Study from China. Pol. J. Ecol. 2014, 62, 263–271. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, H.; Wang, S.L.; Wang, R.Q.; Wang, Y.T.; Liu, J. Factors affecting alien and native plant species richness in temperate nature reserves of northern China. Pol. J. Ecol. 2017, 65, 320–333. [Google Scholar] [CrossRef]
- Lin, Q.L. Adaptability analysis of Pinus taeda and Pinus elliottii. J. Fujian For. Sci. Technol. 2007, 34, 102–105. [Google Scholar]
- Ma, Z.; Liu, Q.; Wang, H. The growth pattern of Pinus elliottii Plantation in central subtropical China. Acta Ecol. Sin. 2011, 31, 1525–1537. [Google Scholar]
| Month | Tleaf (°C) | VPD (kPa) | SLA (m2 kg−1) | |
|---|---|---|---|---|
| MP | Apr | 28.68 ± 3.01 | 1.27 ± 0.10 | 5.66 ± 0.90 |
| Jun | 33.19 ± 4.49 | 2.63 ± 0.79 | 5.67 ± 0.32 | |
| Sep | 31.64 ± 1.15 | 1.54 ± 0.20 | 5.83 ± 0.53 | |
| Nov | 19.15 ± 3.35 | 0.62 ± 0.16 | 5.43 ± 0.32 | |
| SP | Apr | 29.45 | 2.21 | 4.00 |
| Jun | 35.33 ± 0.93 | 2.75 ± 0.25 | 4.02 ± 0.22 | |
| Sep | 32.50 ± 0.43 | 1.83 ± 0.15 | 3.71 ± 0.17 | |
| Nov | 24.69 ± 1.57 | 0.88 ± 0.09 | 3.34 ± 0.05 | |
| p value | 0.020 | 0.052 | 0.000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhou, L.; Wang, S.; Chi, Y.; Chen, J. Leaf Temperature and Vapour Pressure Deficit (VPD) Driving Stomatal Conductance and Biochemical Processes of Leaf Photosynthetic Rate in a Subtropical Evergreen Coniferous Plantation. Sustainability 2018, 10, 4063. https://doi.org/10.3390/su10114063
Li Y, Zhou L, Wang S, Chi Y, Chen J. Leaf Temperature and Vapour Pressure Deficit (VPD) Driving Stomatal Conductance and Biochemical Processes of Leaf Photosynthetic Rate in a Subtropical Evergreen Coniferous Plantation. Sustainability. 2018; 10(11):4063. https://doi.org/10.3390/su10114063
Chicago/Turabian StyleLi, Yue, Lei Zhou, Shaoqiang Wang, Yonggang Chi, and Jinghua Chen. 2018. "Leaf Temperature and Vapour Pressure Deficit (VPD) Driving Stomatal Conductance and Biochemical Processes of Leaf Photosynthetic Rate in a Subtropical Evergreen Coniferous Plantation" Sustainability 10, no. 11: 4063. https://doi.org/10.3390/su10114063
APA StyleLi, Y., Zhou, L., Wang, S., Chi, Y., & Chen, J. (2018). Leaf Temperature and Vapour Pressure Deficit (VPD) Driving Stomatal Conductance and Biochemical Processes of Leaf Photosynthetic Rate in a Subtropical Evergreen Coniferous Plantation. Sustainability, 10(11), 4063. https://doi.org/10.3390/su10114063





