Non-Competitive and Competitive Adsorption of Pb2+, Cd2+ and Zn2+ Ions onto SDS in Process of Micellar-Enhanced Ultrafiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membrane
2.3. Experimental Setup and Analytical Instrument
2.4. Procedure
2.4.1. Unitary System Sorption
2.4.2. Binary System Sorption
2.5. Calculations
3. Results and Discussion
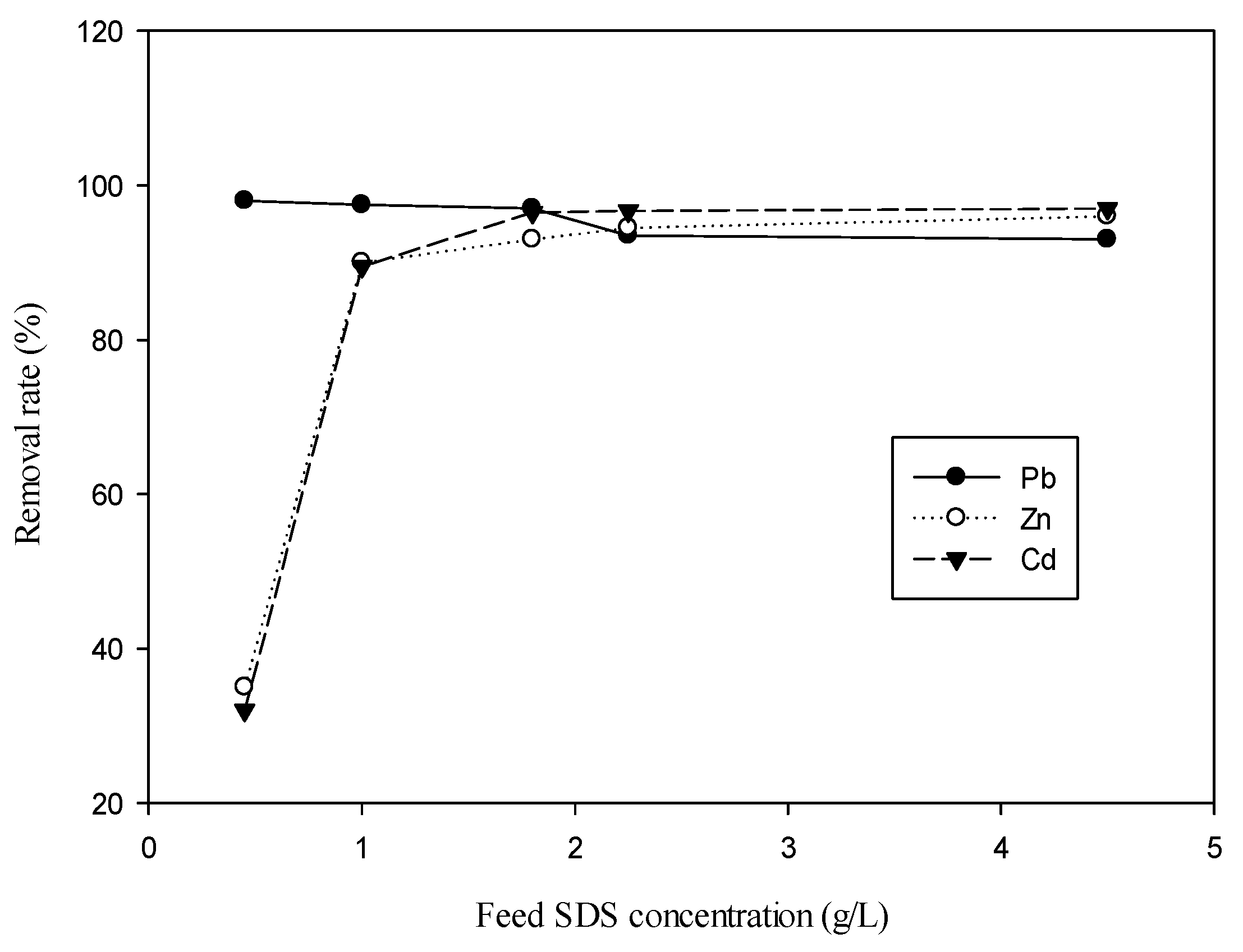
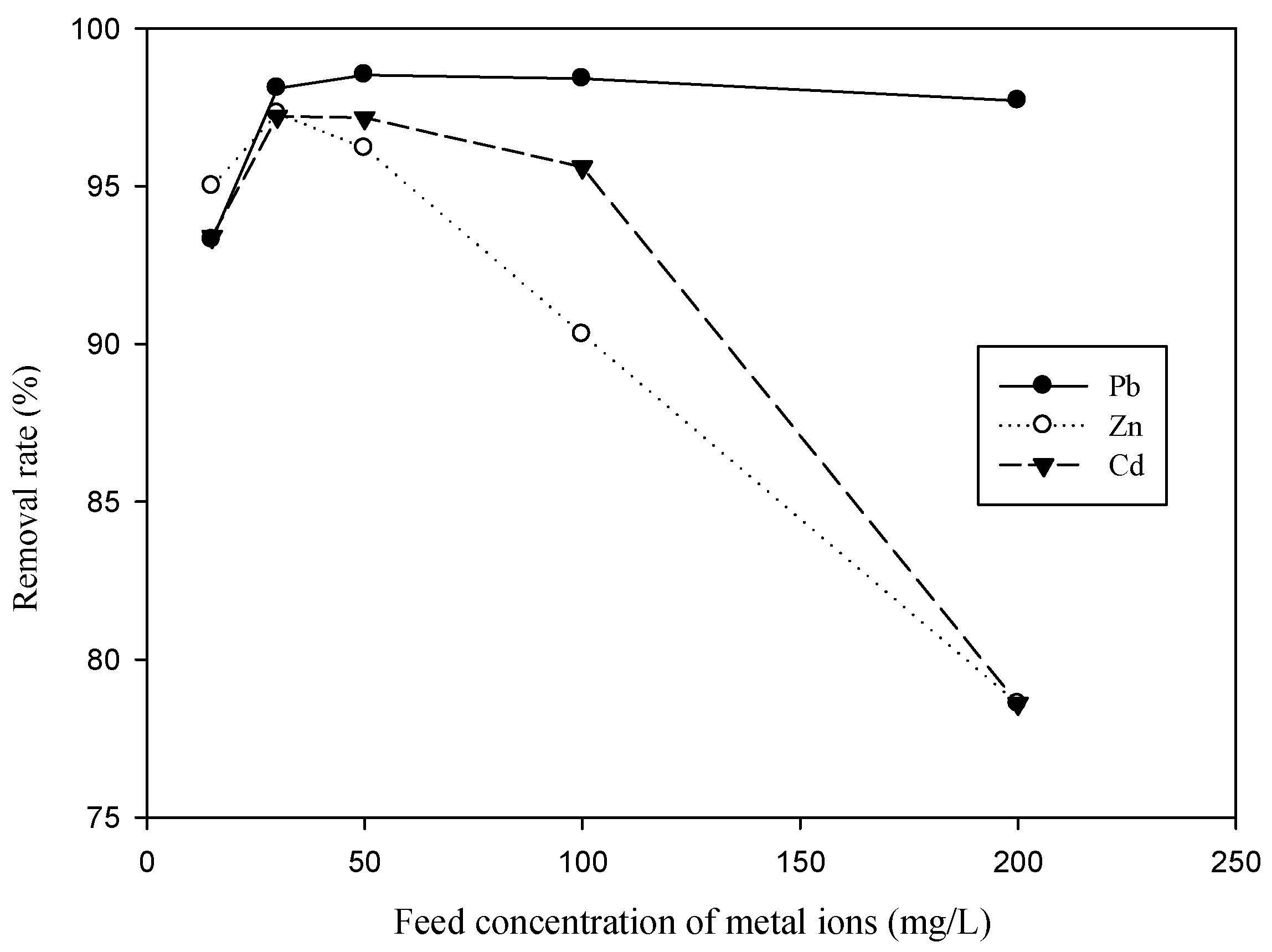
3.1. Effect of Feed SDS Concentration and Feed Concentration of Metal Ion on Metal Removal for Single-Ion Situation
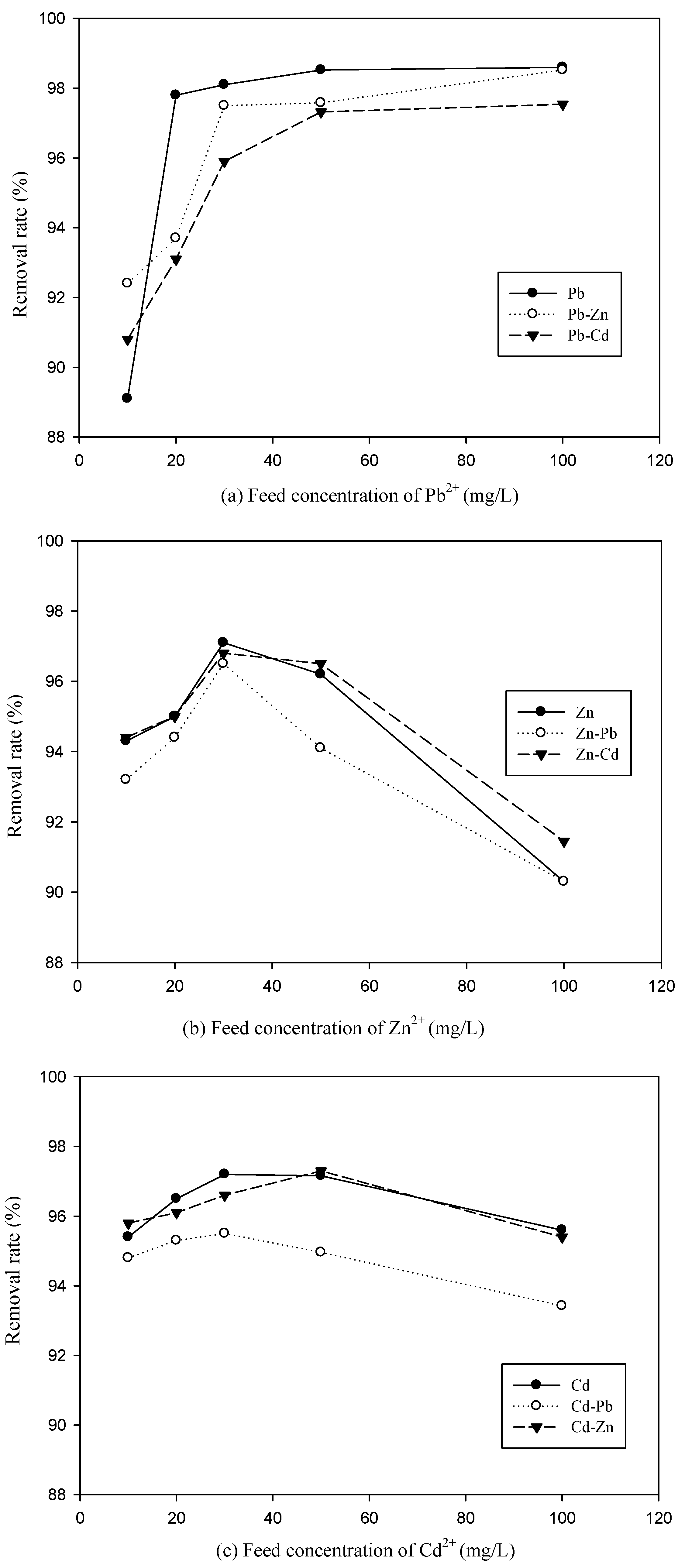
3.2. Effect of Feed Concentration of Metal Ions on Metal Removal Efficiency in Binary System
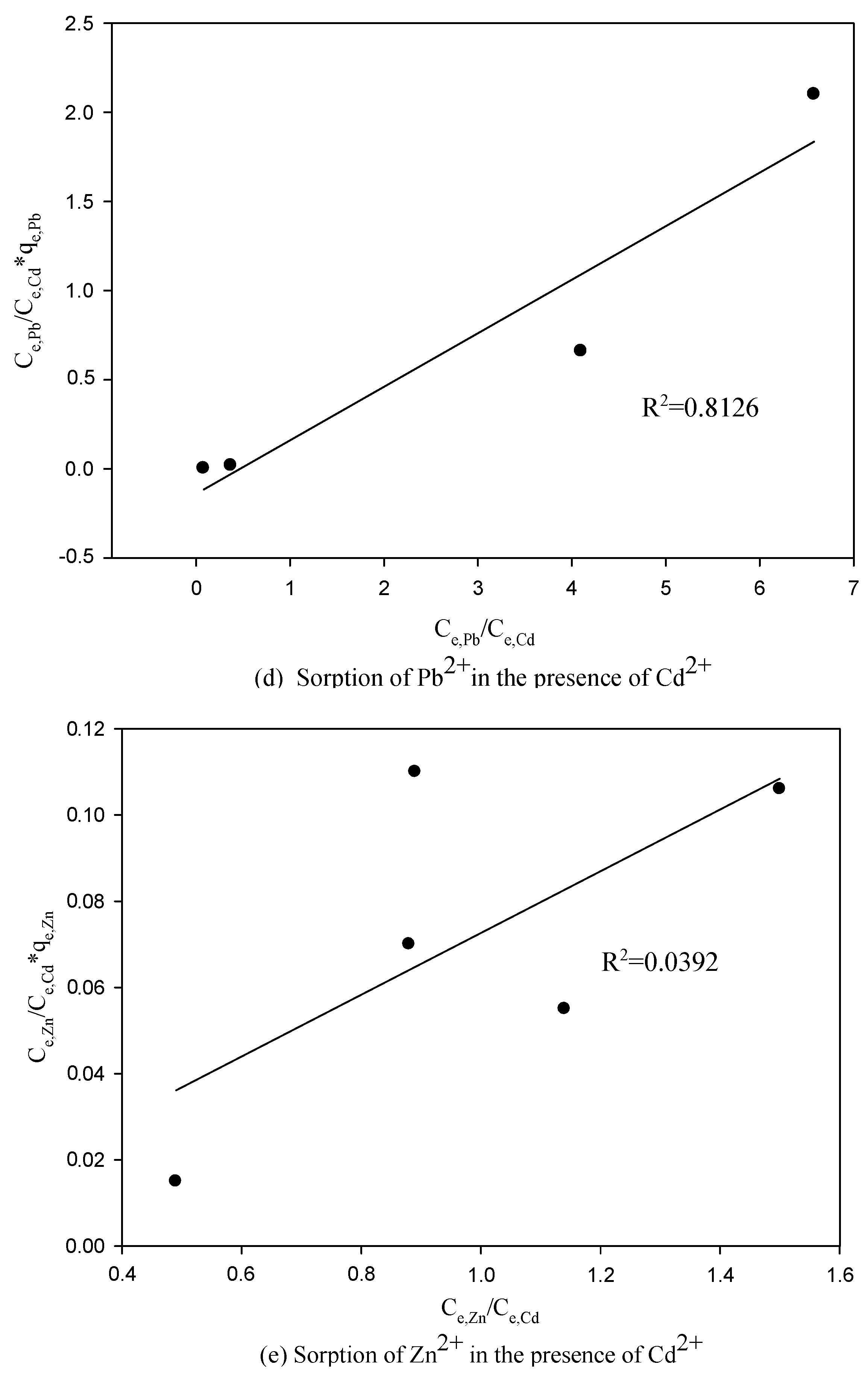
3.3. The Competitive Effects of Ions from Binary System Sorption
3.4. Application of the LCM to Binary System
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, J.H.; Liu, W.C.; Zeng, G.M.; Li, F.; Huang, X.L.; Gu, Y.L.; Wan, J. An exploration of spatial human health risk assessment of soil toxic metals under different land uses using sequential indicator simulation. Ecotoxicol. Environ. Saf. 2016, 129, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Li, F.; Zeng, G.M.; Liu, W.C.; Huang, X.L.; Xiao, Z.H.; He, Y. Integrating hierarchical bioavailability and population distribution into potential eco-risk assessment of heavy metals in road dust: A case study in Xiandao District, Changsha city, China. Sci. Total Environ. 2015, 541, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zeng, G.M.; Huang, J.H.; Zhang, D.M.; Shi, L.J.; He, S.B.; Ruan, M. Simultaneous removal of cadmium ions and phenol with MEUF using SDS and mixed surfactants. Desalination 2011, 276, 136–141. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.D.; Jiang, W.; Liu, C.Y.; Zhang, Z.M.; Zhang, C.D.; Zeng, G. Spatial health risk assessment and hierarchical risk management for mercury in soils from a typical contaminated site, China. Environ. Geochem. Health 2017, 39, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wang, M.M.; Zeng, G.M.; Liu, Y.G.; Huang, D.L.; Zhang, C.; Wu, H.P. Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar] [CrossRef]
- Hasan, S.; Talat, M.; Rai, S. Sorption of cadmium and zinc from aqueous solutions by water hyacinth (Eichchornia crassipes). Bioresour. Technol. 2007, 98, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Tharani, A.; Harish, R.; Mageshkumar, P.; Ramesh, S. Comparative study on removal of heavy metals from textile industry wastewater using various adsorbent. Int. J. Adv. Sci. Eng. Res. 2017, 2, 12–22. [Google Scholar]
- Fagundesklen, M.R.; Vaz, L.G.L.; Veit, M.T.; Borba, C.E.; Silva, E.A.; Kroumov, A.D. Biosorption of the copper and cadmium ions—A study through adsorption isotherms analysis. Int. J. Bioautom. 2007, 7, 23–33. [Google Scholar]
- Mehrizad, A.; Gharbani, P. Study of 1-chloro-4-nitrobenzene adsorption on carbon nanofibers by experimental design. Int. J. Nano Dimens. 2016, 7, 77–84. [Google Scholar]
- Li, X.; Zeng, G.M.; Huang, J.H.; Zhang, C.; Fang, Y.Y.; Qu, Y.H.; Liu, H.L. Recovery and reuse of surfactant SDS from a MEUF retentate containing Cd2+ or Zn2+ by ultrafiltration. J. Membr. Sci. 2009, 337, 92–97. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W. Modeling the Effect of pH on Kinetics of Heavy Metal Ion Biosorption. A Theoretical Approach Based on the Statistical Rate Theory. Langmuir 2009, 25, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Channarong, B.; Seunghwan, L.; Bade, R.; Shipin, O.V. Simultaneous removal of nickel and zinc from aqueous solution by micellar-enhanced ultrafiltration and activated carbon fiber hybrid process. Desalination 2010, 262, 221–227. [Google Scholar] [CrossRef]
- Kedari, C.S.; Pandit, S.S.; Tripathi, S.C. Extraction of Am(III) from aqueous nitrate solutions into micellar pseudo phase of anionic or non-ionic surfactant and separation by ultrafiltration. J. Membr. Sci. 2009, 341, 122–130. [Google Scholar] [CrossRef]
- Huang, J.H.; Yuan, F.; Zeng, G.M.; Li, X.; Gu, Y.L.; Shi, L.X.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.M.; Li, X.; Huang, J.H.; Zhang, C.; Zhou, C.F.; Niu, J.; Li, F. Micellar-enhanced ultrafiltration of cadmium and methylene blue in synthetic wastewater using SDS. J. Hazard. Mater. 2011, 185, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Yang, J.W. Removal of ferriccyanide using micellar enhanced ultrafiltration (MEUF). Dev. Chem. Eng. Miner. Process 2005, 13, 137–146. [Google Scholar] [CrossRef]
- Yenphan, P.; Chanachai, A.; Jiraratananon, R. Experimental study on micellar-enhanced ultrafiltration (MEUF) of aqueous solution and wastewater containing lead ion with mixed surfactants. Desalination 2010, 253, 30–37. [Google Scholar] [CrossRef]
- Akita, S.; Yang, L.; Takeuchi, H. Micellar-enhanced ultrafiltration of gold(III) with nonionic surfactant. J. Membr. Sci. 1997, 133, 189–194. [Google Scholar] [CrossRef]
- Rujirawanich, V.; Chavadej, S.; O’Haver, J.H.; Rujiravanit, R. Removal of trace Cd2+ using continuous multistage ion foam fractionation: Part I-The effect of feed SDS/Cd molar ratio. J. Hazard. Mater. 2010, 182, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Zhao, Y.; Zeng, G.M.; Peng, L.; Li, X.; Liu, L.X.; Yuan, F. Micellar-enhanced ultrafiltration for the solubilization of various phenolic compounds with different surfactants. Water Sci. Technol. 2015, 72, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Shi, L.J.; Zeng, G.M.; Li, X.; He, S.B.; Li, F.; Xie, G.X. Effects of feed concentration and transmembrane pressure on membrane fouling in Cd2+ removal by micellar-enhanced ultrafiltration. Desalination 2012, 294, 67–73. [Google Scholar] [CrossRef]
- Li, F.; Qiu, Z.; Zhang, J.; Liu, W.; Liu, C.; Zeng, G.M. Investigation, Pollution Mapping and Simulative Leakage Health Risk Assessment for Heavy Metals and Metalloids in Groundwater from a Typical Brownfield, Middle China. Int. J. Environ. Res. Public Health 2017, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Yang, J.W. Simultaneous removal of chlorinated aromatic hydrocarbons, nitrate, and chromate using micellar-enhanced ultrafiltration. Chemosphere 2004, 57, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.C.; Yang, Y.M.; Chang, C.H.; Maa, J.R. Removal of copper ions and dissolved phenol from water using micellar-enhanced ultrafiltration with mixed surfactants. Waste Manag. 2002, 22, 695–701. [Google Scholar] [CrossRef]
- Witek, A.; Koltuniewicz, A.; Kurczewski, B.; Radziejowska, M.; Hatalski, M. Simultaneous removal of phenols and Cr3+ using micellar-enhanced ultrafiltration process. Desalination 2006, 191, 111–116. [Google Scholar] [CrossRef]
- Misra, S.K.; Mahatele, A.K.; Tripathi, S.C.; Dakshinamoorthy, A. Studies on the simultaneous removal of dissolved DBP and TBP as well as uranyl ions from aqueous solutions by using Micellar-Enhanced Ultrafiltration Technique. Hydrometallurgy 2009, 96, 47–51. [Google Scholar] [CrossRef]
- Huang, C.P.; Smith, E.H. Removal of Cd(II) from plating waste water by an activated carbon process. In Chemistry in Water Reuse; Cooper, W.J., Ed.; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1981; Volume 2, pp. 355–398. [Google Scholar]
- Huang, J.H.; Zeng, G.M.; Zhou, C.F.; Li, X.; Shi, L.J.; He, S.B. Adsorption of surfactant micelles and Cd2+/Zn2+ in micellar-enhanced ultrafiltration. J. Hazard. Mater. 2010, 183, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Mahamadi, C.; Nharingo, T. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresour. Technol. 2010, 101, 859–864. [Google Scholar] [CrossRef] [PubMed]
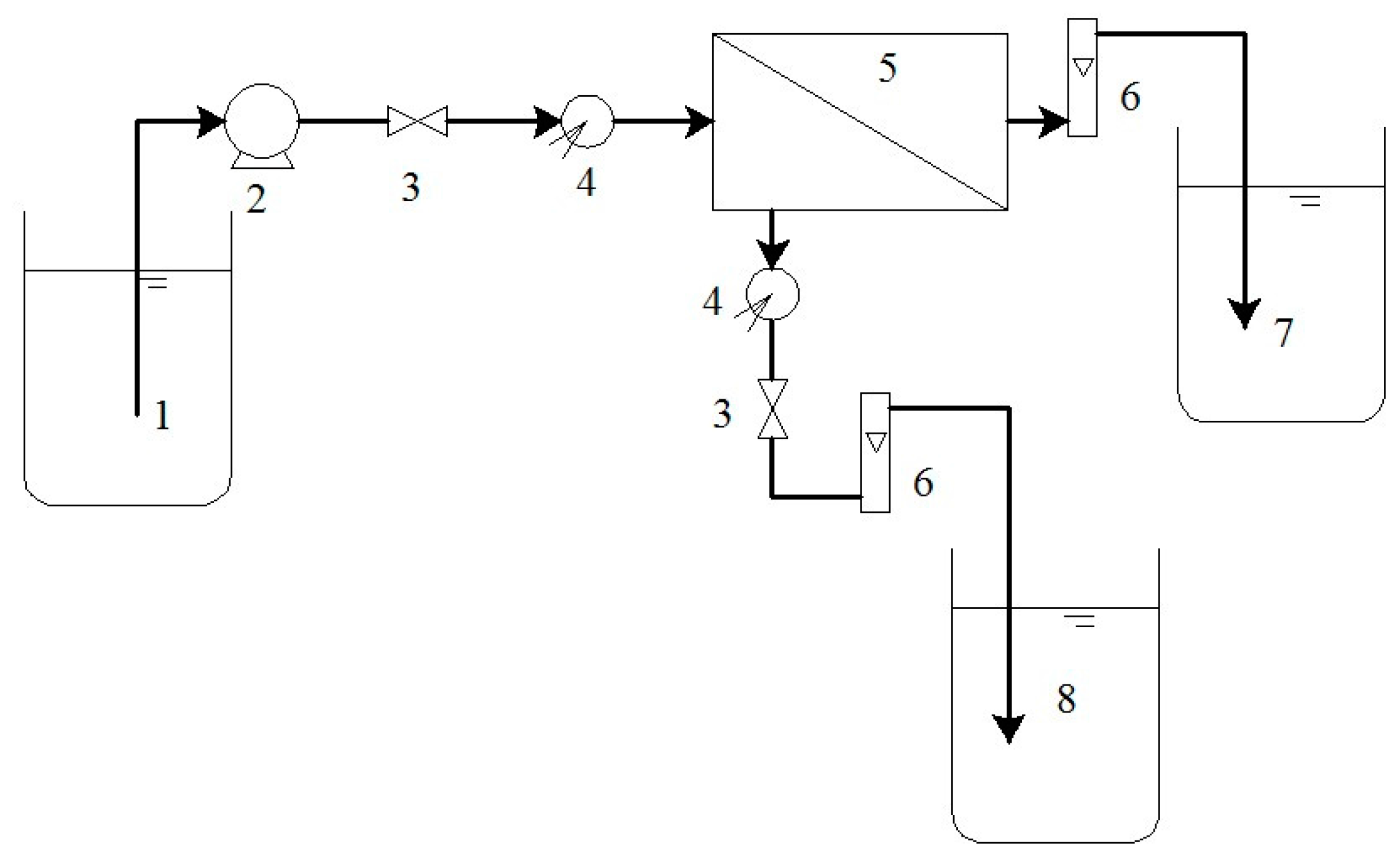

| Type | Membrane Material | MWCO (Da) | Contour Size φ × L (mm) | Effective Membrane Area (m2) | Transmembrane Pressure (MPa) | Operating Temperature (°C) | pH Operating Range |
|---|---|---|---|---|---|---|---|
| PLCGC | cellulose | 10 k | 30 × 188 | 0.005 | <0.65 | 4–25 | 2–14 |
| System | Metal Ion (+Interferent) | qe,exp (mg/g) | Qmax (mg/g) | qe/qm | KL,2/KL,1 | R2 |
|---|---|---|---|---|---|---|
| (a) Pb as primary metal ion | Langmuir model | |||||
| Unitary | Pb | 49.37 | - | 1 | ||
| Binary | Pb-Zn | 29.86 | - | 0.6 | 6.41 | |
| Pb-Cd | 29.94 | - | 0.61 | |||
| (b) Cd as primary metal ion | Langmuir model | |||||
| Unitary | Cd | 47.27 | 151.51 | 1 | 0.99 | |
| LCM | ||||||
| Binary | Cd-Pb | 29.6 | 35.84 | 0.63 | 3.02 | 1 |
| Cd-Zn | 33.47 | - | 0.71 | |||
| (c) Zn as primary metal ion | Langmuir model | |||||
| Unitary | Zn | 44.18 | 136.98 | 1 | 0.98 | |
| LCM | ||||||
| Binary | Zn-Pb | 29.45 | 58.41 | 0.67 | 0.95 | |
| Zn-Cd | 32.58 | - | 0.73 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; He, S.; Feng, C.; Zhu, Y.; Pang, Y.; Hou, J.; Luo, K.; Liao, X. Non-Competitive and Competitive Adsorption of Pb2+, Cd2+ and Zn2+ Ions onto SDS in Process of Micellar-Enhanced Ultrafiltration. Sustainability 2018, 10, 92. https://doi.org/10.3390/su10010092
Li X, He S, Feng C, Zhu Y, Pang Y, Hou J, Luo K, Liao X. Non-Competitive and Competitive Adsorption of Pb2+, Cd2+ and Zn2+ Ions onto SDS in Process of Micellar-Enhanced Ultrafiltration. Sustainability. 2018; 10(1):92. https://doi.org/10.3390/su10010092
Chicago/Turabian StyleLi, Xue, Songbao He, Chongling Feng, Yanke Zhu, Ya Pang, Juan Hou, Kun Luo, and Xingsheng Liao. 2018. "Non-Competitive and Competitive Adsorption of Pb2+, Cd2+ and Zn2+ Ions onto SDS in Process of Micellar-Enhanced Ultrafiltration" Sustainability 10, no. 1: 92. https://doi.org/10.3390/su10010092
APA StyleLi, X., He, S., Feng, C., Zhu, Y., Pang, Y., Hou, J., Luo, K., & Liao, X. (2018). Non-Competitive and Competitive Adsorption of Pb2+, Cd2+ and Zn2+ Ions onto SDS in Process of Micellar-Enhanced Ultrafiltration. Sustainability, 10(1), 92. https://doi.org/10.3390/su10010092





