Functional Diversity of Soil Microbial Communities in Response to the Application of Cefuroxime and/or Antibiotic-Resistant Pseudomonas putida Strain MC1
Abstract
:1. Introduction
2. Materials and Methods
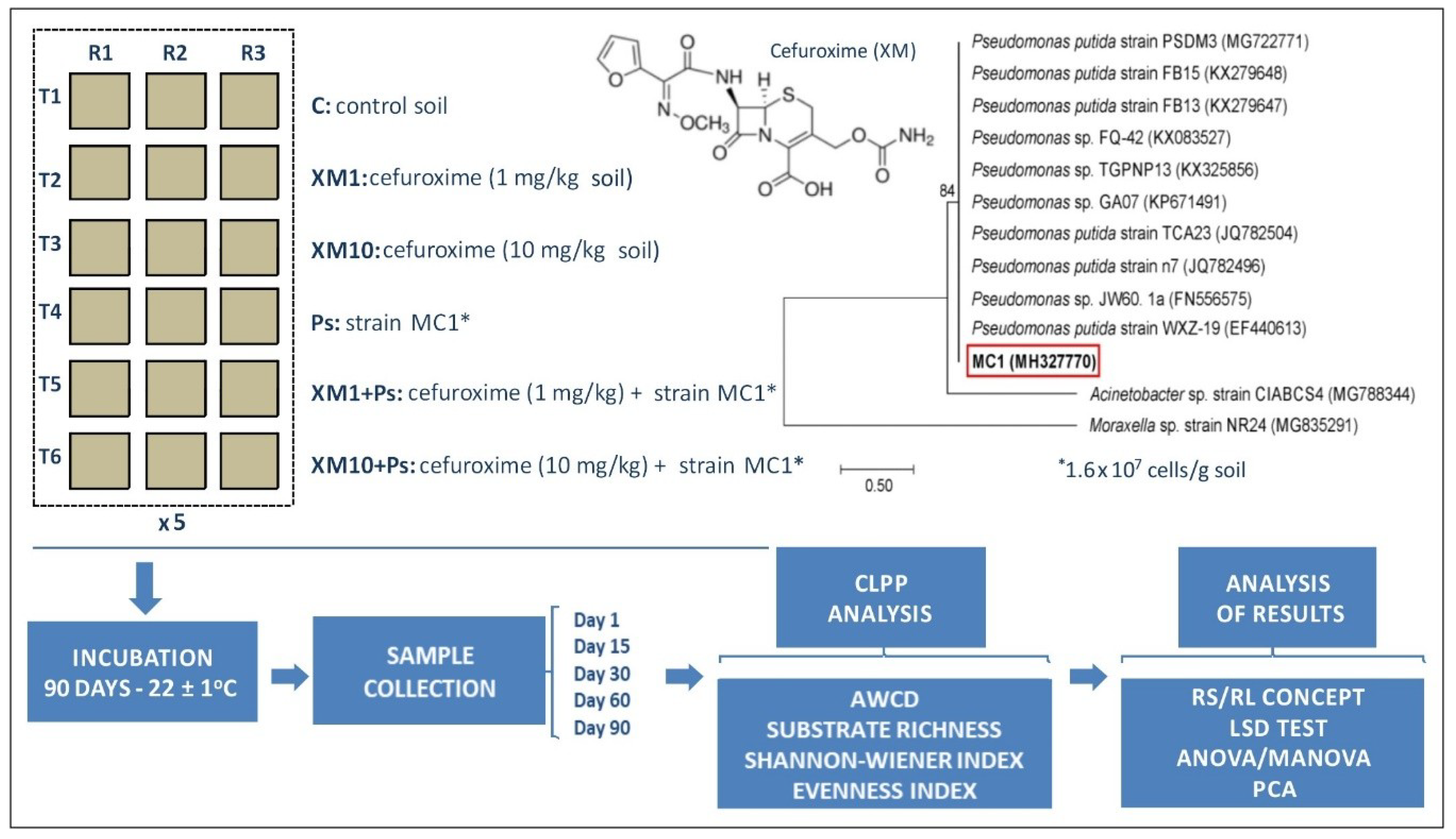
2.1. Experimental Design
2.2. Determination of the Catabolic Activity of Soil Microorganisms
2.3. Analysis of the Data
3. Results
3.1. The CLPP Indices
3.2. Carbon Substrate Utilization Pattern
3.3. Principal Component Analysis
3.4. The Resistance (RS)/Resilience (RL) Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, L.-J.; Ying, G.-G.; Liu, S.; Zhao, J.-L.; Yang, B.; Chen, Z.-F.; Lai, H.-J. Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci. Total Environ. 2013, 452–453, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.; Qin, S.; Luo, Y. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China. Environ. Sci. Pollut. Res. 2015, 22, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Owino, A.A.; Gao, Y.; Yan, X.; Xu, C.; Wang, J. Occurrence, composition and risk assessment of antibiotics in soils from Kenya, Africa. Ecotoxicology 2016, 25, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Leaching behavior of veterinary antibiotics in animal manure-applied soils. Sci. Total Environ. 2017, 579, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, C.; Dolhi, J.M.; Li, S.; He, J.; Qiao, M. Characteristics of oxytetracycline sorption and potential bioavailability in soils with various physical-chemical properties. Chemosphere 2012, 87, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, S.-P.; Fu, J.; Zhou, Z.-Q.; Zhang, N.; Guo, L. Influence of ciprofloxacin on microbial community structure and function in soils. Biol. Fertil. Soils 2014, 50, 939–947. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Adsorption and degradation of five selected antibiotics in agricultural soil. Sci. Total Environ. 2016, 545–546, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Li, H.; Gu, J.; Tuo, X.; Sun, W.; Qian, X.; Wang, X. Effects of biochar on reducing the abundance of oxytetracycline, antibiotic resistance genes, and human pathogenic bacteria in soil and lettuce. Environ. Pollut. 2017, 224, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Orlewska, K.; Markowicz, A.; Zmijowska, A.; Smoleń-Dzirba, J.; Bratosiewicz-Wasik, J.; Wasik, T.J.; Piotrowska-Seget, Z. Vancomycin and/or multidrug-resistant Citrobacter freundii altered the metabolic pattern of soil microbial community. Front. Microbiol. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Braschi, I.; Blasioli, S.; Fellet, C.; Lorenzini, R.; Garelli, A.; Pori, M.; Giacomini, D. Persistence and degradation of new β-lactam antibiotics in the soil and water environment. Chemosphere 2013, 93, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Kay, P.; Blackwell, P.A.; Boxall, A.B.A. Fate of veterinary antibiotics in a macroporous tile drained clay soil. Environ. Toxicol. Chem. 2004, 23, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Popowska, M.; Rzeczycka, M.; Miernik, A.; Krawczyk-Balska, A.; Walsh, F.; Duffy, B. Influence of soil use on prevalence of tetracycline, streptomycin, and erythromycin resistance and associated resistance genes. Antimicrob. Agents Chemother. 2012, 56, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- DeVries, S.L.; Zhang, P. Antibiotics and the Terrestrial Nitrogen Cycle: A Review. Curr. Pollut. Rep. 2016, 2, 51–67. [Google Scholar] [CrossRef] [Green Version]
- Orlewska, K.; Piotrowska-Seget, Z.; Bratosiewicz-Wąsik, J.; Cycoń, M. Characterization of bacterial diversity in soil contaminated with the macrolide antibiotic erythromycin and/or inoculated with a multidrug-resistant Raoultella sp. strain using the PCR-DGGE approach. Appl. Soil Ecol. 2018, 126, 57–64. [Google Scholar] [CrossRef]
- Binh, C.T.T.; Heuer, H.; Gomes, N.C.M.; Kotzerke, A.; Fulle, M.; Wilke, B.M.; Schloter, M.; Smalla, K. Short-term effects of amoxicillin on bacterial communities in manured soil. FEMS Microbiol. Ecol. 2007, 62, 290–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; LaPara, T.M. The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J. 2007, 1, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyselková, M.; Jirout, J.; Chroňáková, A.; Vrchotová, N.; Bradley, R.; Schmitt, H.; Elhottová, D. Cow excrements enhance the occurrence of tetracycline resistance genes in soil regardless of their oxytetracycline content. Chemosphere 2013, 93, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lin, H.; Sun, W.; Wang, Q.; Yu, Q.; Zhao, Y.; Fu, J. Soil microbial systems respond differentially to tetracycline, sulfamonomethoxine, and ciprofloxacin entering soil under pot experimental conditions alone and in combination. Environ. Sci. Pollut. Res. 2014, 21, 7436–7448. [Google Scholar] [CrossRef] [PubMed]
- Wepking, C.; Avera, B.; Badgley, B.; Barrett, J.E.; Franklin, J.; Knowlton, K.F.; Ray, P.P.; Smitherman, C.; Strickland, M.S. Exposure to dairy manure leads to greater antibiotic resistance and increased mass-specific respiration in soil microbial communities. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Keen, P.L.; Patrick, D.M. Tracking change: A look at the ecological footprint of antibiotics and antimicrobial resistance. Antibiotics 2013, 2, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Lou, C.; Wang, S.; Lu, Y.; Liu, M.; Hashmi, M.Z.; Liang, X.; Li, Z.; Liao, Y.; Qin, W.; et al. Effects of long-term manure applications on the occurrence of antibiotics and antibiotic resistance genes (ARGs) in paddy soils: Evidence from four field experiments in south of China. Soil Biol. Biochem. 2015, 90, 179–187. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Pinna, M.V.; Castaldi, P.; Deiana, P.; Pusino, A.; Garau, G. Sorption behavior of sulfamethazine on unamended and manure-amended soils and short-term impact on soil microbial community. Ecotoxicol. Environ. Saf. 2012, 84, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Akimenko, Y.V.; Kazeev, K.S.; Kolesnikov, S.I. Impact assessment of soil contamination with antibiotics (For example, an ordinary chernozem). Am. J. Appl. Sci. 2015, 12, 80–88. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Wang, J.; Zhou, H.; Jiang, C. The combined effect of sulfadiazine and copper on soil microbial activity and community structure. Ecotoxicol. Environ. Saf. 2016, 134, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.; Haapakangas, H.; Van Beelen, P. Effects of antibiotics on soil microorganisms: Time and nutrients influence pollution-induced community tolerance. Soil Biol. Biochem. 2005, 37, 1882–1892. [Google Scholar] [CrossRef]
- Brandt, K.K.; Sjøholm, O.R.; Krogh, K.A.; Halling-Sørensen, B.; Nybroe, O. Increased pollution-induced bacterial community tolerance to sulfadiazine in soil hotspots amended with artificial root exudates. Environ. Sci. Technol. 2009, 43, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Zhang, X.; Wang, J.; Gao, M. Effects of chlortetracycline on soil microbial communities: Comparisons of enzyme activities to the functional diversity via Biolog EcoPlatesTM. Eur. J. Soil Biol. 2015, 68, 69–76. [Google Scholar] [CrossRef]
- Chen, W.; Liu, W.; Pan, N.; Jiao, W.; Wang, M. Oxytetracycline on functions and structure of soil microbial community. J. Soil Sci. Plant Nutr. 2013, 13, 967–975. [Google Scholar] [CrossRef]
- Ma, T.; Pan, X.; Chen, L.; Liu, W.; Christie, P.; Luo, Y.; Wu, L. Effects of different concentrations and application frequencies of oxytetracycline on soil enzyme activities and microbial community diversity. Eur. J. Soil Biol. 2016, 76, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Reichel, R.; Michelini, L.; Ghisi, R.; Thiele-Bruhn, S. Soil bacterial community response to sulfadiazine in the soil-root zone. J. Plant Nutr. Soil Sci. 2015, 178, 499–506. [Google Scholar] [CrossRef]
- Versporten, A.; Coenen, S.; Adriaenssens, N.; Muller, A.; Minalu, G.; Faes, C.; Vankerckhoven, V.; Aerts, M.; Hens, N.; Molenberghs, G.; et al. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient cephalosporin use in Europe (1997–2009). J. Antimicrob. Chemother. 2011, 66, 25–35. [Google Scholar] [CrossRef]
- Ishibiki, K.; Inoue, S.; Suzuki, F.; Okumura, K.; Takeda, K.; Toshimitsu, Y. Investigation of adsorption, metabolism and excretion of cefuroxime axetil in volunteers of gastrectomized patients. Jpn. J. Antibiot. 1990, 43, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hu, Y.; Yin, Y.; Yang, X.; Xiang, C.; Wang, B.; Chen, Y.; Yang, F.; Lei, F.; Wu, N.; et al. Functional screening of antibiotic resistance genes from human gut microbiota reveals a novel gene fusion. FEMS Microbiol. Lett. 2012, 336, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, D.; Dey, S.; Kadam, S.; Kalal, S.; Jali, S.; Koley, H.; Sinha, R.; Nag, D.; Kholkute, S.D.; Roy, S. Isolation of NDM-1-producing multidrug-resistant Pseudomonas putida from a paediatric case of acute gastroenteritis, India. New Microbes New Infect. 2015, 5, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Ferech, M.; Dvorakova, K.; Hendrickx, E.; Suetens, C.; Goossens, H. European Surveillance of Antimicrobial Consumption (ESAC): Outpatient cephalosporin use in Europe. J. Antimicrob. Chemother. 2006, 58, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Iatrou, E.I.; Stasinakis, A.S.; Thomaidis, N.S. Consumption-based approach for predicting environmental risk in Greece due to the presence of antimicrobials in domestic wastewater. Environ. Sci. Pollut. Res. 2014, 21, 12941–12950. [Google Scholar] [CrossRef] [PubMed]
- Oǧuz, M.; Mihçiokur, H. Environmental risk assessment of selected pharmaceuticals in Turkey. Environ. Toxicol. Pharmacol. 2014, 38, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; Zuo, J.; Zhang, M.; Chen, L.; Li, Z. Distribution and persistence of cephalosporins in cephalosporin producing wastewater using SPE and UPLC–MS/MS method. Sci. Total Environ. 2016, 569–570, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Material Safety Data Sheet. Available online: http://www.lupinpharmaceuticals.com/pdf/07-08/MSDS%20Cefuroxime%20Axetil%20Tablets.pdf (accessed on 25 September 2018).
- Orlewska, K.; Piotrowska-Seget, Z.; Cycon, M. Use of the PCR-DGGE method for the analysis of the bacterial community structure in soil treated with the cephalosporin antibiotic cefuroxime and/or inoculated with a multidrug-resistant Pseudomonas putida strain MC1. Front. Microbiol. 2018, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Piotrowska-Seget, Z.; Kozdrój, J. Linuron effects on microbiological characteristics of sandy soils as determined in a pot study. Ann. Microbiol. 2010, 60, 439–449. [Google Scholar] [CrossRef]
- Cycoń, M.; Borymski, S.; Orlewska, K.; Wąsik, T.J.; Piotrowska-Seget, Z. An analysis of the effects of vancomycin and/or vancomycin-resistant Citrobacter freundii exposure on the microbial community structure in soil. Front. Microbiol. 2016, 7, 1015. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Zmijowska, A.; Piotrowska-Seget, Z. Biodegradation kinetics of 2,4-D by bacterial strains isolated from soil. Cent. Eur. J. Biol. 2011, 6, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Insam, H. A new set of substrates proposed for community characterization in environmental samples. In Microbial Communities: Functional Versus Structural Approaches; Insam, H., Rangger, A., Eds.; Springer: Berlin, Germany, 1997; pp. 259–260. [Google Scholar]
- Garland, J.L. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 1997, 24, 289–300. [Google Scholar] [CrossRef]
- Orwin, K.H.; Wardle, D.A. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Cycoń, M.; Markowicz, A.; Piotrowska-Seget, Z. Structural and functional diversity of bacterial community in soil treated with the herbicide napropamide estimated by the DGGE, CLPP and r/K-strategy approaches. Appl. Soil Ecol. 2013, 72, 242–250. [Google Scholar] [CrossRef]
- Floch, C.; Chevremont, A.-C.; Joanico, K.; Capowiez, Y.; Criquet, S. Indicators of pesticide contamination: Soil enzyme compared to functional diversity of bacterial communities via Biolog® Ecoplates. Eur. J. Soil Biol. 2011, 47, 256–263. [Google Scholar] [CrossRef]
- Kong, W.-D.; Zhu, Y.-G.; Fu, B.-J.; Marschner, P.; He, J.-Z. The veterinary antibiotic oxytetracycline and Cu influence functional diversity of the soil microbial community. Environ. Pollut. 2006, 143, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toth, J.D.; Feng, Y.; Dou, Z. Veterinary antibiotics at environmentally relevant concentrations inhibit soil iron reduction and nitrification. Soil Biol. Biochem. 2011, 43, 2470–2472. [Google Scholar] [CrossRef]
- Pino-Otín, M.R.; Muñiz, S.; Val, J.; Navarro, E. Effects of 18 pharmaceuticals on the physiological diversity of edaphic microorganisms. Sci. Total Environ. 2017, 595, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Zhang, X.; Wang, J.; Gao, M. Combined effects of chlortetracycline and dissolved organic matter extracted from pig manure on the functional diversity of soil microbial community. Soil Biol. Biochem. 2014, 74, 148–155. [Google Scholar] [CrossRef]
- Fang, H.; Han, L.; Cui, Y.; Xue, Y.; Cai, L.; Yu, Y. Changes in soil microbial community structure and function associated with degradation and resistance of carbendazim and chlortetracycline during repeated treatments. Sci. Total Environ. 2016, 572, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Demoling, L.A.; Bååth, E.; Greve, G.; Wouterse, M.; Schmitt, H. Effects of sulfamethoxazole on soil microbial communities after adding substrate. Soil Biol. Biochem. 2009, 41, 840–848. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Ying, G.-G.; Luo, Z.; Feng, H. Changes in functional diversity of soil microbial community with addition of antibiotics sulfamethoxazole and chlortetracycline. Appl. Microbiol. Biotechnol. 2012, 95, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, H.; Sun, W.; Xia, Y.; Ma, J.; Fu, J.; Zhang, Z.; Wu, H.; Qian, M. Variations in the fate and biological effects of sulfamethoxazole, norfloxacin and doxycycline in different vegetable-soil systems following manure application. J. Hazard. Mater. 2016, 304, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Piotrowska-Seget, Z. Pyrethroid-degrading microorganisms and their potential for the bioremediation of contaminated soils: A review. Front. Microbiol. 2016, 7, 1463. [Google Scholar] [CrossRef] [PubMed]
- Hirth, N.; Topp, E.; Dörfler, U.; Stupperich, E.; Munch, J.C.; Schroll, R. An effective bioremediation approach for enhanced microbial degradation of the veterinary antibiotic sulfamethazine in an agricultural soil. Chem. Biol. Technol. Agric. 2016, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhou, Y.; Huang, Y.; Wu, L.; Liu, X.; Luo, Y. Residues and risks of veterinary antibiotics in protected vegetable soils following application of different manures. Chemosphere 2016, 152, 229–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.C.; Radl, V.; Schloter-Hai, B.; Jechalke, S.; Heuer, H.; Smalla, K.; Schloter, M. Dynamics of soil bacterial communities in response to repeated application of manure containing sulfadiazine. PLoS ONE 2014, 9, e92958. [Google Scholar] [CrossRef] [PubMed]
- Chessa, L.; Pusino, A.; Garau, G.; Mangia, N.P.; Pinna, M.V. Soil microbial response to tetracycline in two different soils amended with cow manure. Environ. Sci. Pollut. Res. 2016, 23, 5807–5817. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Kucharski, J.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with azoxystrobin. Environ. Monit. Assess. 2015, 187, 615. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.-S.; Renslow, R.S.; Fredrickson, J.K.; Lindemann, S.R. Integrating ecological and engineering concepts of resilience in microbial communities. Front. Microbiol. 2015, 6, 1298. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, A.; Amelung, W.; Hollert, H.; Kaestner, M.; Kandeler, E.; Kruse, J.; Miltner, A.; Ottermanns, R.; Pagel, H.; Peth, S.; et al. The impact of chemical pollution on the resilience of soils under multiple stresses: A conceptual framework for future research. Sci. Total Environ. 2016, 568, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, M.; Wilmes, P.; Schrader, S. Measuring soil sustainability via soil resilience. Sci. Total Environ. 2018, 626, 1484–1493. [Google Scholar] [CrossRef] [PubMed]




| SV | AWCD | R | H | E | Amines | |||||
| VE | p | VE | p | VE | p | VE | p | VE | p | |
| S | <1 | 0.004 ** | <1 | 0.403 | 2 | <0.001 *** | 3 | <0.001 *** | 1 | <0.001 *** |
| C | 11 | <0.001 *** | 2 | <0.001 *** | 5 | <0.001 *** | 8 | <0.001 *** | 6 | <0.001 *** |
| T | 31 | <0.001 *** | 38 | <0.001 *** | 40 | <0.001 *** | 25 | <0.001 *** | 41 | <0.001 *** |
| S × C | <1 | 0.019 * | <1 | 0.586 | <1 | 0.063 | <1 | 0.210 | <1 | <0.001 *** |
| S × T | <1 | <0.001 *** | 3 | <0.001 *** | 1 | 0.001 ** | 4 | 0.005 ** | 1 | <0.001 *** |
| C × T | 55 | <0.001 *** | 47 | <0.001 *** | 46 | <0.001 *** | 36 | <0.001 *** | 46 | <0.001 *** |
| S × C × T | <1 | 0.866 | 2 | 0.029 * | 2 | 0.002 ** | 9 | <0.001 | 2 | <0.001 *** |
| SV | Amino acids | Carbohydrates | Carboxylic acids | Miscellaneous | Polymers | |||||
| VE | p | VE | p | VE | p | VE | p | VE | p | |
| S | <1 | 0.084 | <1 | <0.001 *** | <1 | <0.001 *** | <1 | 0.002 ** | <1 | 0.013 * |
| C | 8 | <0.001 *** | 9 | <0.001 *** | 6 | <0.001 *** | 18 | <0.001 *** | 11 | <0.001 *** |
| T | 41 | <0.001 *** | 53 | <0.001 *** | 44 | <0.001 *** | 9 | <0.001 *** | 26 | <0.001 *** |
| S × C | <1 | <0.001 *** | <1 | 0.077 | <1 | 0.025 * | <1 | <0.001 *** | <1 | 0.001 ** |
| S × T | 2 | <0.001 *** | 1 | <0.001 *** | <1 | <0.001 *** | <1 | 0.080 | 1 | <0.001 *** |
| C × T | 47 | <0.001 *** | 36 | <0.001 *** | 47 | <0.001 *** | 72 | <0.001 *** | 58 | <0.001 *** |
| S × C × T | <1 | <0.001 *** | <1 | 0.005 ** | 1 | <0.001 *** | <1 | <0.001 *** | 1 | 0.002 ** |
| SV | CLPP Indices | Carbon Substrate Groups | ||||||
|---|---|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | |||||
| VE | p | VE | p | VE | p | VE | p | |
| S | 1 | <0.001 *** | 1 | 0.072 | <1 | 0.003 ** | <1 | <0.001 *** |
| C | 8 | <0.001 *** | 4 | 0.005 ** | 11 | <0.001 *** | 2 | <0.001 *** |
| T | 35 | <0.001 *** | 25 | <0.001 *** | 34 | <0.001 *** | 46 | <0.001 *** |
| S × C | <1 | 0.267 | 1 | 0.456 | <1 | 0.045 * | 1 | <0.001 *** |
| S × T | 1 | 0.003 ** | 7 | 0.001 ** | 1 | <0.001 *** | 4 | <0.001 *** |
| C × T | 50 | <0.001 *** | 32 | <0.001 *** | 54 | <0.001 *** | 41 | <0.001 *** |
| S × C × T | 1 | 0.005 ** | 10 | 0.002 ** | <1 | 0.003 ** | 4 | <0.001 *** |
| Day | SV | CLPP Indices | Carbon Substrate Groups | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC 1 | PC 2 | PC 1 | PC 2 | ||||||
| VE | p | VE | p | VE | p | VE | p | ||
| 1 | S | 8 | <0.001 *** | <1 | 0.758 | 2 | 0.032 * | <1 | 0.942 |
| C | 86 | <0.001 *** | 10 | 0.379 | 94 | <0.001 *** | 45 | 0.002 ** | |
| S × C | 3 | 0.014 * | 35 | 0.051 | <1 | 0.955 | 31 | 0.007 ** | |
| 15 | S | 5 | 0.009 ** | <1 | 0.849 | 3 | <0.001 *** | 40 | <0.001 *** |
| C | 86 | <0.001 *** | 4 | 0.725 | 96 | <0.001 *** | 33 | <0.001 *** | |
| S × C | 4 | 0.057 | 26 | 0.154 | <1 | 0.079 | 22 | <0.001 *** | |
| 30 | S | 11 | <0.001 *** | 27 | 0.007 ** | <1 | 0.500 | 7 | 0.033 * |
| C | 81 | <0.001 *** | 39 | 0.007 ** | 98 | <0.001 *** | 33 | <0.001 *** | |
| S × C | 3 | 0.066 | 3 | 0.569 | 1 | 0.194 | 47 | <0.001 *** | |
| 60 | S | <1 | 0.592 | 4 | 0.201 | 1 | 0.438 | 13 | 0.027 * |
| C | 92 | <0.001 *** | <1 | 0.959 | 85 | <0.001 *** | 47 | 0.002 ** | |
| S × C | <1 | 0.907 | 66 | <0.001 *** | 3 | 0.214 | 16 | 0.051 | |
| 90 | S | <1 | 0.332 | 9 | 0.114 | <1 | 0.514 | 59 | <0.001 *** |
| C | 93 | <0.001 *** | 46 | 0.009 ** | 99 | <0.001 *** | 1 | 0.210 | |
| S × C | 1 | 0.334 | 6 | 0.435 | <1 | 0.780 | 36 | <0.001 *** | |
| Parameter | Day | Treatment | |||||
|---|---|---|---|---|---|---|---|
| XM1 | XM10 | Ps | XM1+Ps | XM10+Ps | |||
| AWCD | 1 | 0.374 Bc | 0.196 Ad | 0.731 Ba | 0.571 Bb | 0.229 Bd | 0.420 A |
| 15 | 0.085 Dc | 0.045 Bc | 0.759 Ba | 0.184 Cb | 0.100 Cc | 0.235 B | |
| 30 | 0.161 Cb | 0.086 Bbc | 0.969 Aa | 0.146 Cb | 0.080 Cc | 0.288 B | |
| 60 | 0.927 Aa | 0.244 Ad | 0.956 Aa | 0.842 Ab | 0.427 Ac | 0.679 A | |
| 90 | 0.924 Aab | −0.252 Cc | 0.966 Aa | 0.899 Ab | −0.221 Dc | 0.463 A | |
| Substrate richness (R) | 1 | 0.721 Db | 0.402 Cd | 0.816 BCa | 0.743 Db | 0.564 Bc | 0.649 C |
| 15 | 0.745C Db | 0.626 Bc | 0.862 Ba | 0.919 ABa | 0.626 Bc | 0.755 ABD | |
| 30 | 0.952 Aa | 0.783 Ac | 0.868 Bb | 0.809 Cbc | 0.932 Aa | 0.869 A | |
| 60 | 0.844 Bb | 0.686 Bc | 0.787 Cb | 0.966 Aa | 0.573 Bd | 0.771 AB | |
| 90 | 0.795 BCc | 0.432 Cd | 0.958 Aa | 0.893 Bb | 0.394 Cd | 0.694 CB | |
| Shannon-Wiener index (H) | 1 | 0.838 Cb | 0.711 Ae | 0.796 Cc | 0.925 Ba | 0.752 Ad | 0.804 A |
| 15 | 0.804 Cc | 0.691 ABd | 0.955 ABa | 0.868 Cb | 0.801 Ac | 0.824 A | |
| 30 | 0.822 Cbc | 0.662 Bd | 0.960 Aa | 0.848 Cb | 0.786 Ac | 0.816 A | |
| 60 | 0.925 Bab | 0.723 Ac | 0.978 Aa | 0.979 Aa | 0.697 Bc | 0.860 A | |
| 90 | 0.964 Aa | 0.689 ABc | 0.918 Bb | 0.991 Aa | 0.656 Cc | 0.844 A | |
| Evenness (E) | 1 | 0.936 Aa | 0.920 Aa | 0.564 Bb | 0.955 Aa | 0.922 Aa | 0.859 A |
| 15 | 0.897 Aa | 0.823 ABa | 0.934 Aa | 0.895 Aa | 0.961 Aa | 0.902 A | |
| 30 | 0.834 Aab | 0.623 BCb | 0.911 Aa | 0.912 Aa | 0.804 Aab | 0.817 A | |
| 60 | 0.909 Aa | 0.551 Cb | 0.937 Aa | 0.981 Aa | 0.834 Aa | 0.842 A | |
| 90 | 0.963 Aa | 0.886 Aa | 0.920 Aa | 0.964 Aa | 0.866 Aa | 0.920 A | |
| AWCD—amines | 1 | 0.414 Ba | 0.323 Ab | −0.167 Cc | 0.335 Dab | 0.351 Bab | 0.251 B |
| 15 | 0.137 Cc | 0.051 Ccd | 0.660 Ba | 0.532 Cb | 0.047 Cd | 0.285 B | |
| 30 | 0.264 Cb | 0.147 Bc | 0.927 Aa | 0.353 Db | 0.108 Cc | 0.360 B | |
| 60 | 0.789 Ab | 0.270 Ae | 0.961 Aa | 0.668 Bc | 0.461 Ad | 0.630 A | |
| 90 | 0.774 Ab | −0.113 Dc | 0.952 Aa | 0.863 Aab | −0.166 Dc | 0.462 AB | |
| AWCD—amino acids | 1 | 0.582 Bb | 0.519 Ab | 0.892 Ba | 0.521 Bb | 0.382 Bc | 0.579 AB |
| 15 | 0.079 Cd | 0.024 Cd | 0.673 Ca | 0.354 Cb | 0.153 Cc | 0.257 CD | |
| 30 | 0.024 Cb | 0.020 Cb | 0.597 Da | 0.020 Db | 0.014 Db | 0.135 D | |
| 60 | 0.879 Aa | 0.350 Bb | 0.968 Aa | 0.942 Aa | 0.939 Aa | 0.816 A | |
| 90 | 0.868 Aa | −0.311 Db | 0.953 ABa | 0.914 Aa | −0.303 Eb | 0.424 BC | |
| AWCD—carbohydrates | 1 | 0.514 Bab | 0.126 Bd | 0.611 Ca | 0.477 Bb | 0.299 Bc | 0.406 BC |
| 15 | 0.099 Cc | 0.030 Bc | 0.755 Ba | 0.272 Cb | 0.246 Bb | 0.280 BC | |
| 30 | 0.067 Cb | 0.035 Bb | 0.818 Ba | 0.039 Db | 0.015 Cb | 0.195 C | |
| 60 | 0.757 Ab | 0.963 Aa | 0.655 Cb | 0.920 Aa | 0.980 Aa | 0.855 A | |
| 90 | 0.762 Ab | −0.115 Cc | 0.932 Aa | 0.933 Aa | −0.166 Dc | 0.469 B | |
| AWCD—carboxylic acids | 1 | 0.748 Bb | 0.057 Abc | 0.947 Aa | 0.709 Bb | 0.119 Ac | 0.516 A |
| 15 | 0.086 Cb | 0.028 Bb | 0.728 Ba | 0.042 Db | 0.018 BCb | 0.180 B | |
| 30 | 0.165 Cb | 0.130 Abc | 0.747 Ba | 0.094 Dbc | 0.069 Abc | 0.241 AB | |
| 60 | 0.733 Ba | −0.396 Dc | 0.500 Cb | 0.575 Cb | −0.653 Dd | 0.152 B | |
| 90 | 0.891 Aa | −0.067 Cb | 0.950 Aa | 0.952 Aa | −0.040 Cb | 0.537 A | |
| AWCD—miscellaneous | 1 | 0.339 Ba bc | 0.160 Abc | 0.436 Ca | 0.415 BC ab | 0.212 AB bc | 0.312 AB |
| 15 | 0.057 Cb | 0.045 Bb | 0.819 Aa | 0.037 Db | 0.031 Bb | 0.198 B | |
| 30 | 0.541 Ba | 0.203 ABb | 0.715 ABa | 0.329 Cb | 0.266 Ab | 0.411 AB | |
| 60 | 0.773 Aab | 0.255 Ac | 0.606 BCb | 0.884 Aa | 0.244 Ac | 0.552 A | |
| 90 | 0.964 Aa | −0.396 Cc | 0.851 Aa | 0.576 Bb | −0.362 Cc | 0.326 AB | |
| AWCD—polymers | 1 | 0.346 Db | 0.208 Bc | 0.959 Aa | 0.254 Dc | 0.233 Bc | 0.400 B |
| 15 | 0.121 Ec | 0.105 Cc | 0.670 Ca | 0.226 Db | 0.068 Cc | 0.238 B | |
| 30 | 0.576 Cb | 0.213 Be | 0.648 Ca | 0.347 Ccd | 0.280 Bde | 0.413 B | |
| 60 | 0.842 Aa | 0.337 Ad | 0.774 Bab | 0.731 Bb | 0.609 Ac | 0.659 A | |
| 90 | 0.748 Bb | −0.367 Dd | 0.939 Ba | 0.901 Aa | −0.210 Dc | 0.402 B | |
| SV/Parameter | AWCD | R | H | E | Amines | |||||
| VE | p | VE | p | VE | p | VE | p | VE | p | |
| Tr | 58 | <0.01 ** | 50 | <0.01 ** | 73 | <0.001 *** | 13 | 0.019 * | 37 | <0.01 ** |
| T | 16 | <0.01 ** | 19 | <0.01 ** | 3 | <0.001 *** | 5 | 0.306 | 15 | <0.01 ** |
| Tr × T | 26 | <0.01 ** | 28 | <0.01 ** | 21 | <0.001 *** | 30 | 0.060 | 46 | <0.01 ** |
| SV/Parameter | Amino acids | Carbohydrates | Carboxylic acids | Miscellaneous | Polymers | |||||
| VE | p | VE | p | VE | p | VE | p | VE | p | |
| Tr | 36 | <0.01 ** | 26 | <0.01 ** | 60 | <0.01 ** | 47 | <0.001 *** | 51 | <0.01 ** |
| T | 34 | <0.01 ** | 35 | <0.01 ** | 14 | <0.01 ** | 10 | <0.001 *** | 15 | <0.01 ** |
| Tr × T | 30 | <0.01 ** | 37 | <0.01 ** | 24 | <0.01 ** | 36 | <0.001 *** | 33 | <0.01 ** |
| Parameter | Treatment | |||||
|---|---|---|---|---|---|---|
| XM1 | XM10 | Ps | XM1 + Ps | XM10 + Ps | ||
| AWCD | 0.750 a | −0.206 b | 0.321 ab | 0.690 a | −0.226 b | 0.266 |
| Substrate richness (RS) | 0.302b c | 0.160 cd | 0.711 a | 0.538 ab | −0.095 d | 0.323 |
| Shannon−Wiener index (H) | 0.678 a | −0.011 b | 0.478 c | 0.793 a | −0.158 b | 0.356 |
| Evenness (E) | 0.344 ab | −0.246 b | 0.476 a | 0.053 ab | −0.249 b | 0.076 |
| AWCD—amines | 0.242 c | −0.660 c | 0.938 a | 0.537 b | −0.705 c | 0.070 |
| AWCD—amino acids | −0.278 a | −0.959 b | −0.354 ab | 0.078 a | −0.945 b | −0.491 |
| AWCD—carbohydrates | −0.189 b | −0.693 c | 0.283 ab | 0.456 a | −0.797 c | −0.188 |
| AWCD—carboxylic acids | 0.115 b | −0.442 c | −0.354 c | 0.521 a | −0.471 c | −0.126 |
| AWCD—miscellaneous | 0.960 a | −0.247 d | 0.699 b | 0.495 c | −0.260 d | 0.329 |
| AWCD—polymers | 0.682 a | −0.354 b | 0.021 b | 0.898 a | −0.222 b | 0.205 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlewska, K.; Markowicz, A.; Piotrowska-Seget, Z.; Smoleń-Dzirba, J.; Cycoń, M. Functional Diversity of Soil Microbial Communities in Response to the Application of Cefuroxime and/or Antibiotic-Resistant Pseudomonas putida Strain MC1. Sustainability 2018, 10, 3549. https://doi.org/10.3390/su10103549
Orlewska K, Markowicz A, Piotrowska-Seget Z, Smoleń-Dzirba J, Cycoń M. Functional Diversity of Soil Microbial Communities in Response to the Application of Cefuroxime and/or Antibiotic-Resistant Pseudomonas putida Strain MC1. Sustainability. 2018; 10(10):3549. https://doi.org/10.3390/su10103549
Chicago/Turabian StyleOrlewska, Kamila, Anna Markowicz, Zofia Piotrowska-Seget, Joanna Smoleń-Dzirba, and Mariusz Cycoń. 2018. "Functional Diversity of Soil Microbial Communities in Response to the Application of Cefuroxime and/or Antibiotic-Resistant Pseudomonas putida Strain MC1" Sustainability 10, no. 10: 3549. https://doi.org/10.3390/su10103549
APA StyleOrlewska, K., Markowicz, A., Piotrowska-Seget, Z., Smoleń-Dzirba, J., & Cycoń, M. (2018). Functional Diversity of Soil Microbial Communities in Response to the Application of Cefuroxime and/or Antibiotic-Resistant Pseudomonas putida Strain MC1. Sustainability, 10(10), 3549. https://doi.org/10.3390/su10103549







