The Temperature Influence on Drying Kinetics and Physico-Chemical Properties of Pomegranate Peels and Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Processing
2.2. Drying
2.2.1. Drying Kinetic Adjustment Models
2.2.2. Effective Diffusivity
2.3. Thermodynamic Properties
2.4. Obtaining Flour
Proximal Composition and Physicochemical Characterization of Pomegranate Peels and Seeds Fresh and Flour
3. Results and Discussion
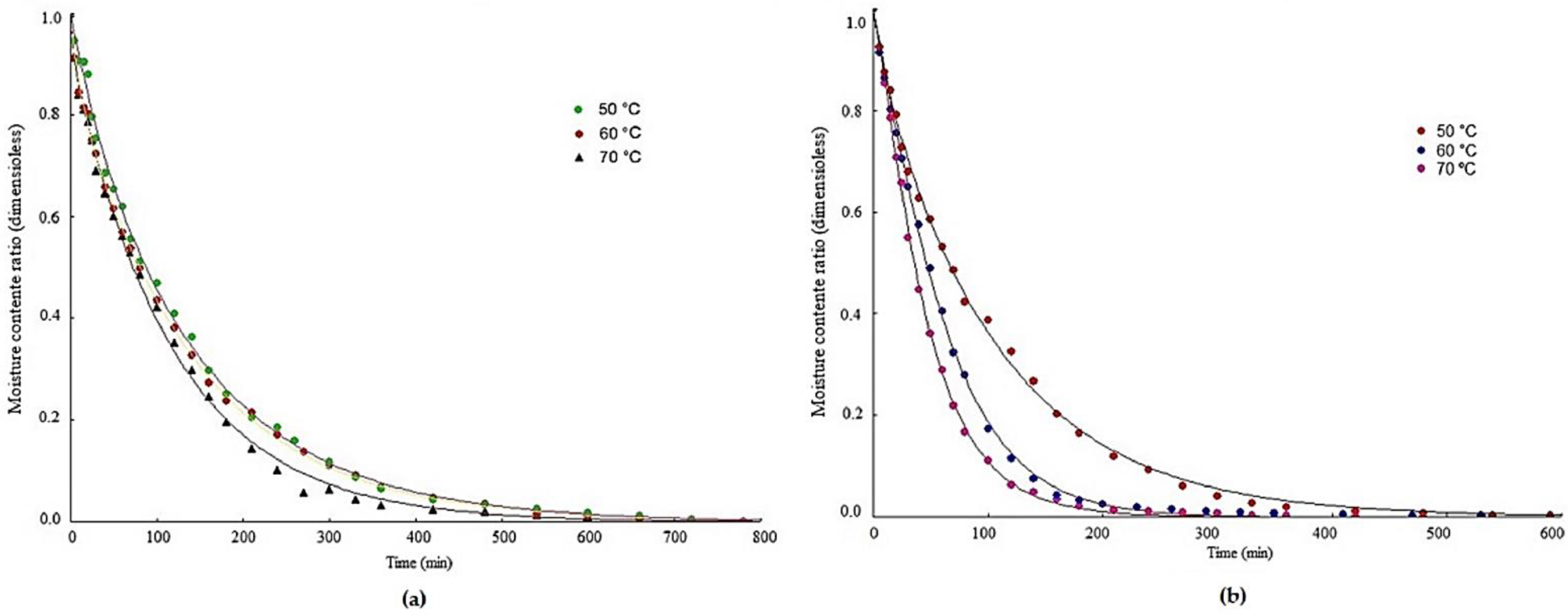
3.1. Drying Kinetics
3.2. Effective Diffusivity
3.3. Thermodynamic Properties
3.4. Proximal Composition and Physicochemical Parameters of Fresh Pomegranate Peels and Seeds and in Their Flours
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, S.S.; Ma, D.X.; Zhu, Y.; Zhao, J.H.; Chen, J.Q.; Sheng, Z.L. Antidiarrheal effect of bioactivity-guided fractions and bioactive components of pomegranate (Punica granatum L.) peels. Neurogastroenterol. Motil. 2018, 30, e13364. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.Y.; Jadhav, R.M.U.S.K.; Rathnayaka, A.K.S. Investigation of nutrient content, phytochemical content, antioxidant activity and antibacterial activity of inedible portion of pomegranate (Punica granatum L.). Eur. J. Med. Plants 2014, 4, 610–622. [Google Scholar] [CrossRef]
- Jbir, R.; Hasnaoui, N.; Mars, M.; Marrakchi, M.; Trifi, M. Characterization of Tunisian pomegranate (Punica granatum L.) cultivars using amplified fragment length polymorphism analysis. Sci. Hortic. 2008, 115, 231–237. [Google Scholar] [CrossRef]
- Melgarejo, P.; Martínez, J.J.; Hernández, F.C.A.; Martínez, R.; Legua, P.; Oncina, R.; Martinez, M.A. Cultivar identification using 18S–28S rDNA intergenic spacer-RFLP in pomegranate (Punica granatum L.). Sci. Hortic. 2009, 120, 500–503. [Google Scholar] [CrossRef]
- Domingues, S.; Schveitzer, B.; Gabardo, G.C.; Dos Santos, K.C.; Fenili, C.L.; Santos, F.A. Nutritional potential of pomegranate fruits. Congrega Urcamp 2016, 16, 909–917. [Google Scholar]
- Pinheiro, A.J.M.C.R.; Gonçalves, J.S.; Dourado, A.W.A.; Sousa, E.M.; Brito, N.M.; Silva, L.K.; Batista, M.C.A.; Sá, J.C.; Monteiro, C.R.A.V.; Fernandes, E.D.; et al. Punica granatum L. Leaf extract attenuates lung inflammation in mice with acute lung injury. J. Immunol. Res. 2018, 2018, 6879183. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, F.; Wang, S. A polysaccharide from pomegranate peels induces the apoptosis of human osteosarcoma cells via the mitochondrial apoptotic pathway. Tumor Biol. 2014, 35, 7475–7482. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improve shelf life of chicken products. Int. J. Food Sci. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Kharaghani, A.; Lech, K.; Figiel, A.; Carbonell-Barrachina, Á.A.; Tsotsas, E. Drying kinetics and microstructural and sensory properties of black chokeberry (Aronia melanocarpa) as affected by drying method. Food Bioproc. Tech. 2015, 8, 63–74. [Google Scholar] [CrossRef]
- Radünz, L.L.; Amaral, A.S.; Mossi, A.J.; Castro, E.; Rocha, R.P. Evaluation of the drying kinetics of carqueja. Eng. Agric. 2011, 19, 19–27. [Google Scholar]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R. Potential applications of grape (Vittis vinífera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, 20th ed.; Method 934.01; AOAC: Arlington, TX, USA, 2016. [Google Scholar]
- Golestani, R.; Raisi, A.; Aroujalian, A. Mathematical modeling on air drying of apples considering shrinkage and variable diffusion coefficient. Dry. Technol. 2013, 31, 40–51. [Google Scholar] [CrossRef]
- Akpinar, E.K.A.; Krzykowski, D.; Dziki, S.; Rudy, U.; Gawlik-Dziki, R.; Polak, B. Mathematical modelling and experimental investigation on sun and solar drying of white mulberry. J. Mech. Sci. Technol. 2008, 22, 1544–1553. [Google Scholar] [CrossRef]
- Jittanit, W. Kinetics and temperature dependent moisture diffusivities of pumpkin seeds during drying. Kasetsart J. Nat. Sci. 2011, 45, 147–158. [Google Scholar]
- Ertekin, C.; Yaldiz, O. Drying of eggplant and selection of a suitable thin layer drying model. J. Food Eng. 2004, 63, 349–359. [Google Scholar] [CrossRef]
- Madhiyanon, T.; Phila, A.; Soponronnarit, S. Models of fluidized bed drying for thin-layer chopped coconut. Appl. Therm. Eng. 2009, 29, 2849–2854. [Google Scholar] [CrossRef]
- Diamante, L.M.; Ihns, R.; Savage, G.P.; Vanhanen, L. A new mathematical model for thin layer drying of fruits. Int. J. Food Sci. 2010, 45, 1956–1962. [Google Scholar] [CrossRef]
- Serhat, S.T.; Küçüköner, E.; Karacabey, E. Improvements in drying characteristics and quality parameters of tomato by carbonic maceration pretreatment. J. Food Process. Preserv. 2018, 42, e13282. [Google Scholar] [CrossRef]
- Yaldiz, O.; Ertekin, C.; Uzun, H.I. Mathematical modeling of thin layer solar drying of sultana grapes. Energy 2001, 26, 457–465. [Google Scholar] [CrossRef]
- Leite, D.D.F.; Queiroz, A.J.M.; Figueiredo, R.M.F.; Santos, F.S.; Silva, S.N.; Santos, D.C. Mathematical modeling and thermodynamic properties in the drying of citron watermelon seeds. Rev Bras Eng Agr Amb. 2022, 26, 67–74. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum Rate of Air Drying Shelled Corn in Thin-Layers. Master’s Thesis, Purdue University, West Lafayette, Indiana, 1949. [Google Scholar]
- Verma, B.; Shukla, N.P. Removal of Nickel (II) from electroplating industry effluent by agrowaste carbons. Indian J. Environ. Health 2000, 42, 145–150. [Google Scholar]
- Botelho, F.M.; Corrêa, P.C.; Goneli, A.L.D.; Martins, M.A.; Magalhães, F.E.A.; Campos, S.C. Periods of constant and falling-rate for infrared drying of carrot slices. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 845–852. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H. Mathematical modeling of thin layer drying of pistachio by using solar energy. Energy Convers. Manag. 2003, 44, 1111–1122. [Google Scholar] [CrossRef]
- Silva, W.P.; Rodrigues, A.F.; Silva, C.M.D.P.S.; Castro, D.S.; Gomes, J.P. Comparison between continuous and intermittent drying of whole bananas using empirical and diffusion models to describe the processes. J. Food Eng. 2015, 166, 230–236. [Google Scholar] [CrossRef]
- Lewis, W.K. The rate of drying of solid materials. J. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Oliveira, W.P.; Park, K.J. Determination of the effective diffusivity of chicory root. Agric. Eng. 2006, 26, 181–189. [Google Scholar]
- Brooker, D.B.; Bakker-Arkema, F.W.; Hall, C.W. Drying and Storage of Grains and Oilseeds, 2nd ed.; Springer: New York, NY, USA, 1992; p. 450. [Google Scholar]
- Araújo, W.D.; Goneli, A.L.D.; Corrêa, P.C.; Hartmann Filho, C.P.; Martins, E.A.S. Mathematical modeling of the drying of peanut fruits in a thin layer. Agron. Sci. 2017, 48, 448–457. [Google Scholar] [CrossRef]
- Corrêa, P.C.; Oliveira, G.H.H.; Botelho, F.M.; Goneli, A.L.D.; Carvalho, F.M. Mathematical modeling and determination of the thermodynamic properties of coffee (Coffea arabica L.) during the drying process. Rev. Ceres 2010, 57, 595–601. [Google Scholar] [CrossRef]
- Benassi, M.T.; Antunes, A.J.A. Comparison of metaphosphoric and oxalic acids as extractant solutions for the determination of vitamin C in selected vegetables. Biol. Tech. Arch. 1988, 31, 507–513. [Google Scholar]
- Bligh, E.G.; Dyer, W.J.A. Rapid method of total lipid extraction and purification. Can. J. Biochem. 1959, 27, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Atwater, W.O.; Woods, C.D. The chemical Composition of American Food Materials; Department of Agriculture. Office of Experiment Stations: Washington, DC, USA, 1899; Volume 10, pp. 461–462. [Google Scholar]
- Guo, H.L.; Chen, Y.; Xu, W.; Xu, M.T.; Sun, Y.; Wang, X.C.; Wang, X.Y.; Luo, J.; Zhang, H.; Xiong, Y.K. Assessment of Drying Kinetics, Textural and Aroma Attributes of Mentha haplocalyx Leaves during the Hot Air Thin-Layer Drying Process. Foods 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Doymaz, I. Experimental study on drying characteristics of pomegranate peels. Food Sci. Biotechnol. 2011, 20, 965–970. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Pathare, P.B.; Opara, U.L. Drying kinetics of pomegranate fruit peel (cv. Wonderful). Sci. Afr. 2019, 5, e00145. [Google Scholar] [CrossRef]
- Santos, T.; Cunha, R. Kinetic modeling and influence of drying temperature on pomegranate peel extract yield (Punica granatum L). In Proceedings of the Brazilian Congress of Chemical Engineering in Scientific Initiation, ABEQ, São Carlos, Brazil, 19 July 2017; Volume 1, pp. 3287–3292. [Google Scholar]
- Resende, K.K.O.; Silva, S.S.E.; Guedes, S.F.; Loss, R.A. Drying kinetics and physicochemical evaluation of breadfruit (Artocarpus Altilis). Seminiferous Variety. Neotrop. Agric. J. 2019, 6, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Fogaça, M.B.; Franco, T.S.; Flores, S.T.H. Study of drying kinetics of Phormium tenax leaves for use in polymeric composites. Eng. Tech. 2021, 13, 105–114. [Google Scholar]
- Brazil National Health Surveillance Agency. RDC Resolution No. 263 of September 22, 2005. Technical Regulation for Cereal Products, Starches, Flours and Bran; Official Gazette of the Union: Brasilia, Brazil, 2005. [Google Scholar]
- Farias, M.M. Study of the Addition of Pulp and Pomegranate Peel Flour (Punica granatum L.) in the Quality of Bread Type Forma. Master’s Thesis, Federal University of Ceará, Fortaleza, Brazil, 2018. [Google Scholar]
- Diógenes, A.M.G.; Queiroz, A.J.M.; Figueirêdo, R.M.F.; Santos, D.C. Pumpkin grain drying kinetics. Rev. Caatinga 2013, 26, 71–80. [Google Scholar]
- Kara, C.; Doymaz, I. Thin layer drying kinetics of by-products from pomegranate juice processing. J. Food Process. Preserv. 2015, 39, 480–487. [Google Scholar] [CrossRef]
- Barros, S.; Câmara, G.; Leite, D.; Santos, N.; Santos, F.; Soares, T.; Lima, A.; Soares, T.; Oliveira, M.; Vasconcelos, U.; et al. Mathematical modeling of the drying kinetics of kino peels (Cucumis metuliferus). Res. Soc. Dev. 2020, 9, e60911608. [Google Scholar] [CrossRef]
- Gonçalves, J.Q.; Silva, M.A.P.; Plácido, G.R.; Silva, M.C.; Marins, R.; Moura, L.C.; Souza, D.G. Drying kinetics of green banana peel and pulp (Musa acuminata): Properties physical and functional of flour. Glob. Sci-Tech. 2016, 9, 62–72. [Google Scholar]
- Goneli, A.L.D.; Vieira, M.C.; Vilhasanti, H.C.B.; Gonçalves, A.A. Mathematical modeling and effective diffusivity of mastic leaves during drying. Pesq. Agropec. Trop. 2014, 44, 56–64. [Google Scholar] [CrossRef]
- Alves, J.J.L.; Resende, O.; Oliveira, D.E.C.; Branquinho, N.A.A. Cinética de secagem das folhas de Hyptis suaveolens. Rev. Bras. Plantas Med. 2017, 19, 168–176. [Google Scholar] [CrossRef]
- Moscon, E.S.; Martin, S.; Spehar, C.R.; Devilla, I.A. Quinoa grain drying kinetics (Chenopodium quinoa W.). Eng. Agric. 2017, 25, 318–328. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.Z.; Yang, X.H.; Mujumdar, A.S.; Zhao, J.H.; Wang, D.; Zhang, Q.; Wang, J.; Gao, Z.J.; Xiao, H.W. Red pepper (Capsicum annuum L.) drying: Effects of different drying methods on drying kinetics, physicochemical properties, antioxidant capacity, and microstructure. Dry. Technol. 2017, 36, 893–907. [Google Scholar] [CrossRef]
- Olanipekun, B.F.; Tunde-Akintunde, T.Y.; Oyelade, O.J.; Adebisi, M.G.; Adenaya, T.A. Mathematical modeling of thin-layer pineapple drying. J. Food Process. Preserv. 2015, 39, 1431–1441. [Google Scholar] [CrossRef]
- Madamba, P.S.; Driscoll, R.H.; Bruckle, K.A. The thin-layer drying characteristics of garlic slices. J. Food Eng. 1996, 29, 75–97. [Google Scholar] [CrossRef]
- Kaveh, M.; Golpour, I.; Gonçalves, J.C.; Ghafouri, S.; Guiné, R. Determination of drying kinetics, specific energy consumption, shrinkage, and colour properties of pomegranate arils submitted to microwave and convective drying. Open Agric. 2021, 6, 230–242. [Google Scholar] [CrossRef]
- Süfer, O.; Palazoğlu, T.K. A study on hot-air drying of pomegranate. J. Therm. Anal. Calorim. 2019, 137, 1981–1990. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Zhang, M.; Mu, G.; Li, X.; Zhang, G.; Xiong, S. Heat pump drying of kelp (Laminaria japonica): Drying kinetics and thermodynamic properties. Processes 2022, 10, 514. [Google Scholar] [CrossRef]
- Anabel, F.; Celia, R.; Germán, M.; Rosa, R. Determination of effective moisture diffusivity and thermodynamic properties variation of regional wastes under different atmospheres. Case Stud. Therm. Eng. 2018, 12, 248–257. [Google Scholar] [CrossRef]
- Ghibate, R.; Senhaji, O.; Taouil, R.R. Kinetic and thermodynamic approaches on Rhodamine B adsorption onto pomegranate peel. CSCEE 2021, 3, e100078. [Google Scholar] [CrossRef]
- Koukouch, A.; Idlimam, A.; Asbik, M.; Sarh, B.; Izrar, B.; Bostyn, S.; Bah, A.; Ansari, O.; Zegaoui, O.A.A. Experimental determination of the effective moisture diffusivity and activation energy during convective solar drying of olive pomace waste. Renew. Energy 2017, 101, 565–574. [Google Scholar] [CrossRef]
- Alves, N.; Arruda, S.T.A.; Santos, S.B.D.; Galle, N.B.; Silva, I.D.; Silva, M.I. Drying kinetics and thermodynamic properties of ‘baru’ almond. Rev. Bras. Eng. Agrícola Ambient. 2020, 25, 30–36. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Valleo, N. Thermodynamic analysis of experimental sorption isotherms of loquat and quince fruits. J. Food Eng. 2008, 88, 514–521. [Google Scholar] [CrossRef]
- Matos, J.D.P.; Figueirêdo, R.M.F.; Queiroz, A.J.M.; Moraes, M.S.; Silva, S.N.; Silva, L.P.F.R. Foam mat drying kinetics of jambolan and acerola mixed pulp. Rev. Bras. Eng. Agrícola Ambient. 2022, 26, 502–512. [Google Scholar] [CrossRef]
- Santos, D.C.; Leite, D.D.F.; Lisbôa, J.F.; Ferreira, J.P.L.; Santos, F.S.; Lima, T.L.B.; Figueiredo, R.M.F.; Costa, T.N. Modeling and thermodynamic properties of drying acuri slices. Braz. J. Food Technol. 2019, 22, e2018031. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Hesham, A.; Ismail, A.M.; Hameed, M.M.; Refaey, A.S.; Ahmed, A.A. Rheological, physio-chemical and organoleptic characteristics of ice cream enriched with doum syrup and pomegranate peel. Arab. J. Chem. 2020, 13, 7346–7356. [Google Scholar] [CrossRef]
- Sharifi, A.; Hamidi, E.Z.; Gavlighi, H.A.; Saberian, H. Assisted ohmic heating extraction of pectin from pomegranate peel. Chem. Eng. Process. 2022, 172, 108760. [Google Scholar] [CrossRef]
- Santos, J.A.; Acevedo, A.F.G.; Lacerda, V.R.; Vieites, R.L. Physical-chemical, nutritional characterization and bioactive compounds of pomegranate caracterización físico-química, nutricional y compuestos bioactivos de la granada. Res. Soc. Dev. 2022, 11, e18511222777. [Google Scholar] [CrossRef]
- Jan, K.; Ahmad, M.; Rehman, S.; Gani, A.; Khaqan, K. Effect of roasting on physicochemical and antioxidant properties of kalonji (Nigella sativa) seed flour. J. Food Meas. Charact. 2019, 13, 1364–1372. [Google Scholar] [CrossRef]
- Marchi, L.B. Evaluation of Antioxidant Activity and Microbial Activity of Antimicrobial Pomegranate Extracts. Master’s Thesis, State University of Maringá, Maringá, Brazil, 2014. [Google Scholar]
- Paul, A.; Radhakrishnan, M. Pomegranate seed oil in food industry: Extraction, characterization, and applications. Trends Food Sci. Technol. 2020, 105, 273–283. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Abdel, M.S.S.; Awadalla, I.M. Nutritional and chemical evaluation of dried pomegranate (Punica granatum L.) peels and studying the impact of level of inclusion in ration formulation on productive performance of growing Ossimi lambs. Bull. Natl. Res. Cent. 2019, 43, 182. [Google Scholar] [CrossRef]
- Rowayshed, G.; Salama, A.; Abul, F.M.; Akila, H.S.; Emad, A. Nutritional and chemical evaluation for pomegranate (Punica granatum L.) fruit peel and seeds powders by products. Middle East J. Appl. Sci. 2013, 3, 169–179. [Google Scholar]
- Martínez, J.J.; Hernández, F.; Abdelmajid, H.; Legua, P.; Martínez, R.; Amine, A.; Melgarejo, P. Physico-chemical characterization of six pomegranate cultivars from Morocco: Processing and fresh market aptitudes. Sci. Hortic. 2012, 140, 100–106. [Google Scholar] [CrossRef]
- Hernández, F.; Legua, P.; Martínez, R.; Melgarejo, P.; Martínez, J.J. Fruit quality characterization of seven pomegranate accessions (Punica granatum L.) grown in Southeast of Spain. Sci. Hortic. 2014, 175, 174–180. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Bera, M.B.; Kumar, P. Nutritional composition of detanninated and fresh pomegranate peel powder. IOSR J. Environ. Sci. Toxicol. Food Technol. 2013, 7, 38–42. [Google Scholar] [CrossRef]
- Campos, L.; Seixas, L.; Henriques, M.H.; Peres, A.M.; Veloso, A.C. Pomegranate peels and seeds as a source of phenolic compounds: Effect of cultivar, by-product, and extraction solvent. Int. J. Food Sci. 2022, 2022, 9189575e. [Google Scholar] [CrossRef] [PubMed]
- Jalal, H.; Ashraf, M.P.; Ahmad, S.R.; Rather, M.; Mudasir, H.S. Physico-chemical and functional properties of pomegranate peel and seed powder. J. Pharm. Innov. 2018, 7, 1127–1131. [Google Scholar]
- Taco. Tabela Brasileira de Composição de Alimentos, 4th ed.; NEPA-UNICAMP: Campinas, Brazil, 2011; p. 161. [Google Scholar]
- Ismail, T.; Akhtar, S.; Riaz, M.; Ismail, A. Effect of pomegranate peel supplementation on nutritional, organoleptic and stability properties of cookies. Int. J. Food Sci. Nutr. 2014, 65, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Ranjitha, J.; Bhuvaneshwari, G.; Deepa, T.; Kavya, K. Nutritional composition of fresh pomegranate peel powder. Int. J. Chem. Stud. 2018, 6, 692–696. [Google Scholar]
- Nogueira, L.P.S.; Calvacant, M.T.; Souza, P.A.; Fonseca, J.V.S.; Andrade, M.L. Processing and characterization of organic pomegranate waste for agro-industrial use. Rev. Bras. Environ. Manag. 2020, 14, 60–64. [Google Scholar]
- Chisté, R.C.; Cohen, K.D.O.; Mathias, E.D.A.; Ramoa, J.A.G.A. Quality of cassava flour from a dry group. Food Sci. Technol. 2006, 26, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Mekni, M.; Kharroubi, W.; Cheraief, I.; Hammami, M. Pomological, organoleptic and biochemical characterizations of tunisian pomegranate fruits (Punica granatum L.). Am. J. Plant Sci. 2019, 10, 1181–1195. [Google Scholar] [CrossRef] [Green Version]
- Ataíde, E.M.; Silva, M.S.; Bastos, D.C.; Souza, J.M.A. Postharvest quality of pomegranate marketed in the semiarid region of Pernambuco. Agrar. Acad. 2018, 5, 429–436. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Neves, J.O.R.; Helena, E.T.S. Polypharmacy and pattern of drug use in pomerode, SC. Arq. Catarin. Med. 2018, 47, 124–136. [Google Scholar]
- Tozzi, F.; Núñez, G.D.; Legua, P.; Bubba, M.; Giordani, E.; Melgarejo, P. Qualitative and varietal characterization of pomegranate peel: High-value co-product or waste of production? Sci. Hortic. 2022, 291, e110601. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. BioMed Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef] [PubMed]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
| Model | Equation | Equation | Reference |
|---|---|---|---|
| Diffusion Approximation | (2) | [16] | |
| Two terms | (3) | [17] | |
| Two-term exponential | (4) | [18] | |
| Henderson and Pabis Modified | (5) | [19] | |
| Henderson and Pabis | (6) | [20] | |
| Logarithmic | (7) | [21] | |
| Logistic | (8) | [22] | |
| c | (9) | [23] | |
| Page | (10) | [24] | |
| Verma | (11) | [25] | |
| Thompson | (12) | [26] | |
| Midilli | (13) | [27] |
| Pomegranate Sample | Drying Temperature (°C) | Drying Time (min) | Water Content (% bu) | Water Content (% bs) |
|---|---|---|---|---|
| Peels | 50 | 840 | 11.84 | 13.44 |
| 60 | 780 | 8.30 | 9.09 | |
| 70 | 720 | 7.14 | 7.70 | |
| Seeds | 50 | 710 | 2.42 | 2.48 |
| 60 | 650 | 1.64 | 1.67 | |
| 70 | 540 | 1.26 | 1.28 |
| Model | T (°C) | Parameters | R2 | MSD | χ2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | k | b | ||||||||
| Diffusion Approximation | 50 | 0.1043 | 0.0304 | 0.2254 | - | - | - | 0.9984 | 0.0134 | 0.0002 |
| 60 | 0.1185 | 0.1174 | 0.0601 | - | - | - | 0.9994 | 0.0077 | 0.0001 | |
| 70 | 0.0781 | 0.2629 | 0.0321 | - | - | - | 0.9982 | 0.0138 | 0.0002 | |
| Model | T (°C) | a | k0 | b | k1 | R2 | MSD | χ2 | ||
| Two Terms | 50 | 0.8978 | 0.0069 | 0.1068 | 0.0327 | - | - | 0.9985 | 0.0133 | 0.0002 |
| 60 | 0.1159 | 0.1089 | 0.8798 | 0.0070 | - | - | 0.9994 | 0.0076 | 0.0001 | |
| 70 | 0.5535 | 0.0087 | 0.3905 | 0.0087 | 0.9968 | 0.0185 | 0.0004 | |||
| Model | T (°C) | a | k | R2 | MSD | χ2 | ||||
| Exponential | 50 | 0.0363 | 0.2018 | - | - | - | - | 0.9978 | 0.0158 | 0.0003 |
| 60 | 0.1321 | 0.0535 | - | - | - | 0.9986 | 0.0122 | 0.0002 | ||
| 70 | 0.0793 | 0.1071 | - | - | - | - | 0.9977 | 0.0156 | 0.0002 | |
| Model | T (°C) | a | k | R2 | MSD | χ2 | ||||
| Henderson and Pabis | 50 | 0.9776 | 0.0074 | - | - | - | - | 0.9975 | 0.0167 | 0.0003 |
| 60 | 0.9273 | 0.0075 | - | - | - | - | 0.9966 | 0.0187 | 0.0004 | |
| 70 | 0.9439 | 0.0087 | - | - | - | - | 0.9968 | 0.0185 | 0.0004 | |
| Model | T (°C) | a | k0 | b | k1 | c | k2 | R2 | MSD | χ2 |
| Modified Henderson and Pabis | 50 | 0.1068 | 0.0327 | 0.7618 | 0.0068 | 0.1359 | 0.0068 | 0.9981 | 0.0133 | 0.0002 |
| 60 | 0.0858 | 0.0348 | 0.8481 | 0.0068 | 0.0666 | 0.2907 | 0.9995 | 0.0066 | 0.0001 | |
| 70 | 0.3144 | 0.0087 | 0.3144 | 0.0087 | 0.3149 | 0.0087 | 0.9968 | 0.0185 | 0.0004 | |
| Model | T (°C) | a | k | c | R2 | MSD | χ2 | |||
| Logarithmic | 50 | 0.9729 | 0.0076 | 0.0069 | - | - | - | 0.9977 | 0.0163 | 0.0003 |
| 60 | 0.9205 | 0.0078 | 0.0102 | - | - | - | 0.9968 | 0.0181 | 0.0004 | |
| 70 | 0.9466 | 0.0086 | 0.0038 | 0.9968 | 0.0184 | 0.0004 | ||||
| Model | T (°C) | a0 | a | k | R2 | MSD | χ2 | |||
| Logistic | 50 | 0.0538 | 0.0550 | 0.0075 | - | - | - | 0.9976 | 0.0167 | 0.0003 |
| 60 | 0.1635 | 0.1763 | 0.0076 | - | - | - | 0.9966 | 0.0209 | 0.0004 | |
| 70 | 0.1475 | 0.1562 | 0.0087 | 0.9968 | 0.0185 | 0.0004 | ||||
| Model | T (°C) | k | R2 | MSD | χ2 | |||||
| Newton | 50 | 0.0077 | - | - | - | - | - | 0.9969 | 0.0189 | 0.0004 |
| 60 | 0.0085 | - | - | - | - | - | 0.9883 | 0.0344 | 0.0012 | |
| 70 | 0.0095 | - | - | 0.9923 | 0.0288 | 0.0009 | ||||
| Model | T (°C) | k | n | R2 | MSD | χ2 | ||||
| Page | 50 | 0.0110 | 0.9256 | - | - | - | - | 0.9981 | 0.0139 | 0.0002 |
| 60 | 0.0194 | 0.8245 | - | - | - | - | 0.9985 | 0.0121 | 0.0002 | |
| 70 | 0.0160 | 0.8868 | - | 0.9958 | 0.0210 | 0.0005 | ||||
| Model | T (°C) | a | k | k1 | R2 | MSD | χ2 | |||
| Verma | 50 | 0.1042 | 0.0304 | 0.0068 | - | - | - | 0.9984 | 0.0134 | 0.0002 |
| 60 | 0.1184 | 0.1174 | 0.0070 | - | - | - | 0.9994 | 0.0077 | 0.0001 | |
| 70 | 0.0780 | 0.2633 | 0.0084 | 0.9982 | 0.0138 | 0.0002 | ||||
| Model | T (°C) | a | b | R2 | MSD | χ2 | ||||
| Thompson | 50 | −11.741 | 0.0946 | - | - | - | 0.9980 | 0.0150 | 0.0002 | |
| 60 | −3.8820 | 0.0415 | - | - | - | 0.9958 | 0.0208 | 0.0005 | ||
| 70 | −10.385 | 0.1037 | 0.9935 | 0.0263 | 0.0008 | |||||
| Model | T (°C) | a | k | n | b | R2 | MSD | χ2 | ||
| Midilli | 50 | 1.0053 | 0.0118 | 0.9118 | 0.000007 | - | - | 0.9983 | 0.0138 | 0.0002 |
| 60 | 0.9803 | 0.0177 | 0.8354 | 0.000022 | - | - | 0.9992 | 0.0090 | 0.0001 | |
| 70 | 0.9586 | 0.0113 | 0.9480 | 0.000013 | 0.9970 | 0.0178 | 0.0004 | |||
| Model | T (°C) | Parameters | R2 | MSD | χ2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | k | b | ||||||||
| Diffusion Approximation | 50 | 0.0725 | 0.0891 | 0.1054 | 0.9987 | 0.0118 | 0.0002 | |||
| 60 | 0.0070 | 0.0251 | 0.9932 | 0.9995 | 0.0103 | 0.0001 | ||||
| 70 | 0.2045 | 0.0836 | 0.2878 | 0.9995 | 0.0074 | 0.0001 | ||||
| Model | T (°C) | a | k0 | b | k1 | R2 | MSD | χ2 | ||
| Two Terms | 50 | 0.0729 | 0.0896 | 0.9276 | 0.0094 | - | - | 0.9987 | 0.0041 | 0.0002 |
| 60 | 0.5113 | 0.0161 | 0.5113 | 0.0161 | - | - | 0.9967 | 0.0195 | 0.0045 | |
| 70 | 0.5208 | 0.0211 | 0.5207 | 0.0211 | - | - | 0.9976 | 0.0152 | 0.0003 | |
| Model | T (°C) | a | k | R2 | MSD | χ2 | ||||
| Exponential | 50 | 0.0633 | 0.1497 | - | - | - | - | 0.9986 | 0.0123 | 0.0002 |
| 60 | 0.0033 | 4.7941 | - | - | - | - | 0.9960 | 0.0214 | 0.0001 | |
| 70 | 0.0026 | 7.5399 | - | - | - | - | 0.9958 | 0.0216 | 0.0005 | |
| Model | T (°C) | a | k | R2 | MSD | χ2 | ||||
| Henderson and Pabis | 50 | 0.9655 | 0.0098 | - | - | - | - | 0.9979 | 0.0152 | 0.0002 |
| 60 | 1.0230 | 0.0161 | - | - | - | - | 0.9967 | 0.0195 | 0.0004 | |
| 70 | 1.0415 | 0.0211 | - | - | - | - | 0.9976 | 0.0163 | 0.0003 | |
| Model | T (°C) | a | k0 | b | k1 | c | k2 | R2 | MSD | χ2 |
| Modified Henderson and Pabis | 50 | −0.3878 | 0.0050 | 1.2822 | 0.0075 | 0.1072 | 0.0690 | 0.9992 | 0.0101 | 0.0001 |
| 60 | 0.3410 | 0.0161 | 0.3410 | 0.0161 | 0.3410 | 0.0161 | 0.9967 | 0.0196 | 0.0005 | |
| 70 | 0.3543 | 0.0174 | 0.3543 | 0.0174 | 0.3543 | 0.0174 | 0.9976 | 0.0164 | 0.0003 | |
| Model | T (°C) | a | k | c | R2 | MSD | χ2 | |||
| Logarithmic | 50 | 0.9672 | 0.0098 | −0.0026 | - | - | - | 0.9979 | 0.0151 | 0.0003 |
| 60 | 1.0290 | 0.0158 | −0.0086 | - | - | - | 0.9963 | 0.0188 | 0.0004 | |
| 70 | 1.0436 | 0.0209 | 0.0031 | - | - | - | 0.9976 | 0.0162 | 0.0003 | |
| Model | T (°C) | a0 | a | k | R2 | MSD | χ2 | |||
| Logistic | 50 | 0.1596 | 0.1653 | 0.0099 | - | - | - | 0.9979 | 0.0152 | 0.0002 |
| 60 | 0.1263 | 0.1235 | 0.0161 | - | - | - | 0.9967 | 0.0195 | 0.0004 | |
| 70 | 0.1038 | 0.0997 | 0.0211 | - | - | - | 0.9976 | 0.0163 | 0.0003 | |
| Model | T (°C) | k | R2 | MSD | χ2 | |||||
| Newton | 50 | 0.0104 | - | - | - | - | - | 0.9964 | 0.0201 | 0.0004 |
| 60 | 0.0157 | - | - | - | - | - | 0.9962 | 0.0211 | 0.0005 | |
| 70 | 0.0200 | - | - | - | - | - | 0.9960 | 0.021 | 0.0005 | |
| Model | T (°C) | k | n | R2 | MSD | χ2 | ||||
| Page | 50 | 0.0151 | 0.9171 | - | - | - | - | 0.9981 | 0.0142 | 0.0002 |
| 60 | 0.0092 | 1.1290 | - | - | - | - | 0.9987 | 0.0122 | 0.0002 | |
| 70 | 0.0112 | 1.1523 | - | - | - | - | 0.9993 | 0.0086 | 0.0001 | |
| Model | T (°C) | a | k | k1 | R2 | MSD | χ2 | |||
| Verma | 50 | 0.0725 | 0.0891 | 0.0094 | - | - | - | 0.9987 | 0.0118 | 0.0002 |
| 60 | −6.5085 | 0.0260 | 0.0242 | - | - | - | 0.9991 | 0.0103 | 0.0001 | |
| 70 | 0.0422 | 0.0200 | 0.0200 | - | - | - | 0.9960 | 0.0211 | 0.0005 | |
| Model | T (°C) | a | b | R2 | MSD | χ2 | ||||
| Thompson | 50 | −13.716 | 0.1429 | - | - | - | - | 0.9972 | 0.0176 | 0.0003 |
| 60 | −2445.7 | 1.9007 | - | - | - | - | 0.9962 | 0.0211 | 0.0005 | |
| 70 | −2054.4 | 1.9073 | - | - | - | - | 0.9960 | 0.0211 | 0.0005 | |
| Model | T (°C) | a | k | n | b | R2 | MSD | χ2 | ||
| Midilli | 50 | 0.9884 | 0.0142 | 0.9248 | 0.0000 | - | - | 0.9984 | 0.0131 | 0.0002 |
| 60 | 0.9723 | 0.0068 | 1.1928 | 0.0000 | - | - | 0.9981 | 0.0101 | 0.0001 | |
| 70 | 1.0011 | 0.0111 | 1.1537 | 0.0000 | - | - | 0.9996 | 0.0082 | 0.0001 | |
| Pomegranate | T (°C) | Def (m2/s) | R2 |
|---|---|---|---|
| Peels | 50 | 3.7583 × 10−12 | 0.9829 |
| 60 | 4.1243 × 10−12 | 0.9957 | |
| 70 | 4.6803 × 10−12 | 0.9901 | |
| Seeds | 50 | 1.3106 × 10−9 | 0.9874 |
| 60 | 2.0267 × 10−9 | 0.9690 | |
| 70 | 2.5907 × 10−9 | 0.9667 |
| Pomegranate | Def0 | Ea (kJ/mol) | R2 |
|---|---|---|---|
| Peels | 1.599 × 10−5 | 10.60 | 0.9889 |
| Seeds | 1.659 × 10−5 | 31.39 | 0.9798 |
| Pomegranate | T (°C) | ΔH (kJ/mol) | ΔS (kJ/mol K) | ΔG (kJ/mol) |
|---|---|---|---|---|
| Peels | 50 | 7.9133 | −0.43311 | 147.8717 |
| 60 | 7.8302 | −0.43306 | 152.2040 | |
| 70 | 7.7471 | −0.43301 | 156.5389 | |
| Seeds | 50 | 28.7033 | −0.41562 | 163.0110 |
| 60 | 28.6202 | −0.41225 | 165.9611 | |
| 70 | 28.5371 | −0.41045 | 169.3844 |
| Parameter (bu) | Freshsample | Flours | |||
|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | |||
| Water content (%) | Peels | 70.74 ± 0.06 aA | 11.41 ± 0.16 aB | 10.41 ± 0.15 aC | 9.42 ± 0.06 aD |
| Seeds | 36.60 ± 0.09 bA | 4.71 ± 0.10 bB | 4.06 ± 0.04 bC | 3.73 ± 0.12 bC | |
| Lipids (%) | Peels | 0.98 ± 0.01 bB | 0.54 ± 0.01 bA | 0.61 ± 0.01 bA | 0.60 ± 0.01 bA |
| Seeds | 3.14 ± 0.02 aD | 8.43 ± 0.05 aC | 9.28 ± 0.04 aB | 10.62 ± 0.15 aA | |
| Proteins (%) | Peels | 3.40 ± 0.09 bC | 5.45 ± 0.30 bB | 6.50 ± 0.10 bA | 6.64 ± 0.27 bA |
| Seeds | 7.38 ± 0.04 aD | 10.61 ± 0.37 aC | 12.21 ± 0.24 aB | 13.64 ± 0.29 aA | |
| Fibers (%) | Peels | 11.00 ± 0.37 bD | 21.06 ± 0.03 bC | 22.60 ± 1.54 bB | 26.19 ± 0.39 bA |
| Seeds | 20.85 ± 0.09 aD | 41.31 ± 0.27 aC | 43.81 ± 0.14 aB | 45.94 ± 0.25 aA | |
| Ashes (%) | Peels | 1.03 ± 0.02 aC | 3.73 ± 0.05 aA | 3.72 ± 0.08 aA | 3.53 ± 0.06 aB |
| Seeds | 0.86 ± 0.01 bC | 1.48 ± 0.03 bB | 1.59 ± 0.01 bA | 1.55 ± 0.04 bA | |
| Carbohydrates (%) | Peels | 24.74 ± 0.38 bC | 78.90 ± 0.41 aB | 78.76 ± 0.51 aB | 79.81 ± 0.42 aA |
| Seeds | 51.99 ± 0.33 aD | 73.21 ± 0.55 bA | 71.49 ± 0.38 bB | 68.81 ± 0.56 bC | |
| Energetic value (kcal/100 g) | Peels | 113.30 ± 0.14 bD | 342.27 ± 0.18 bC | 346.53 ± 0.15 bB | 351.18 ± 0.11 bA |
| Seeds | 265.86 ± 0.08 aD | 411.14 ± 0.06 aC | 418.37 ± 0.09 aB | 425.97 ± 0.20 aA | |
| Water activity (aw) | Peels | 0.987 ± 0.001 aA | 0.380 ± 0.001 aB | 0.369 ± 0.001 aC | 0.362 ± 0.001 aD |
| Seeds | 0.972 ± 0.001 bA | 0.244 ± 0.001 bB | 0.235 ± 0.002 bC | 0.228 ± 0.001 bD | |
| Total titratable acidity (%citric acid) | Peels | 1.52 ± 0.03 aC | 6.96 ± 0.04 aA | 6.87 ± 0.04 aAB | 6.79 ± 0.07 aB |
| Seeds | 0.13 ± 0.01 bB | 0.73 ± 0.04 bA | 0.73 ± 0.08 bA | 0.75 ± 0.07 bA | |
| pH | Peels | 4.07 ± 0.01 bA | 3.70 ± 0.01 bD | 3.77 ± 0.01 bC | 3.82 ± 0.01 bB |
| Seeds | 5.45 ± 0.01 aA | 4.97 ± 0.01 aD | 5.17 ± 0.01 aC | 5.28 ± 0.01 aB | |
| Total sugars (% glucose) | Peels | 1.81 ± 0.004 bD | 16.80 ± 0.03 aA | 15.70 ± 0.04 aB | 12.72 ± 0.03 aC |
| Seeds | 3.22 ± 0.004 aD | 6.24 ± 0.02 bA | 4.18 ± 0.01 bC | 4.68 ± 0.05 bB | |
| Reducing sugars (% glucose) | Peels | 0.22 ± 0.004 | 0.15 ± 0.002 | 0.14 ± 0.004 | 0.13 ± 0.002 |
| Seeds | 0.21 ± 0.001 | 0.08 ± 0.002 | 0.05 ± 0.002 | 0.05 ± 0.002 | |
| Non-reducing sugars (% sucrose) | Peels | 1.50 ± 0.01 bD | 15.81 ± 0.04 aA | 14.77 ± 0.05 aB | 11.95 ± 0.04 aC |
| Seeds | 2.82 ± 0.001 aD | 5.85 ± 0.02 bA | 3.91 ± 0.01 bC | 4.39 ± 0.05 bB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanderley, R.d.O.S.; de Figueirêdo, R.M.F.; Queiroz, A.J.d.M.; dos Santos, F.S.; Paiva, Y.F.; Ferreira, J.P.d.L.; de Lima, A.G.B.; Gomes, J.P.; Costa, C.C.; da Silva, W.P.; et al. The Temperature Influence on Drying Kinetics and Physico-Chemical Properties of Pomegranate Peels and Seeds. Foods 2023, 12, 286. https://doi.org/10.3390/foods12020286
Wanderley RdOS, de Figueirêdo RMF, Queiroz AJdM, dos Santos FS, Paiva YF, Ferreira JPdL, de Lima AGB, Gomes JP, Costa CC, da Silva WP, et al. The Temperature Influence on Drying Kinetics and Physico-Chemical Properties of Pomegranate Peels and Seeds. Foods. 2023; 12(2):286. https://doi.org/10.3390/foods12020286
Chicago/Turabian StyleWanderley, Roberta de Oliveira Sousa, Rossana Maria Feitosa de Figueirêdo, Alexandre José de Melo Queiroz, Francislaine Suelia dos Santos, Yaroslávia Ferreira Paiva, João Paulo de Lima Ferreira, Antônio Gilson Barbosa de Lima, Josivanda Palmeira Gomes, Caciana Cavalcanti Costa, Wilton Pereira da Silva, and et al. 2023. "The Temperature Influence on Drying Kinetics and Physico-Chemical Properties of Pomegranate Peels and Seeds" Foods 12, no. 2: 286. https://doi.org/10.3390/foods12020286






