Colorimetric Chemosensor Based on Fe3O4 Magnetic Molecularly Imprinted Nanoparticles for Highly Selective and Sensitive Detection of Norfloxacin in Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of Fe3O4 MMIP NPs
2.3. Peroxidase-Like Catalytic Activity of Fe3O4 MMIP NPs
2.4. In Situ Colorimetric Measurement
2.5. Preparation of Milk Samples
2.6. Calculation of LOD
3. Results and Discussion
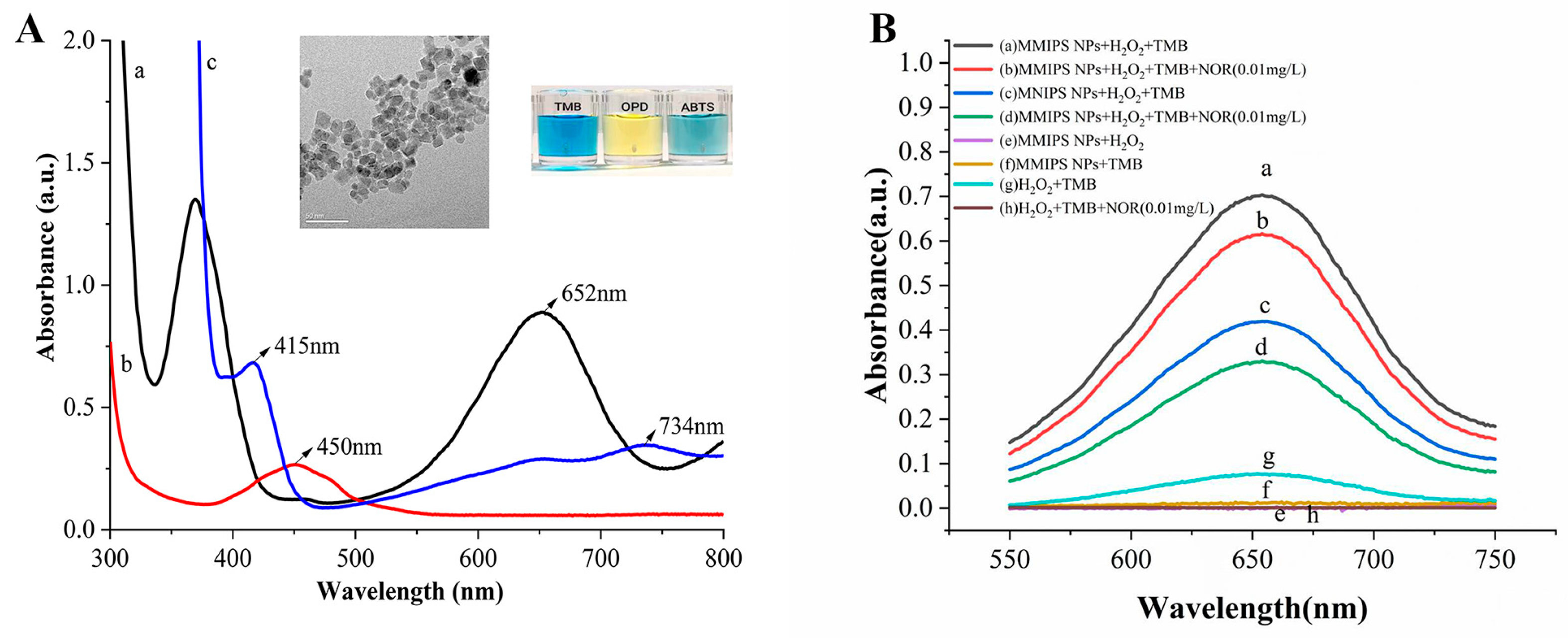
3.1. The Kinetic Parameters of TMB Oxidation Catalyzed by Fe3O4 MMIP NPs
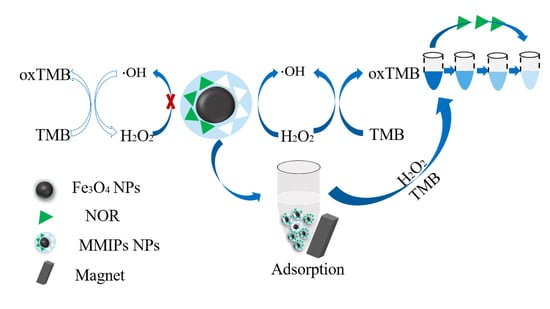
3.2. Detection Feasibility
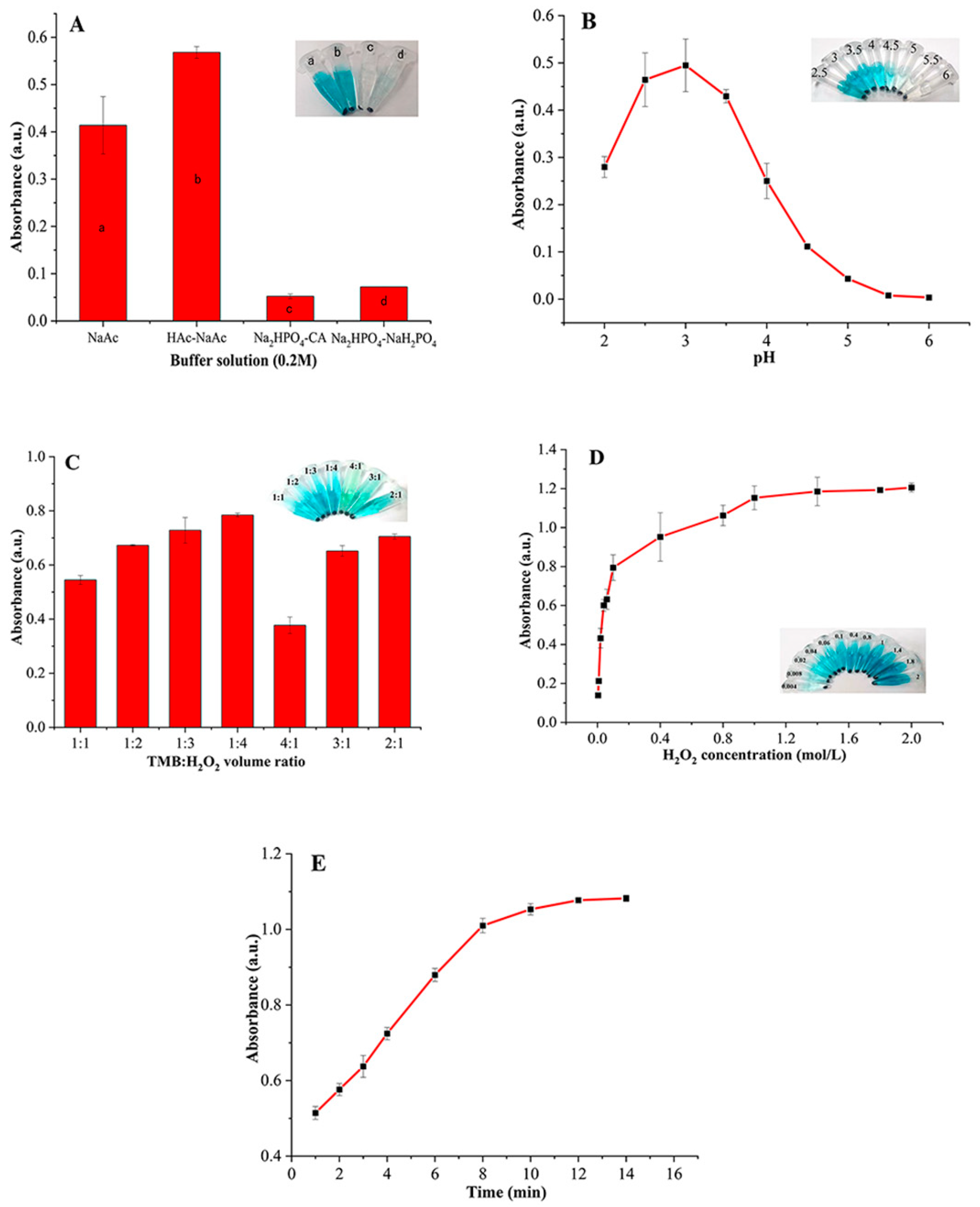
3.3. Optimization of the Experimental Conditions
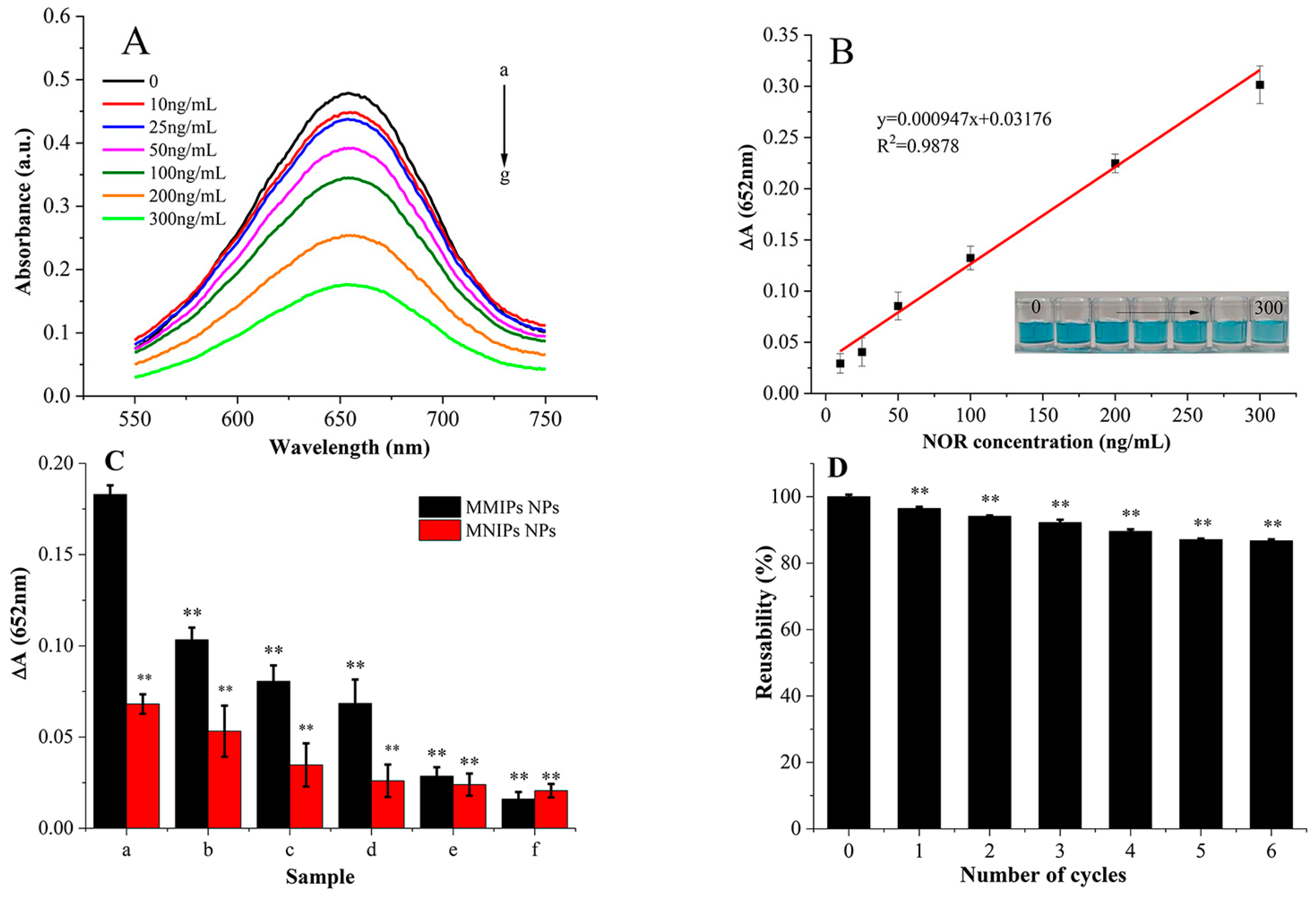
3.4. In Situ Colorimetric Measurements
3.5. Detection of NOR in Milk
3.6. Comparison of Detection Methods for NOR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gadebusch, H.H.; Shungu, D.L. Norfloxacin, the First of a New Class of Fluoroquinolone Antimicrobials, Revisited. Int. J. Antimicrob. Agents 1991, 1, 3–28. [Google Scholar] [CrossRef]
- Wang, Z.; Song, B.; Li, J.; Teng, X. Degradation of Norfloxacin Wastewater Using Kaolin/Steel Slag Particle Electrodes: Performance, Mechanism and Pathway. Chemosphere 2021, 270, 128652. [Google Scholar] [CrossRef]
- Li, Y.F.; Sheng, Y.J.; Tsao, H.K. Evaporation stains: Suppressing the coffee-ring effect by contact angle hysteresis. Langmuir 2013, 25, 7802–7811. [Google Scholar] [CrossRef]
- Announcement No. 2292/2015 of the Ministry of Agriculture of the People’s Republic of China. Gazette of the Ministry of Agriculture of the People’s Republic of China. Available online: http://www.moa.gov.cn/govpublic/SYJ/201509/t20150907_4819267.htm (accessed on 1 September 2015).
- Annunziata, L.; Visciano, P.; Stramenga, A.; Colagrande, M.N.; Campana, G.; Scortichini, G.; Migliorati, G.; Compagnone, D. Development and Validation of a Method for the Determination of Quinolones in Muscle and Eggs by Liquid Chromatography-Tandem Mass Spectrometry. Food Anal. Methods 2016, 9, 2308–2320. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Demetriou, C.E.; Papadoyannis, I.N. Direct Determination of Four Fluoroquinolones, Enoxacin, Norfloxacin, Ofloxacin, and Ciprofloxacin, in Pharmaceuticals and Blood Serum by HPLC. Anal. Bioanal. Chem. 2003, 375, 623–629. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, X.; Yang, J.; Lu, X. Preparation of an Amperometric Sensor for Norfloxacin Based on Molecularly Imprinted Grafting Photopolymerization. Anal. Bioanal. Chem. 2013, 405, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Z.; Han, Z.; Fan, L.; Liu, S.; Yang, H.; Chen, Z.; Sun, T.; Ning, B. A Highly Sensitive and Dual-Readout Immunoassay for Norfloxacin in Milk Based on QDs-FM@ ALP-SA and Click Chemistry. Talanta 2021, 234, 122703. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, J.; Deng, H.; Zhang, L. Progress of Mimetic Enzymes and Their Applications in Chemical Sensors. Crit. Rev. Anal. Chem. 2016, 46, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-nanoparticle-based Transphosphorylation Catalysts. Angew. Chem. 2004, 116, 6291–6295. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Wu, D.; Liu, J.; Wu, Y. Recent Advances on Emerging Nanomaterials for Controlling the Mycotoxin Contamination: From Detection to Elimination. Food Front. 2020, 1, 360–381. [Google Scholar] [CrossRef]
- Jagtiani, E. Advancements in Nanotechnology for Food Science and Industry. Food Front. 2022, 3, 56–82. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.; Ren, D.; Sun, L.; Zhuang, Y.; Yi, L.; Wang, S. Recent Advances in Nanomaterial-assisted Electrochemical Sensors for Food Safety Analysis. Food Front. 2022, 153, 112046. [Google Scholar] [CrossRef]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.; Müller, W.E.G.; Tremel, W. V2O5 Nanowires with an Intrinsic Peroxidase-like Activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Liu, A.; Wang, L.; Li, G.; Lin, X. Peroxidase-like Activity of Cupric Oxide Nanoparticle. ChemCatChem 2011, 3, 1151–1154. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhao, H.; Zhang, L.; Su, Y.; Lv, Y. BSA-Templated MnO2 Nanoparticles as Both Peroxidase and Oxidase Mimics. Analyst 2012, 137, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zhang, L.; Zhao, M.; Wang, Y. Co3O4 Nanoparticles as an Efficient Catalase Mimic: Properties, Mechanism and Its Electrocatalytic Sensing Application for Hydrogen Peroxide. J. Mol. Catal. A Chem. 2013, 378, 30–37. [Google Scholar] [CrossRef]
- Qin, W.; Su, L.; Yang, C.; Ma, Y.; Zhang, H.; Chen, X. Colorimetric Detection of Sulfite in Foods by a TMB–O2–Co3O4 Nanoparticles Detection System. J. Agric. Food Chem. 2014, 62, 5827–5834. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, Y.; Chen, M.; Wang, M.; Yuan, H.; Hu, L. A Colorimetric Heparin Assay Based on the Inhibition of the Oxidase Mimicking Activity of Cerium Oxide Nanoparticles. Microchim. Acta 2019, 186, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhang, J.; Lu, N.; Kim, M.J.; Ghale, K.; Xu, Y.; McKenzie, E.; Liu, J.; Ye, H. Pd–Ir Core–Shell Nanocubes: A Type of Highly Efficient and Versatile Peroxidase Mimic. ACS Nano 2015, 9, 9994–10004. [Google Scholar] [CrossRef]
- Biswas, S.; Tripathi, P.; Kumar, N.; Nara, S. Gold Nanorods as Peroxidase Mimetics and Its Application for Colorimetric Biosensing of Malathion. Sens. Actuators B Chem. 2016, 231, 584–592. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Z.; Ren, C.; Liu, G.; Chen, X. A Novel Colorimetric Determination of Reduced Glutathione in A549 Cells Based on Fe3O4 Magnetic Nanoparticles as Peroxidase Mimetics. Analyst 2012, 137, 485–489. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeong, H.Y.; Kim, M.I.; Park, T.J. Colorimetric Detection System for Salmonella Typhimurium Based on Peroxidase-like Activity of Magnetic Nanoparticles with DNA Aptamers. J. Nanomater. 2015, 2015, 527126. [Google Scholar] [CrossRef]
- Ren, H.; Ma, T.; Zhao, J.; Zhou, R. Vc-Functionalized Fe3O4 Nanocomposites as Peroxidase-like Mimetics for H2O2 and Glucose Sensing. Chem. Res. Chin. Univ. 2018, 34, 260–268. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, Y.; Zhang, L.; Ge, S.; Yu, J. A Novel Microfluidic Paper-Based Colorimetric Sensor Based on Molecularly Imprinted Polymer Membranes for Highly Selective and Sensitive Detection of Bisphenol A. Sens. Actuators B Chem. 2017, 243, 130–136. [Google Scholar] [CrossRef]
- Fan, C.; Liu, J.; Zhao, H.; Li, L.; Liu, M.; Gao, J.; Ma, L. Molecular Imprinting on PtPd Nanoflowers for Selective Recognition and Determination of Hydrogen Peroxide and Glucose. RSC Adv. 2019, 9, 33678–33683. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, H.; Zhang, C.; Qu, L.; Yu, L. A Novel Molecularly Imprinted Sensor Based on PtCu Bimetallic Nanoparticle Deposited on PSS Functionalized Graphene with Peroxidase-like Activity for Selective Determination of Puerarin. Talanta 2020, 210, 120621. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, Z.; Luo, L.; Li, J.; Li, P.; Liu, X. Rapid and Selective Extraction of Norfloxacin from Milk Using Magnetic Molecular Imprinting Polymers Nanoparticles. Food Chem. 2021, 353, 129464. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tang, G.; Xiong, X.; Cao, Y.; Chen, L.; Xu, F.; Tan, H. Carbon Coated Magnetite Nanoparticles with Improved Water-Dispersion and Peroxidase-like Activity for Colorimetric Sensing of Glucose. Sens. Actuators B Chem. 2015, 215, 86–92. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Wei, T.; Bao, J.; Zhu, Q.; Dai, Z. Fe-Porphyrin-Based Covalent Organic Framework as a Novel Peroxidase Mimic for a One-Pot Glucose Colorimetric Assay. ACS Appl. Bio Mater. 2018, 1, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, F.; Wang, X.; Wan, Y.; Xue, Y.; Cai, N.; Chen, W.; Yu, F. Hierarchically Structured Fe3O4-Doped MnO2 Microspheres as an Enhanced Peroxidase-like Catalyst for Low Limit of Detection. Process Biochem. 2019, 83, 35–43. [Google Scholar] [CrossRef]
- Peng, F.F.; Zhang, Y.; Gu, N. Size-Dependent Peroxidase-like Catalytic Activity of Fe3O4 Nanoparticles. Chinese Chem. Lett. 2008, 19, 730–733. [Google Scholar] [CrossRef]
- Xiong, Y.H.; Chen, S.H.; Ye, F.G.; Su, L.J.; Shen, S.F.; Zhao, S.L. Preparation of magnetic core–shell nanoflower Fe3O4@MnO2 as reusable oxidase mimetics for colorimetric detection of phenol. Anal. Methods 2014, 7, 1300–1306. [Google Scholar] [CrossRef]
- Lian, J.J.; Liu, P.; Li, X.C.; Gao, L.N.; Luo, X.L.; Zhang, X.; Shi, Z.Q.; Liu, Q.Y. Perylene diimide-modified magnetic γ-Fe2O3/CeO2 nanoparticles as peroxidase mimics for highly sensitive colorimetric detection of Vitamin C. Appl. Organomet. Chem. 2019, 33, e4884. [Google Scholar] [CrossRef]
- Mendes, C.; Buttchevitz, A.; Kruger, J.H.; Bernardl, L.S.; Oliveira, P.R.; Silva, M. Quantitative analysis of norfloxacin in beta-Cyclodextrin inclusion complexes-development and validation of a stability-indicating HPLC method. Anal. Sci. 2015, 31, 1083–1089. [Google Scholar] [CrossRef]
- Kowalski, C.; Roliński, Z.; Sawik, T.; Gód, B.K.; Roliń, Z. determination of norfloxacin in chicken tissues by HPLC with fluorescence detection determination of norfloxacin in chicken tissues by HPLC with fluorescence detection. Liq. Chromatogr. R. T. 2005, 28, 121–135. [Google Scholar] [CrossRef]
- Cui, J.L.; Zhang, K.; Huang, Q.X.; Yu, Y.Y.; Peng, X.Z. An indirect competitive enzyme-linked immunosorbent assay for determination of norfloxacin in waters using a specific polyclonal antibody. Anal. Chim. Acta. 2011, 688, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Su, X.; Yuan, C.; Jia, C.Q.; Yang, Y. A magnetic phosphorescence molecularly imprinted polymers probe based on manganese-doped ZnS quantum dots for rapid detection of trace norfloxacin residual in food. Spectroch. Acta. A. 2021, 253, 119577. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, R.C.; Aguilar-Caballos, M.P.; Gómez-Hens, A. Sensitive Determination of Fluoroquinolone Antibiotics in Milk Samples Using Time-Resolved Methodology. Anal. Lett. 2004, 37, 1163–1175. [Google Scholar] [CrossRef]
- Qiu, X.Q.; Gu, J.; Yang, T.Q.; Ma, C.Q.; Li, L.; Wu, Y.M.; Zhu, C.; Gao, H.; Yang, Z.C.; Wang, Z.R.; et al. Sensitive determination of Norfloxacin in milk based on beta-cyclodextrin functionalized silver nanoparticles SERS substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 276, 121212. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, Y.; Shui, H.Z.; Wu, Z.J.; Yu, X.H.; Zhang, C.G.; Feng, S. Preparation of molecularly imprinted polymers based on covalent organic frameworks and their application to selective recognition of trace norfloxacin in milk. Chin. J. Chromatogr. 2022, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Catalyst | Km/mM | Vmax/10−8 M s−1 | References | ||
|---|---|---|---|---|---|
| TMB | H2O2 | TMB | H2O2 | ||
| HRP | 0.434 | 3.7 | 10 | 8.7 | [28] |
| Fe3O4 | 0.098 | 154 | 3.44 | 9.78 | [29] |
| Fe3O4 NPs | 0.313 | 0.013 | 13.35 | 2.95 | [30] |
| Fe-COF | 0.02 | 0.143 | 3.83 | 4.74 | [31] |
| VcFe3O4 NPs | 0.067 | 2.96 | 1.93 | 2.05 | [24] |
| Fe3O4-MnO2 | 0.101 | 0.041 | 0.57 | 2.94 | [32] |
| Fe3O4@C | 0.27 | 0.035 | 12 | 3.34 | [28] |
| MMIP NPs | 0.088 | 0.16 | 3.44 | 6.25 | This work |
| Milk Sample | Spiked (ng/mL) | Detected (ng/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 10.00 | 8.98 | 89.83 | 4.32 |
| 50.00 | 42.74 | 85.48 | 3.47 | |
| 100.00 | 78.29 | 78.29 | 9.35 | |
| 2 | 10.00 | 9.09 | 90.88 | 9.46 |
| 50.00 | 44.53 | 89.07 | 6.36 | |
| 100.00 | 82.83 | 82.83 | 3.89 | |
| 3 | 10.00 | 9.58 | 95.81 | 9.88 |
| 50.00 | 41.15 | 82.31 | 6.64 | |
| 100.00 | 83.71 | 83.71 | 4.22 |
| Method | Sample | Liner Ranges (ng/mL) | LOD (ng/mL) | Recovery (%) | Reference |
|---|---|---|---|---|---|
| HPLC analysis | Milk | 1 × 103–30 × 103 | 260.0 | 99.50–101.20 | [36] |
| HPLC with fluorescence | chicken tissues | 10–1 × 103 | 2.5 | 79.20–93.40 | [37] |
| ELISA method | water | 0.1–10 | 0.016 | 74-105 | [38] |
| Fluorescence analysis | Fish, milk | 1–90 | 0.80 | 90.92–111.53 | [39] |
| Fluorescence analysis based on immunoassay | Milk | 13–3986 | 0.0034 | 86.44–101.30 | [8] |
| Fluorescence analysis based on time-resolved methodology | Milk | 0.5–1000 | 0.14 | 91.90–110.50 | [40] |
| Raman spectroscopy | Milk | 7.98–159.67 | 1.701 | 101.29–104.00 | [41] |
| Colorimetric chemosensor based on Fe3O4 MMIP NPs | Milk | 10–300 | 8.90 | 75.97–95.81 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Luo, L.; Li, J.; Xiong, Y.; Wang, L.; Liu, X. Colorimetric Chemosensor Based on Fe3O4 Magnetic Molecularly Imprinted Nanoparticles for Highly Selective and Sensitive Detection of Norfloxacin in Milk. Foods 2023, 12, 285. https://doi.org/10.3390/foods12020285
Li M, Luo L, Li J, Xiong Y, Wang L, Liu X. Colorimetric Chemosensor Based on Fe3O4 Magnetic Molecularly Imprinted Nanoparticles for Highly Selective and Sensitive Detection of Norfloxacin in Milk. Foods. 2023; 12(2):285. https://doi.org/10.3390/foods12020285
Chicago/Turabian StyleLi, Maiquan, Lingli Luo, Jiayin Li, Yingzi Xiong, Ling Wang, and Xia Liu. 2023. "Colorimetric Chemosensor Based on Fe3O4 Magnetic Molecularly Imprinted Nanoparticles for Highly Selective and Sensitive Detection of Norfloxacin in Milk" Foods 12, no. 2: 285. https://doi.org/10.3390/foods12020285
APA StyleLi, M., Luo, L., Li, J., Xiong, Y., Wang, L., & Liu, X. (2023). Colorimetric Chemosensor Based on Fe3O4 Magnetic Molecularly Imprinted Nanoparticles for Highly Selective and Sensitive Detection of Norfloxacin in Milk. Foods, 12(2), 285. https://doi.org/10.3390/foods12020285