Exposing Sustainable Mortars with Nanosilica, Zinc Stearate, and Ethyl Silicate Coating to Sulfuric Acid Attack
Abstract
:1. Introduction
2. Materials and Methods
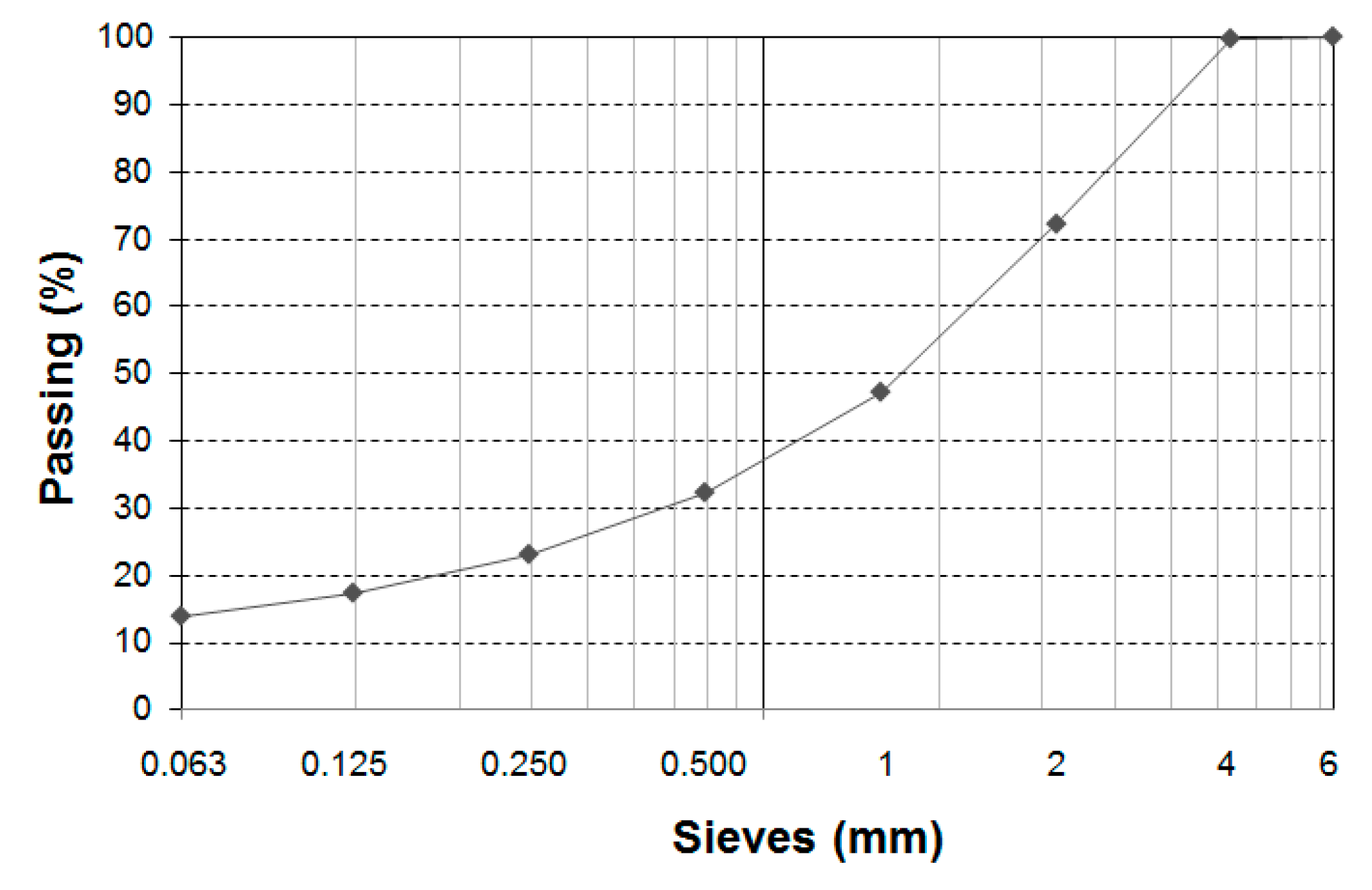
2.1. Materials
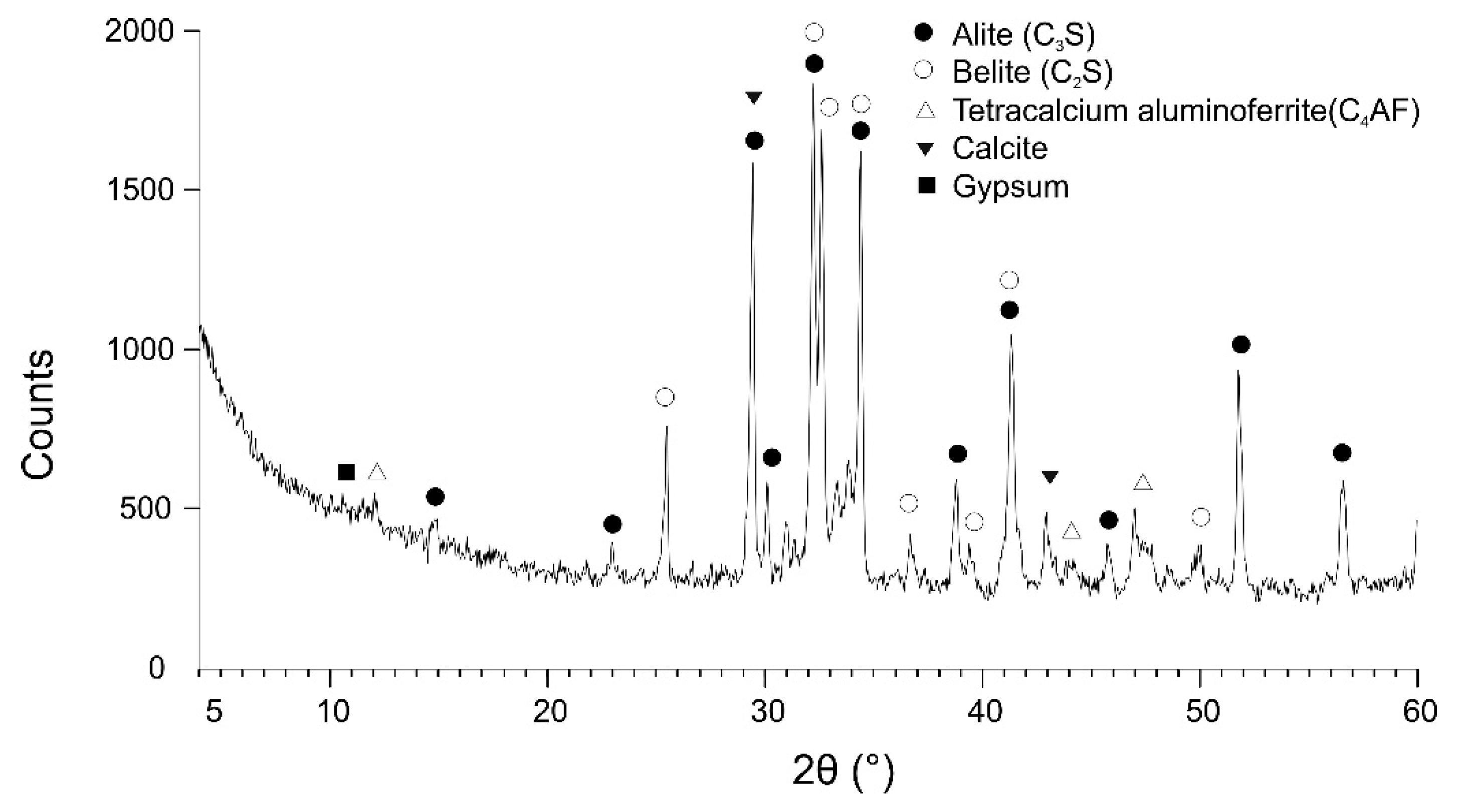
Chemical–Physical Characterization of the Binder
2.2. Methods
2.2.1. Manufacturing and Curing Process of the Mortars and Sulfuric Acid Attack Simulation
- The specimens were extracted from the H2SO4 solution and were gently brushed under a weak flow of tap water to eliminate material residue that may have adhered to the surface.
- The H2SO4 solution was replaced with a new solution.
- The specimens were reintroduced into the new solution.
2.2.2. Physical and Mechanical Properties of the Mortars After the Sulfuric Acid Attack
- C = capillary water absorption coefficient, kg/(m2∙min0.5)
- M1 = specimen mass after the immersion for 10 min, kg
- M2 = specimen mass after the immersion for 90 min, kg
- A = surface of the specimen face submerged in the water, m2
- t2 = 90 min
- t1 = 10 min
2.2.3. Scanning Electron Microscopy (SEM) Examination
3. Results and Discussion
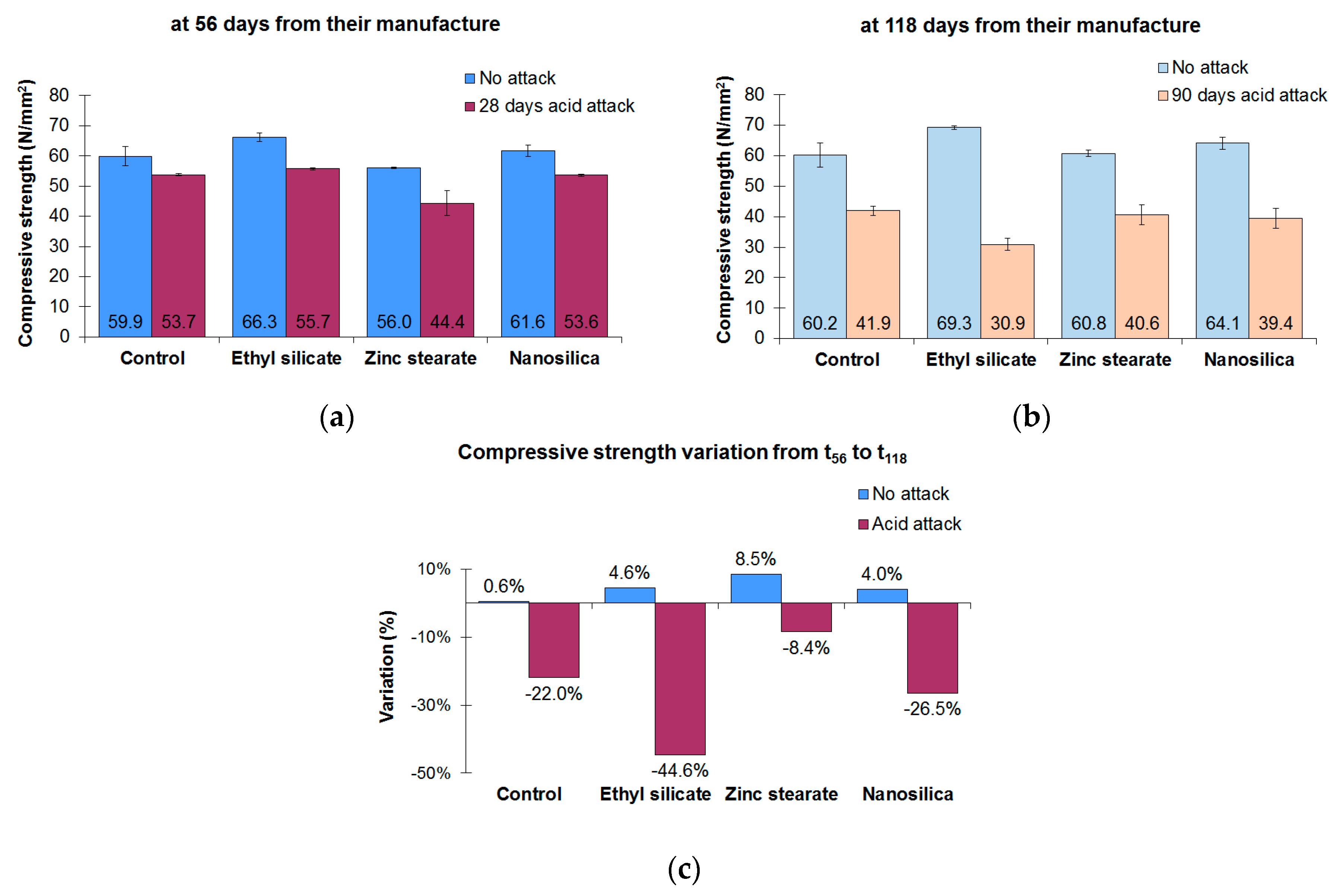
3.1. Compressive Strength
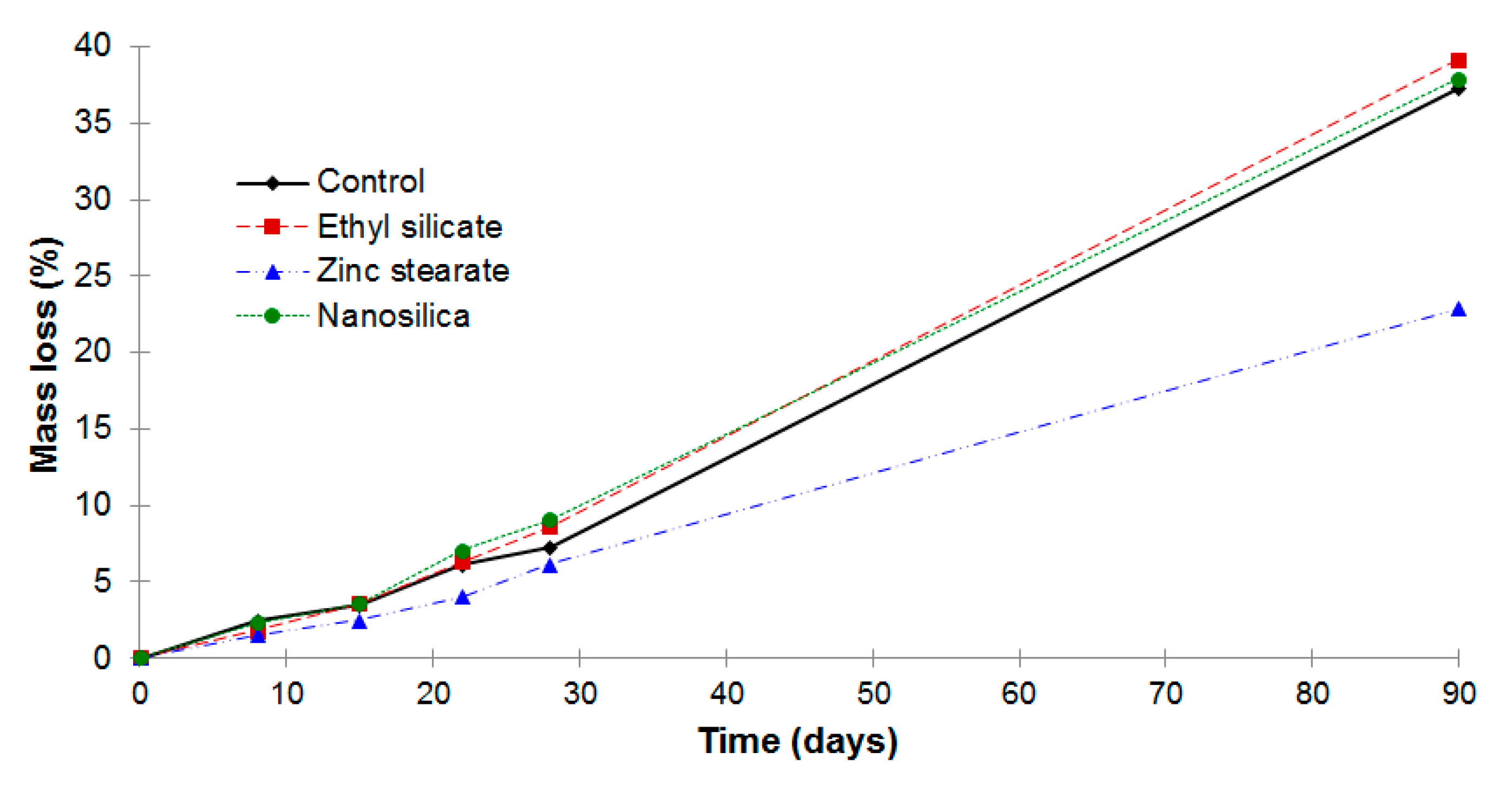
3.2. Mass Loss Due to the Sulfuric Acid Exposure
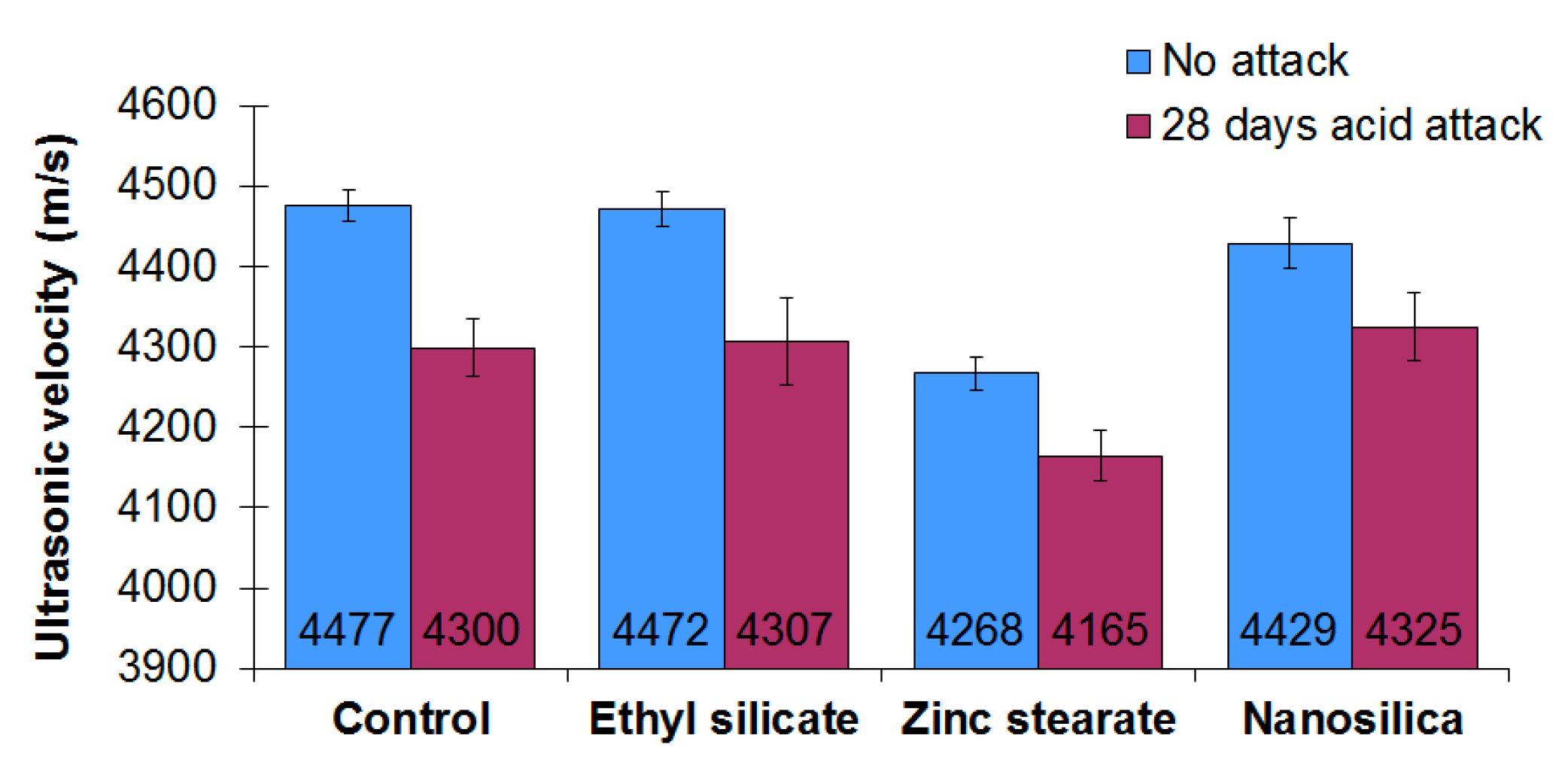
3.3. Ultrasonic Pulse Velocity
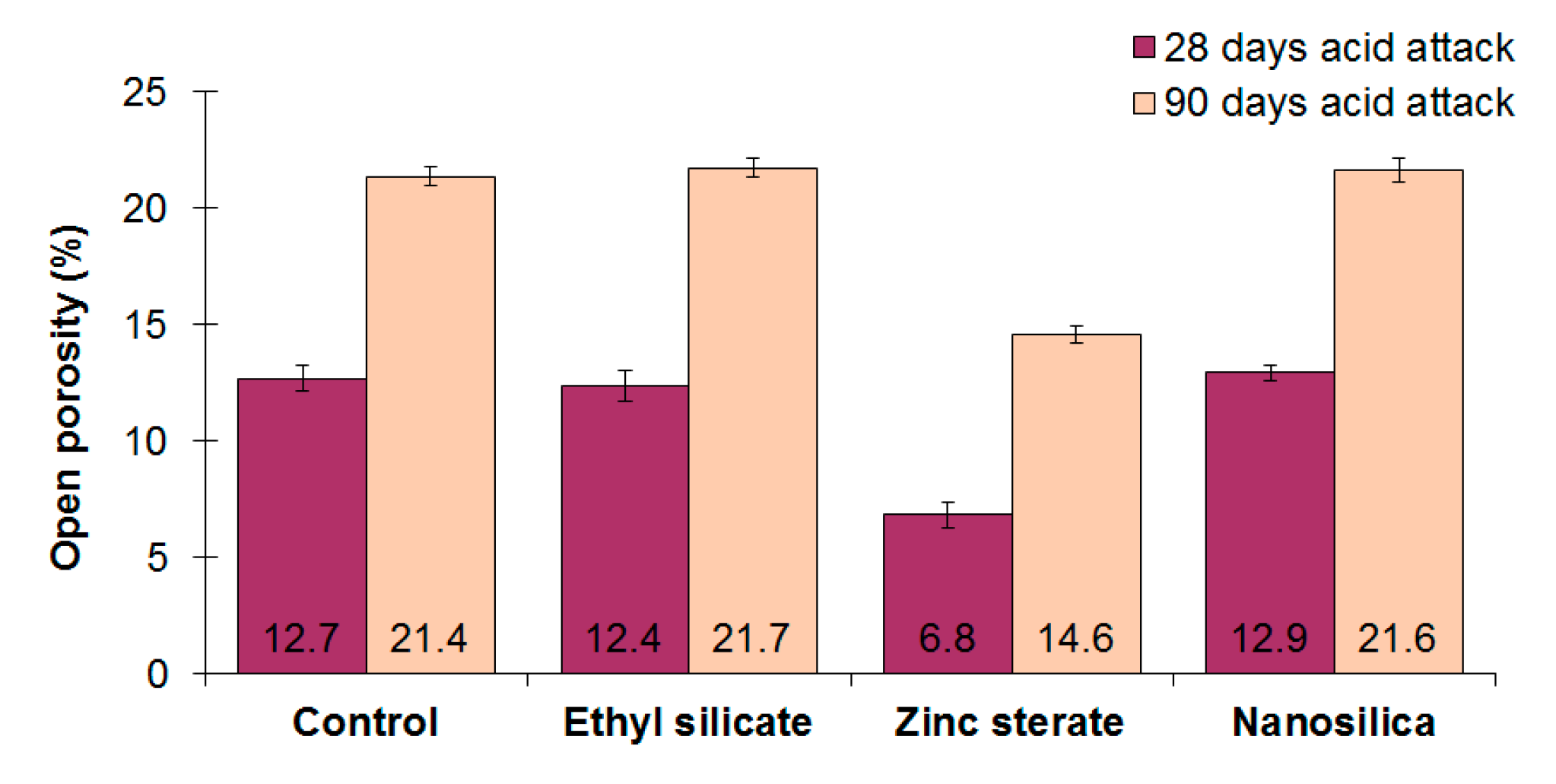
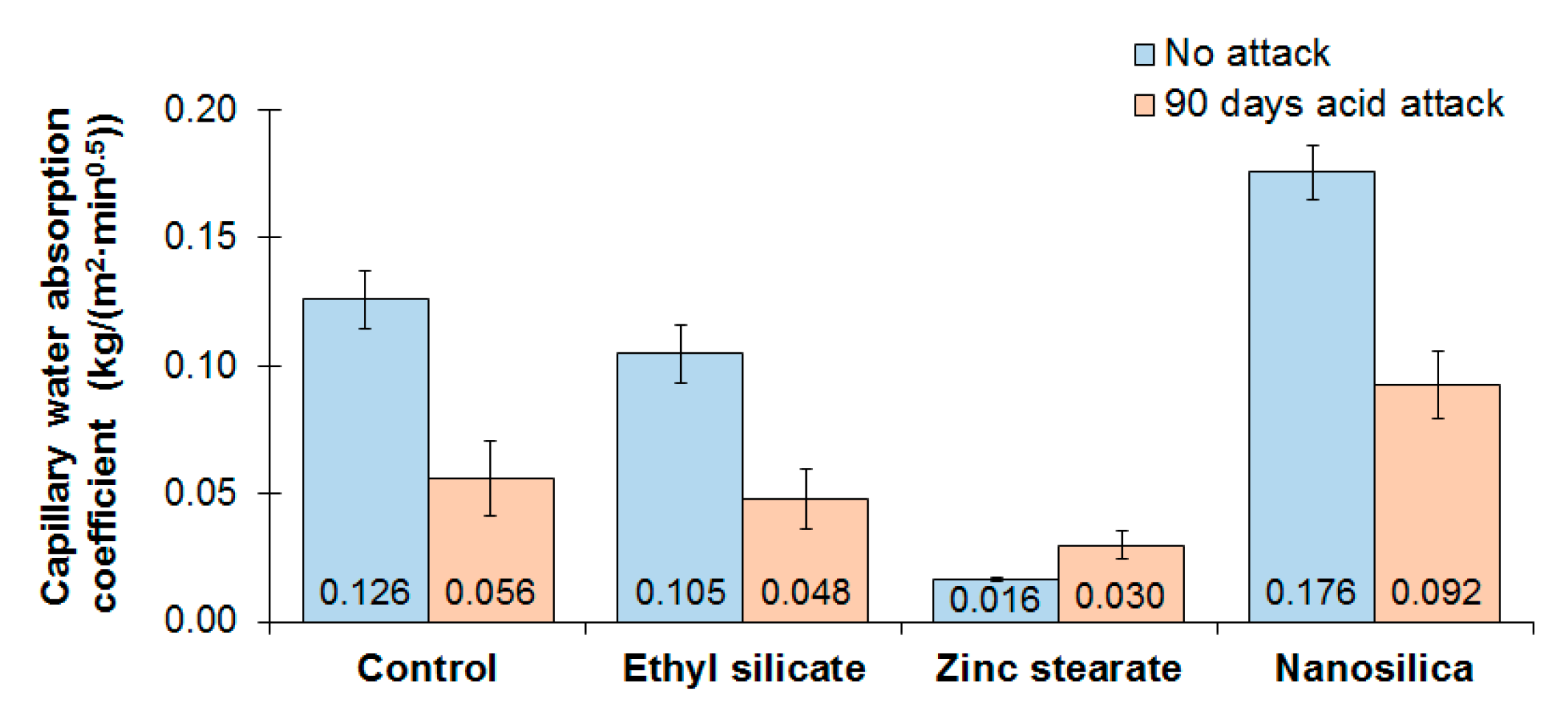
3.4. Open Porosity and Capillary Water Absorption Coefficient
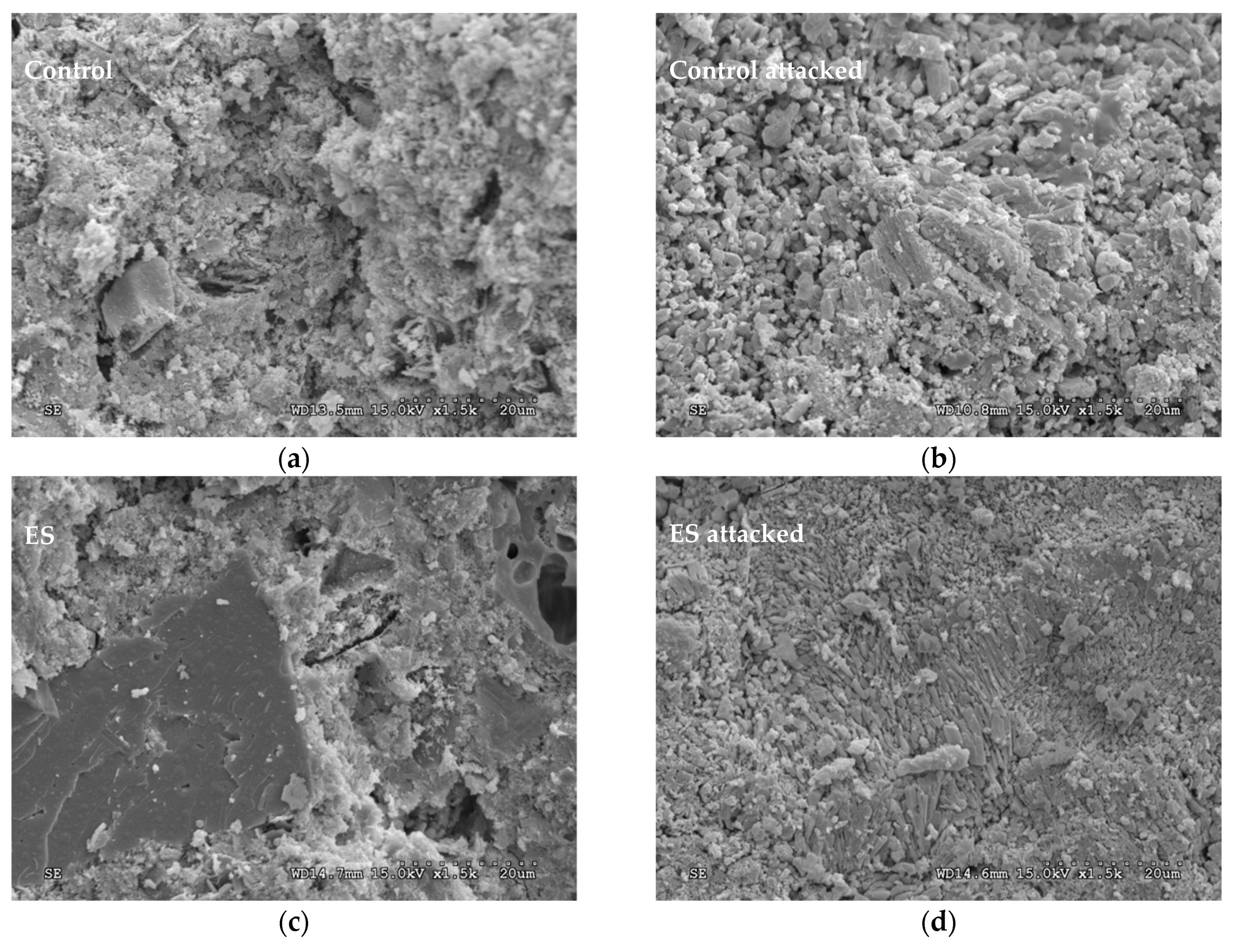
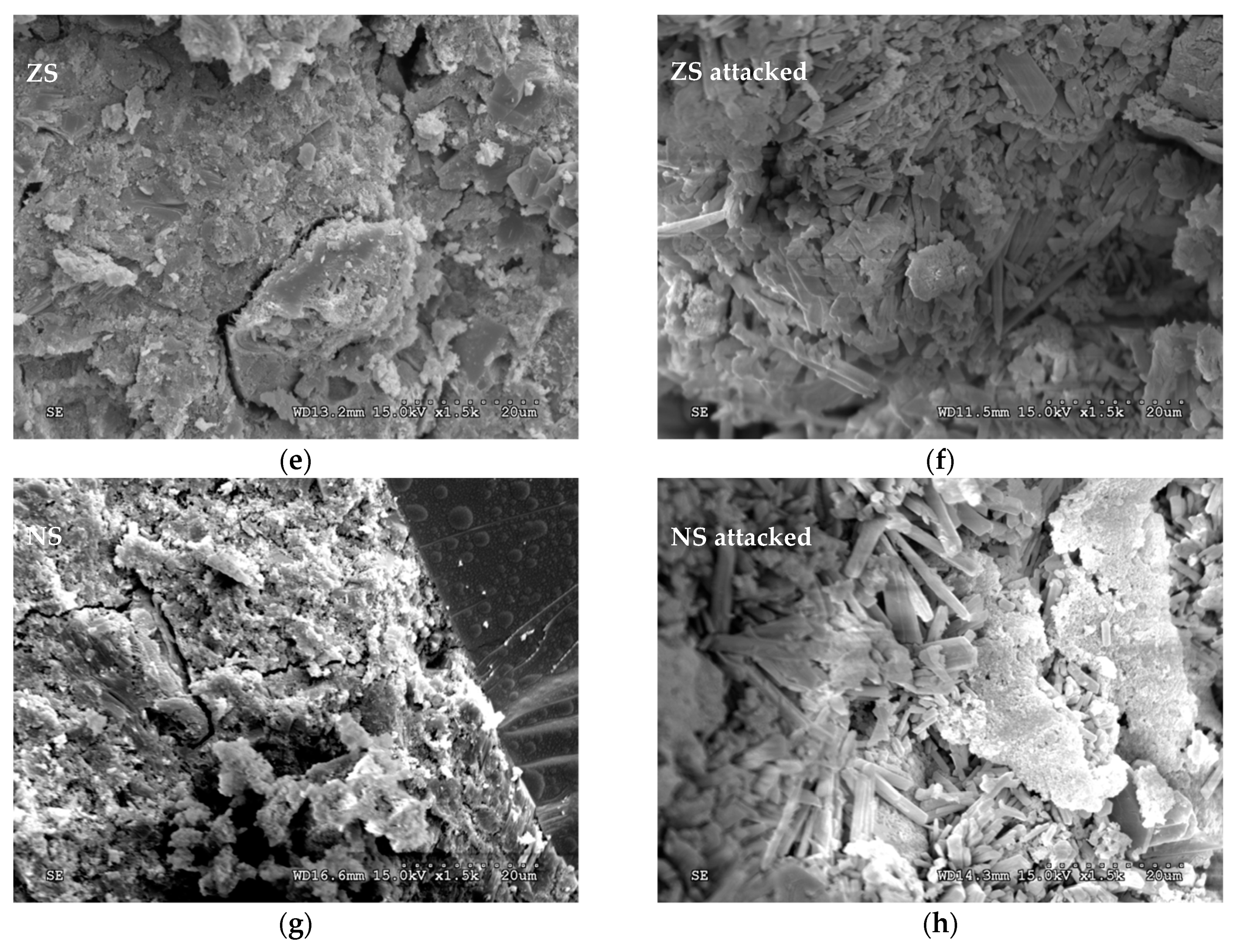
3.5. Scanning Electron Microscopy (SEM) Examination
4. Conclusions
- The compressive strength of the four mortars studied decreased when they were exposed to a sulfuric acid attack. The effect on the strength reduction of the mortars was greater with longer periods of acid exposure. The control mortar had the highest compressive strength after the acid attack. However, the zinc stearate mortar had the lowest percentage of compressive strength loss after 90 days of sulfuric acid attack.
- All the mortars reduced their mass after the sulfuric acid exposure. The lowest mass loss of zinc stearate mortar proves that this mortar best resisted the acid attack.
- The zinc stearate mortar demonstrated its hydrophobic effect with the lowest coefficient of capillary water absorption, both in a non-aggressive and aggressive environment.
- Nanosilica mortar and the mortar with an ethyl silicate coating do not present positive effects when they are exposed to sulfuric acid.
- As a consequence of the sulfuric acid attack, an increase in the open porosity of mortars makes them more vulnerable to aggressive environments. Moreover, a decrease in the ultrasonic pulse velocity is detected after the acid attack.
- The results support that an alternative and reliable method of obtaining the capillary water absorption coefficient for mortars is by calculating the slope of the linear regression lines for the water absorption data as a function of time.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ortega, J.M.; Esteban, M.D.; Williams, M.; Sánchez, I.; Climent, M.A. Short-term performance of sustainable silica fume mortars exposed to sulfate attack. Sustainability 2018, 10, 2517. [Google Scholar] [CrossRef]
- Ortega Álvarez, J.; Esteban Pérez, M.; Rodríguez Escribano, R.; Pastor Navarro, J.; Sánchez Martín, I. Microstructural Effects of Sulphate Attack in Sustainable Grouts for Micropiles. Materials 2016, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Ponikiewski, T.; Golaszewski, J. The effect of high-calcium fly ash on selected properties of self-compacting concrete. Arch. Civ. Mech. Eng. 2014, 14, 455–465. [Google Scholar] [CrossRef]
- Glinicki, M.A.; Jozwiak-Niedzwiedzka, D.; Gibas, K.; Dabrowski, M. Influence of Blended Cements with Calcareous Fly Ash on Chloride Ion Migration and Carbonation Resistance of Concrete for Durable Structures. Materials 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Estokova, A.; Kovalcikova, M.; Luptakova, A.; Prascakova, M. Testing Silica Fume-Based Concrete Composites under Chemical and Microbiological Sulfate Attacks. Materials 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Qudoos, A.; Kim, H.G.; Ryou, J.-S. Influence of titanium dioxide nanoparticles on the sulfate attack upon ordinary Portland cement and slag-blended mortars. Materials 2018, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Sun, W.; Scrivener, K. Mechanism of expansion of mortars immersed in sodium sulfate solutions. Cement Concr. Res. 2013, 43, 105–111. [Google Scholar] [CrossRef]
- Monteiro, P.J.; Kurtis, K.E. Time to failure for concrete exposed to severe sulfate attack. Cement Concr. Res. 2003, 33, 987–993. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhu, Z.M.; Sun, X.; Wang, X.M. Deterioration of fracture toughness of concrete under acid rain environment. Eng. Fail. Anal. 2017, 77, 76–84. [Google Scholar] [CrossRef]
- Whittaker, M.; Black, L. Current knowledge of external sulfate attack. Adv. Cement Res. 2015, 27, 532–545. [Google Scholar] [CrossRef] [Green Version]
- Skalny, J.; Marchand, J.; Odler, I. Sulfate Attack on Concrete; Spon Press: London, UK; New York, NY, USA, 2002; ISBN 0-419-24550-2. [Google Scholar]
- Hadigheh, S.A.; Gravina, R.J.; Smith, S.T. Effect of acid attack on FRP-to-concrete bonded interfaces. Constr. Build. Mater. 2017, 152, 285–303. [Google Scholar] [CrossRef]
- Li, L.G.; Zhu, J.; Huang, Z.H.; Kwan, A.K.H.; Li, L.J. Combined effects of micro-silica and nano-silica on durability of mortar. Constr. Build. Mater. 2017, 157, 337–347. [Google Scholar] [CrossRef]
- CEN EN 206-1-2013+A1:2018. Concrete—Part 1: Specification, Performance, Production and Conformity; AENOR: Madrid, Spain, 2018. [Google Scholar]
- EHE-08—Code on Structural Concrete. Articles and Annexes; Ministerio de Fomento, Gobierno de España: Madrid, Spain, 2008; Available online: https://www.fomento.gob.es/organos-colegiados/mas-organos-colegiados/comision-permanente-del-hormigon/cph/instrucciones/ehe-08-version-en-ingles (accessed on 16 October 2018).
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Durability related transport properties of OPC and slag cement mortars hardened under different environmental conditions. Constr. Build. Mater. 2012, 27, 176–183. [Google Scholar] [CrossRef]
- Norhasri, M.S.M.; Hamidah, M.S.; Fadzil, A.M. Applications of using nano material in concrete: A review. Constr. Build. Mater. 2017, 133, 91–97. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, W. Nano-mechanical behavior of a green ultra-high performance concrete. Constr. Build. Mater. 2014, 63, 150–160. [Google Scholar] [CrossRef]
- Nik, A.S.; Bahari, A. Nano-Particles in Concrete and Cement Mixtures. Appl. Mech. Mater. 2011, 110–116, 3853–3855. [Google Scholar] [CrossRef]
- Aitcin, P.-C. Cements of yesterday and today. Cement Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H.J.H. Effect of nano-silica on the hydration and microstructure development of Ultra-High Performance Concrete (UHPC) with a low binder amount. Constr. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Adak, D.; Sarkar, M.; Mandal, S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Constr. Build. Mater. 2014, 70, 453–459. [Google Scholar] [CrossRef]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C 2014, 45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.S.; Alsayed, S.H.; Aqel, M. Hybrid effect of carbon nanotube and nano-clay on physico-mechanical properties of cement mortar. Constr. Build. Mater. 2011, 25, 145–149. [Google Scholar] [CrossRef]
- Navarro-Blasco, I.; Pérez-Nicolás, M.; Fernández, J.M.; Duran, A.; Sirera, R.; Alvarez, J.I. Assessment of the interaction of polycarboxylate superplasticizers in hydrated lime pastes modified with nanosilica or metakaolin as pozzolanic reactives. Constr. Build. Mater. 2014, 73, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.S.; Sarker, P.K.; Barbhuiya, S. Sorptivity and acid resistance of ambient-cured geopolymer mortars containing nano-silica. Cement Concr. Compos. 2016, 72, 235–245. [Google Scholar] [CrossRef]
- Falchi, L.; Varin, C.; Toscano, G.; Zendri, E. Statistical analysis of the physical properties and durability of water-repellent mortars made with limestone cement, natural hydraulic lime and pozzolana-lime. Constr. Build. Mater. 2015, 78, 260–270. [Google Scholar] [CrossRef]
- Lanzón, M.; García-Ruiz, P.A. Evaluation of capillary water absorption in rendering mortars made with powdered waterproofing additives. Constr. Build. Mater. 2009, 23, 3287–3291. [Google Scholar] [CrossRef]
- Li, W.; Wittmann, F.; Jiang, R.; Zhao, T.; Wolfseher, R. Metal Soaps for the Production of Integral Water Repellent Concre. In Hydrophobe VI: Water Repellent Treatment of Building Materials; Borrelli, E., Fassina, V., Eds.; Aedificatio Publishers: Rome, Italy, 2011; pp. 145–154. [Google Scholar]
- Soroushian, P.; Chowdhury, H.; Ghebrab, T. Evaluation of Water-Repelling Additives for Use in Concrete-Based Sanitary Sewer Infrastructure. J. Infrastruct. Syst. 2009, 15, 106–110. [Google Scholar] [CrossRef]
- Falchi, L.; Zendri, E.; Muller, U.; Fontana, P. The influence of water-repellent admixtures on the behaviour and the effectiveness of Portland limestone cement mortars. Cement Concr. Compos. 2015, 59, 107–118. [Google Scholar] [CrossRef]
- Lanzón, M.; Martínez, E.; Mestre, M.; Madrid, J.A. Use of zinc stearate to produce highly-hydrophobic adobe materials with extended durability to water and acid-rain. Constr. Build. Mater. 2017, 139, 114–122. [Google Scholar] [CrossRef]
- Pigino, B.; Leemann, A.; Franzoni, E.; Lura, P. Ethyl silicate for surface treatment of concrete—Part II: Characteristics and performance. Cement Concr. Compos. 2012, 34, 313–321. [Google Scholar] [CrossRef]
- CEN EN 933-1:2012. Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieveing Method; AENOR: Madrid, Spain, 2012. [Google Scholar]
- CEN EN 196-1:2005. Methods of Testing Cement—Part 1: Determination of Strength; AENOR: Madrid, Spain, 2005. [Google Scholar]
- CEN EN 197-1:2011. Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements; AENOR: Madrid, Spain, 2011. [Google Scholar]
- CEN EN 1936:2007. Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity; AENOR: Madrid, Spain, 2007. [Google Scholar]
- CEN EN 12504-4:2006. Testing Concrete—Part 4: Determination of Ultrasonic Pulse Velocity; AENOR: Madrid, Spain, 2006. [Google Scholar]
- CEN EN-1015-18:2003. Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar; AENOR: Madrid, Spain, 2003. [Google Scholar]
- Tixier, R.; Mobasher, B. Modeling of Damage in Cement-Based Materials Subjected to External Sulfate Attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, C.; Yang, C.; Zhou, L.; Yin, J. Accelerated sulfate attack on mortars using electrical pulse. Constr. Build. Mater. 2015, 95, 875–881. [Google Scholar] [CrossRef]
- Ma, H.; Li, Z. Microstructures and mechanical properties of polymer modified mortars under distinct mechanisms. Constr. Build. Mater. 2013, 47, 579–587. [Google Scholar] [CrossRef]
- Bonakdar, A.; Mobasher, B. Multi-parameter study of external sulfate attack in blended cement materials. Constr. Build. Mater. 2010, 24, 61–70. [Google Scholar] [CrossRef]
- Lanzon, M.; Garcia-Ruiz, P.A. Deterioration and damage evaluation of rendering mortars exposed to sulphuric acid. Mater. Struct. 2010, 43, 417–427. [Google Scholar] [CrossRef]
- Komlosš, K.; Popovics, S.; Nürnbergerová, T.; Babál, B.; Popovics, J.S. Ultrasonic pulse velocity test of concrete properties as specified in various standards. Cement Concr. Compos. 1996, 18, 357–364. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Sui, L.; Xing, F. Effect of sulfate attack on the stress–strain relationship of FRP-confined concrete. Constr. Build. Mater. 2016, 110, 235–250. [Google Scholar] [CrossRef]
- Atahan, H.N.; Arslan, K.M. Improved durability of cement mortars exposed to external sulfate attack: The role of nano & micro additives. Sustain. Cities Soc. 2016, 22, 40–48. [Google Scholar] [CrossRef]
- Yaman, I.; Inci, G.; Yesiller, N.; Aktan, H. Ultrasonic pulse velocity in concrete using direct and indirect transmission. ACI Mater. J. 2001, 98, 450–457. [Google Scholar]
- Toutanji, H. Ultrasonic wave velocity signal interpretation of simulated concrete bridge decks. Mater. Struct. 2000, 33, 207–215. [Google Scholar] [CrossRef]
- Genovés, V.; Vargas, F.; Gosálbez, J.; Carrión, A.; Borrachero, M.V.; Payá, J. Ultrasonic and impact spectroscopy monitoring on internal sulphate attack of cement-based materials. Mater. Des. 2017, 125, 46–54. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulfate attack on concrete. Cement Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Hall, C.; Hoff, W.D. Water Transport in Brick, Stone, and Concrete; Spon Press: London, UK, 2002. [Google Scholar]
- García-Vera, V.E.; Lanzón, M. Physical-chemical study, characterisation and use of image analysis to assess the durability of earthen plasters exposed to rain water and acid rain. Constr. Build. Mater. 2018, 187, 708–717. [Google Scholar] [CrossRef]

| Percentage (%) | |
|---|---|
| Na2O | 0.23 |
| MgO | 3.29 |
| Al2O3 | 3.36 |
| SiO2 | 14.89 |
| P2O5 | 0.14 |
| SO3 | 4.62 |
| Cl | 0.11 |
| K2O | 1.06 |
| CaO | 55.36 |
| TiO2 | 0.25 |
| MnO | 0.04 |
| Fe2O3 | 3.06 |
| SrO | 0.12 |
| Other elements | <0.30 |
| No Attack | Sulfuric Acid Attack | ||||
|---|---|---|---|---|---|
| Regression Line Slope | R2 | Regression Line Slope | R2 | Variation | |
| Control | 0.129 | 1.00 | 0.055 | 0.99 | −57.6% |
| Ethyl silicate | 0.105 | 1.00 | 0.045 | 0.97 | −56.6% |
| Zinc stearate | 0.015 | 0.98 | 0.029 | 0.94 | 89.2% |
| Nanosilica | 0.175 | 1.00 | 0.090 | 0.99 | −48.3% |
| Control | Control Attacked | ES | ES Attacked | ZS | ZS Attacked | NS | NS Attacked | |
|---|---|---|---|---|---|---|---|---|
| Element | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% |
| C | 12.4 | 13.8 | 12.3 | 9.9 | 18.9 | 6.8 | 9.9 | 10.5 |
| O | 49.1 | 53.1 | 51.5 | 49.2 | 49.2 | 55.5 | 49.9 | 46.3 |
| F | - | - | 0.2 | - | - | - | - | - |
| Mg | - | - | - | - | - | - | 0.3 | - |
| Al | 1.3 | - | 1.1 | - | 0.2 | - | 1.1 | - |
| Si | 3.56 | 2.0 | 3.3 | 2.7 | 0.9 | 0.5 | 3.6 | 5.0 |
| S | 1.1 | 16.9 | 0.4 | 16.4 | - | 13.9 | 0.7 | 17.7 |
| Ca | 31.5 | 20.2 | 30.2 | 21.8 | 30.7 | 23.2 | 33.4 | 20.6 |
| Fe | 1.1 | - | 1.0 | - | 0.1 | - | 1.1 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Vera, V.E.; Tenza-Abril, A.J.; Lanzón, M.; Saval, J.M. Exposing Sustainable Mortars with Nanosilica, Zinc Stearate, and Ethyl Silicate Coating to Sulfuric Acid Attack. Sustainability 2018, 10, 3769. https://doi.org/10.3390/su10103769
García-Vera VE, Tenza-Abril AJ, Lanzón M, Saval JM. Exposing Sustainable Mortars with Nanosilica, Zinc Stearate, and Ethyl Silicate Coating to Sulfuric Acid Attack. Sustainability. 2018; 10(10):3769. https://doi.org/10.3390/su10103769
Chicago/Turabian StyleGarcía-Vera, Victoria Eugenia, Antonio José Tenza-Abril, Marcos Lanzón, and José Miguel Saval. 2018. "Exposing Sustainable Mortars with Nanosilica, Zinc Stearate, and Ethyl Silicate Coating to Sulfuric Acid Attack" Sustainability 10, no. 10: 3769. https://doi.org/10.3390/su10103769
APA StyleGarcía-Vera, V. E., Tenza-Abril, A. J., Lanzón, M., & Saval, J. M. (2018). Exposing Sustainable Mortars with Nanosilica, Zinc Stearate, and Ethyl Silicate Coating to Sulfuric Acid Attack. Sustainability, 10(10), 3769. https://doi.org/10.3390/su10103769






