Antifungal Activity of Mycogenic Silver Nanoparticles on Clinical Yeasts and Phytopathogens
Abstract
:1. Introduction
2. Results and Discussion
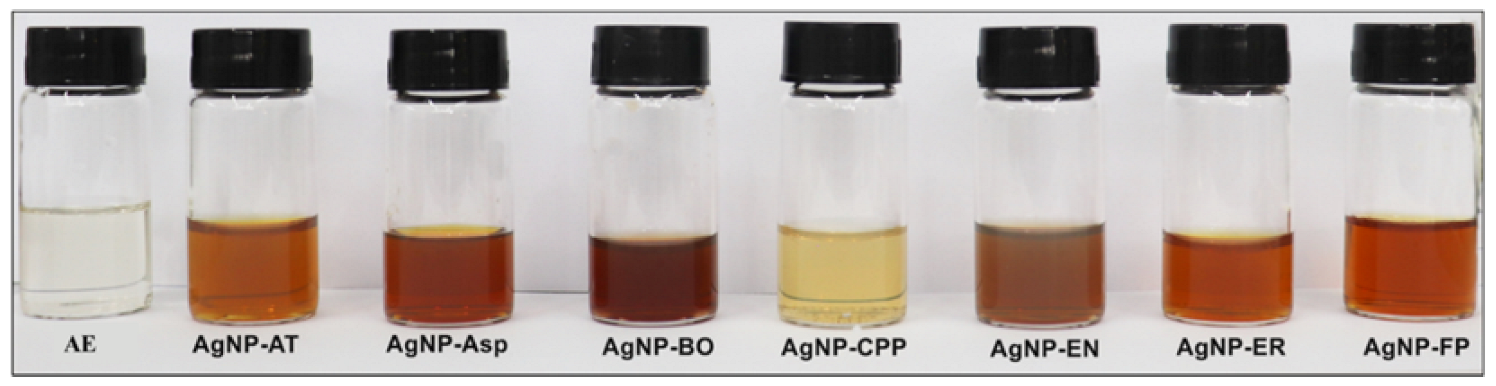
2.1. Physicochemical Characterization of the AgNPs
2.2. Antifungal Activity
3. Materials and Methods
3.1. Fungal Strains
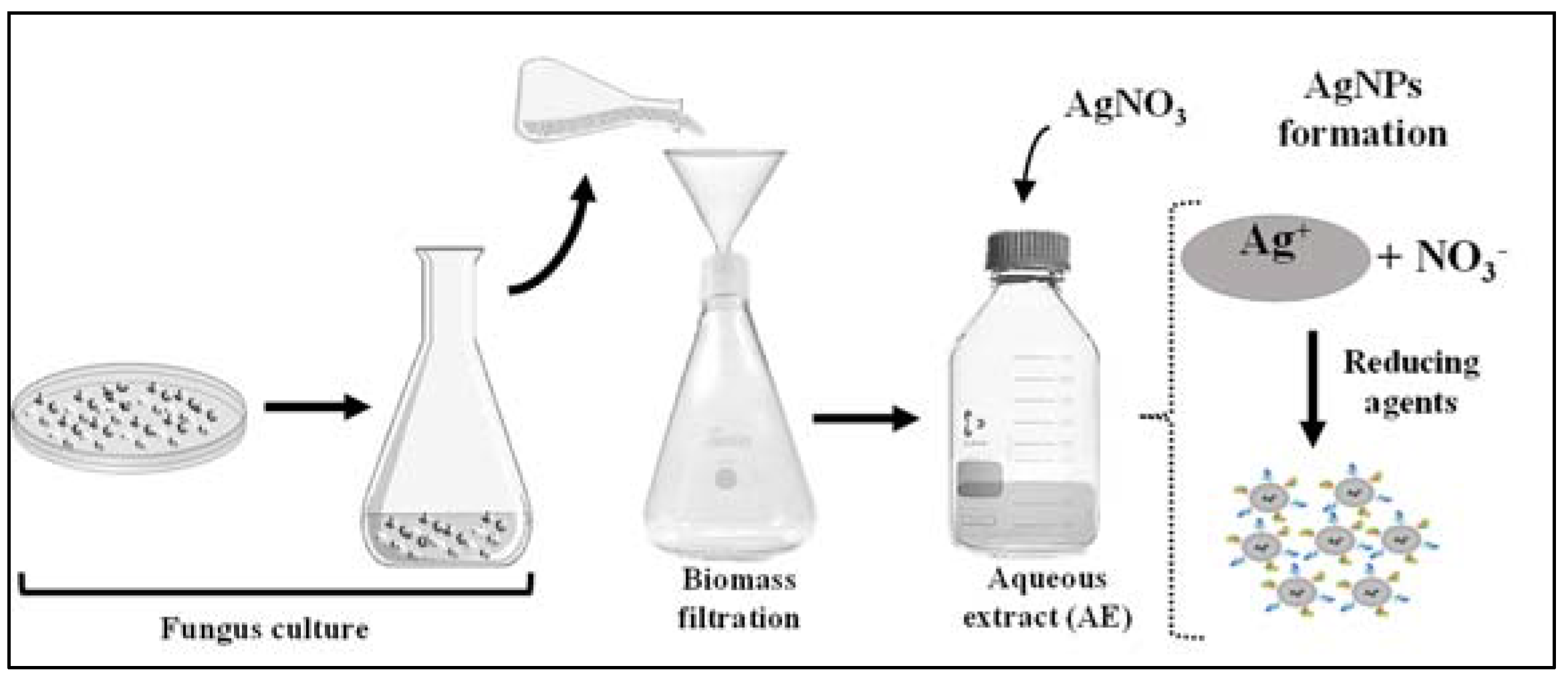
3.2. Biosynthesis and Physicochemical Analysis of the AgNPs
3.3. Antifungal Activity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W.; Perlin, D.S.; Muldoon, E.G.; Colombo, A.L.; Chakrabarti, A.; Richardson, M.D.; Sorrell, T.C. Delivering on antimicrobial resistance agenda not possible without improving fungal diagnostic capabilities. Emerg. Infect. Dis. 2017, 23, 177–183. [Google Scholar] [CrossRef]
- Cui, X.; Wang, L.; Lü, Y.; Yue, C. Development and research progress of anti-drug resistant fungal drugs. J. Infect. Public Health 2022, 15, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Baek, K.H. Control of phytopathogens using sustainable biogenic nanomaterials: Recent perspectives, ecological safety, and challenging gaps. J. Clean. Prod. 2022, 372, 133–729. [Google Scholar] [CrossRef]
- Pradhan, A.; Ghosh, S.; Sahoo, D.; Jha, G. Fungal effectors, the double edge sword of phytopathogens. Curr. Genet. 2020, 67, 27–40. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Guillot, J.; Bond, R. Malassezia yeasts in veterinary dermatology, An updated overview. Front. Cell. Infect. Microbiol. 2020, 10, 79. [Google Scholar] [CrossRef]
- Wang, Y.; Pruitt, R.N.; Nürnberger, T.; Wang, Y. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Zahra, T.; Raja, H.; Amin, M.; Dilshad, E.; Naveed, M.; Ahmed, I. Major natural sinks for harboring microorganisms with altered antibiotic resistance versus major human contributing sources of antibiotic resistance: A detailed insight. In Advances in Environmental Pollution Research Series, Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 70–98. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Magalhães, S.; Martins, A.; Lacerda, S.; Filipe, R.; Prista-Leão, B.; Pinheiro, D.; Silva-Pinto, A.; Santos, L. Candidemia in a Portuguese tertiary care hospital: Analysis of a 2-year period. J. Mycol. Med. 2019, 29, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.; Araújo, D.; Ribeiro, E.; Henriques, M.; Silva, S. Candida albicans adaptation on simulated human body fluids under different pH. Microorganisms 2020, 8, 511. [Google Scholar] [CrossRef] [Green Version]
- Cortés, J.A.; Ruiz, J.F.; Melgarejo-Moreno, L.N.; Lemos, E.V. Candidemia en Colombia. Biomédica 2020, 40, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichne, L.; Reboli, A.C.; Schuster, M.G.; Vasquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 2016, 62, 1–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.J.; Dobson, D.W. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Lanubile, A.; Giorni, P.; Bertuzz, T.; Marocco, A.; Battilani, P. Fusarium verticillioides and Aspergillus flavus co-occurrence influences plant and fungal transcriptional profiles in maize kernels and in vitro. Toxins 2021, 13, 680. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, M.; Das, D.B.; Salem, Z.A.; Beherei, H.H. Nanomaterials for Biomedical Applications: Production, Characterisations, Recent Trends and Difficulties. Molecules 2021, 26, 1077. [Google Scholar] [CrossRef]
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef]
- Zafar, N.; Madni, A.; Khalid, A.; Khan, T.; Kousar, R.; Naz, S.S.; Wahid, F. Pharmaceutical and biomedical applications of green synthesized metal and metal oxide nanoparticles. Curr. Pharm. Des. 2020, 26, 5844–5865. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Trzcińska-Wencel, J.; Wypij, M.; Bonde, S.; Yadav, A.; Kratošová, G.; Golińska, P. Biogenic silver nanoparticles: What we know and what do we need to know? Nanomaterails 2021, 11, 2901. [Google Scholar] [CrossRef]
- Lin, Y.; Betts, H.; Keller, S.; Cariou, K.; Gasser, G. Recent developments of metal-based compounds against fungal pathogens. Chem. Soc. Rev. 2021, 50, 10346–10402. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; Lima, R. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [Green Version]
- Ballottin, D.; Fulaz, S.; Souza, M.L.; Corio, P.; Rodrigues, A.G.; Souza, A.O.; Gaspari, P.M.; Gomes, A.F.; Gozzo, F.; Tasic, L. Elucidating protein involvement in the stabilization of the biogenic silver nanoparticles. Nano Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.O. Overview of nanomaterials and cellular interactions. Biointerface Res. Appl. Chem. 2022, 13, 367. [Google Scholar] [CrossRef]
- Jackson, T.C.; Patani, B.O.; Israel, M.B. Nanomaterials and cell interactions: A review. J. Biomater. Nanobiotechnol. 2017, 8, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Barabadi, H.; Tajani, B.; Moradi, M.; Damavandi, K.K.; Meena, R.; Honary, S.; Mahjoub, M.A.; Saravanan, M. Penicillium family as emerging nanofactory for biosynthesis of green nanomaterials, A journey into the world of microorganisms. J. Clust. Sci. 2019, 30, 843–856. [Google Scholar] [CrossRef]
- Evanoff, D.D., Jr.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. Chemphyschem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.L.; Bueno, C.C.; Da Róz, A.L.; Ziemath, E.C.; Oliveira, O.N. Theoretical models for surface forces and adhesion and their measurement using atomic force microscopy. Int. J. Mol. Sci. 2012, 13, 12773–12856. [Google Scholar] [CrossRef]
- Prakash, S.; Soni, N. Factors affecting the geometry of silver nanoparticles synthesis in Chrysosporium Tropicum and Fusarium Oxysporum. Am. J. Nanotech. 2011, 2, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.G.; Ping, L.Y.; Marcato, P.D.; Alves, O.L.; Silva, M.C.P.; Ruiz, R.C.; Melo, I.S.; Tasic, L.; De Souza, A.O. Biogenic antimicrobial silver nanoparticles produced by fungi. Appl. Microbiol. Biotechnol. 2013, 97, 775–782. [Google Scholar] [CrossRef]
- Shariq, A.M.; Soundhararajan, R.; Akther, T.; Kashif, M.; Khan, J.; Waseem, M.; Srinivasan, H. Biogenic AgNPs synthesized via endophytic bacteria and its biological applications. Environ. Sci. Pollut. Res. Int. 2019, 26, 26939–26946. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.G.; Ruiz, R.C.; Selari, P.J.R.G.; Araújo, W.L.; De Souza, A.O. Anti-Biofilm action of biological silver nanoparticles produced by Aspergillus tubingensis and antimicrobial activity of fabrics carrying it. Biointerface Res. Appl. Chem. 2021, 11, 14764–14774. [Google Scholar] [CrossRef]
- Rodrigues, A.G.; Romano, O.G.P.J.; Ottoni, C.A.; Ruiz, R.C.; Morgano, M.A.; Araújo, W.L.; Melo, I.S.; De Souza, A.O. Functional textiles impregnated with biogenic silver nanoparticles from Bionectria ochroleuca and its antimicrobial activity. Biomed. Microdevices 2019, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Majeed, R.A.; Shahid, A.A.; Ashfaq, M.; Saleem, M.Z.; Haider, M.S. First report of Curvularia lunata causing brown leaf spots of rice in Punjab, Pakistan. Plant Dis. 2015, 100, 219. [Google Scholar] [CrossRef]
- Bansal, Y.; Chander, J.; Kaistha, N.; Singla, N.; Sood, S.; Van Diepeningen, A.D. Fusarium sacchari, a cause of mycotic keratitis among sugarcane farmers—A series of four cases from North India. Mycoses 2016, 59, 705–709. [Google Scholar] [CrossRef]
- Bleackley, M.R.; Samuel, M.; Garcia-Ceron, D.; McKenna, J.A.; Lowe, R.G.T.; Pathan, M.; Zhao, K.; Ang, C.-S.; Mathivanan, S.; Anderson, M.A. Extracellular vesicles from the cotton pathogen Fusarium oxysporum f. sp. vasinfectum induce a phytotoxic response in plants. Front. Plant Sci. 2020, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, Y.; Ha, A.; Kim, J.I.; Park, A.R.; Yu, N.H.; Son, H.; Choi, G.J.; Park, H.W.; Lee, C.W.; et al. Chemosensitization of Fusarium graminearum to chemical fungicides using cyclic lipopeptides produced by Bacillus amyloliquefaciens strain JCK-12. Front. Plant Sci. 2017, 8, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Huang, J.; Luo, X.; Yan, J. Agriculture: Science and technology safeguard sustainability. Nat. Sci. Rev. 2019, 6, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Zwar, I.P.; Trotta, C.V.; Ziotti, A.B.S.; Lima, N.M.; Araújo, W.L.; De Melo, I.S.; Ottoni, C.A.; De Souza, A.O. Biosynthesis of silver nanoparticles using actinomycetes, phytotoxicity on rice seeds, and potential application in the biocontrol of phytopathogens. J. Basic Microbiol. 2023, 6, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Cespedes, C.L. Antifungal activity of alkanols: Inhibition of growth of spoilage yeasts. Phytochem. Rev. 2013, 12, 961–977. [Google Scholar] [CrossRef]
- Stuart, R.M.; Romão, A.S.; Pizzirani-Kleiner, A.A.; Azevedo, J.L.; Araújo, W.L. Culturable endophytic filamentous fungi from leaves of transgenic imidazolinone-tolerant sugarcane and its non-transgenic isolines. Arch. Microbiol. 2010, 192, 307–313. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agent. Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI standard M38. CLSI: Wayne, PA, USA, 2017; Available online: https://clsi.org/media/1894/m38ed3_sample.pdf (accessed on 27 November 2022).
- Clinical and Laboratory Standards Institute (CLSI). Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, 3rd ed.; CLSI Guideline M44. CLSI: Wayne, PA, USA, 2018; Available online: https://clsi.org/standards/products/microbiology/documents/m44/ (accessed on 27 November 2022).
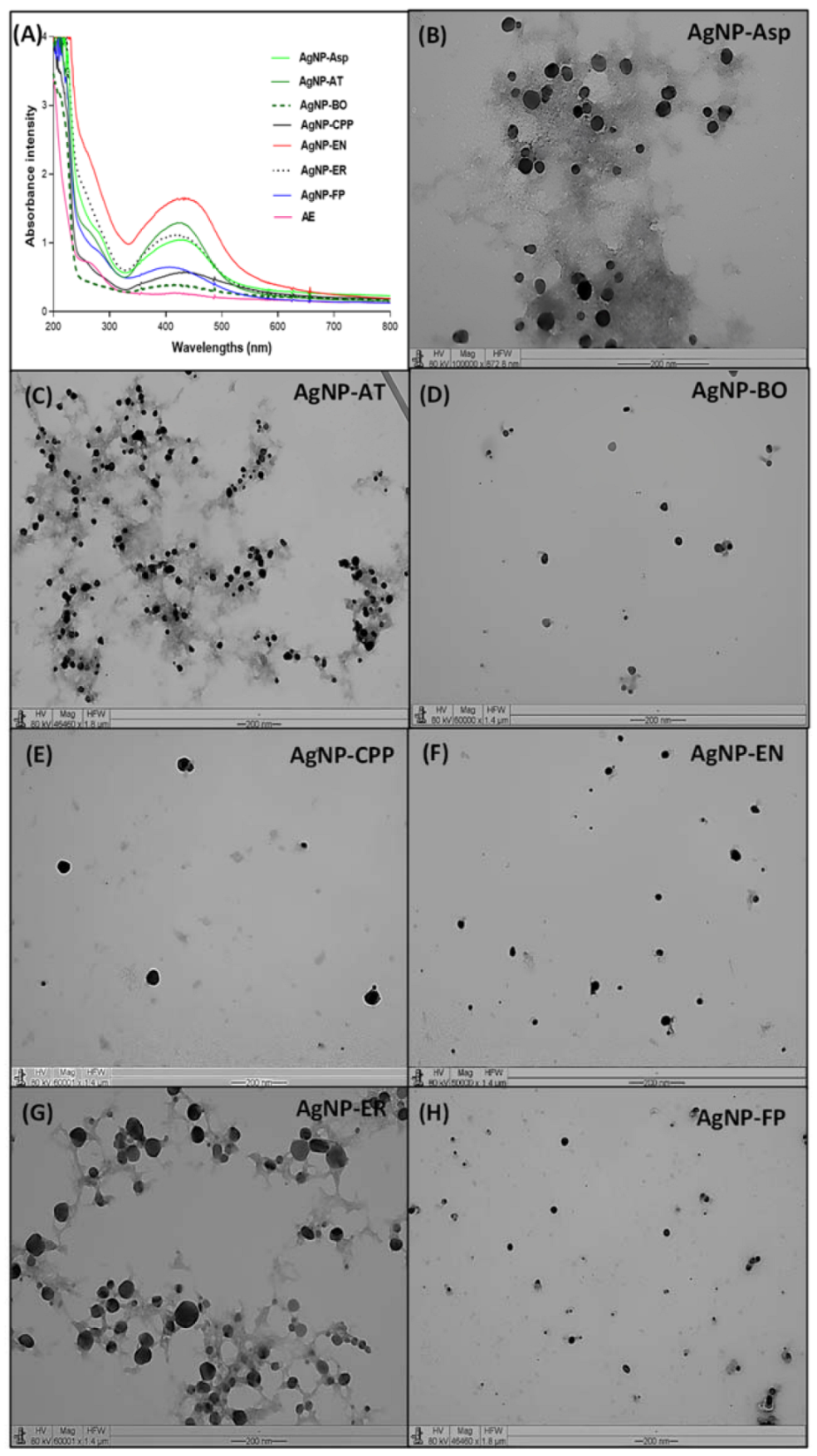
| AgNPs | Size (DLS) * | Size (TEM) * | PDI | Zeta Potential | pH |
|---|---|---|---|---|---|
| AgNP-Asp | 44.9 ± 4.1 | 33.3 ± 2.7 | 0.343 | −1.04 | 6.0 |
| AgNP-AT | 43.4 ± 3.3 | 25.0 ± 6.5 | 0.080 | −22.26 | 7.0 |
| AgNP-BO | 120.6 ± 3.5 | 21.8 ± 4.1 | 0.257 | −2.19 | 7.5 |
| AgNP-CPP | 87.1 ± 3.4 | 35.8 ± 5.16 | 0.334 | −1.59 | 5.0 |
| AgNP-EN | 71.2 ± 6.7 | 28.0 ± 6.31 | 0.404 | −33.28 | 5.0 |
| AgNP-ER | 86.4 ± 6.4 | 22.1 ± 2.9 | 0.230 | −14.40 | 4.5 |
| AgNP-FP | 59.6 ± 2.1 | 26.7 ± 5.3 | 0.199 | −15.33 | 6.0 |
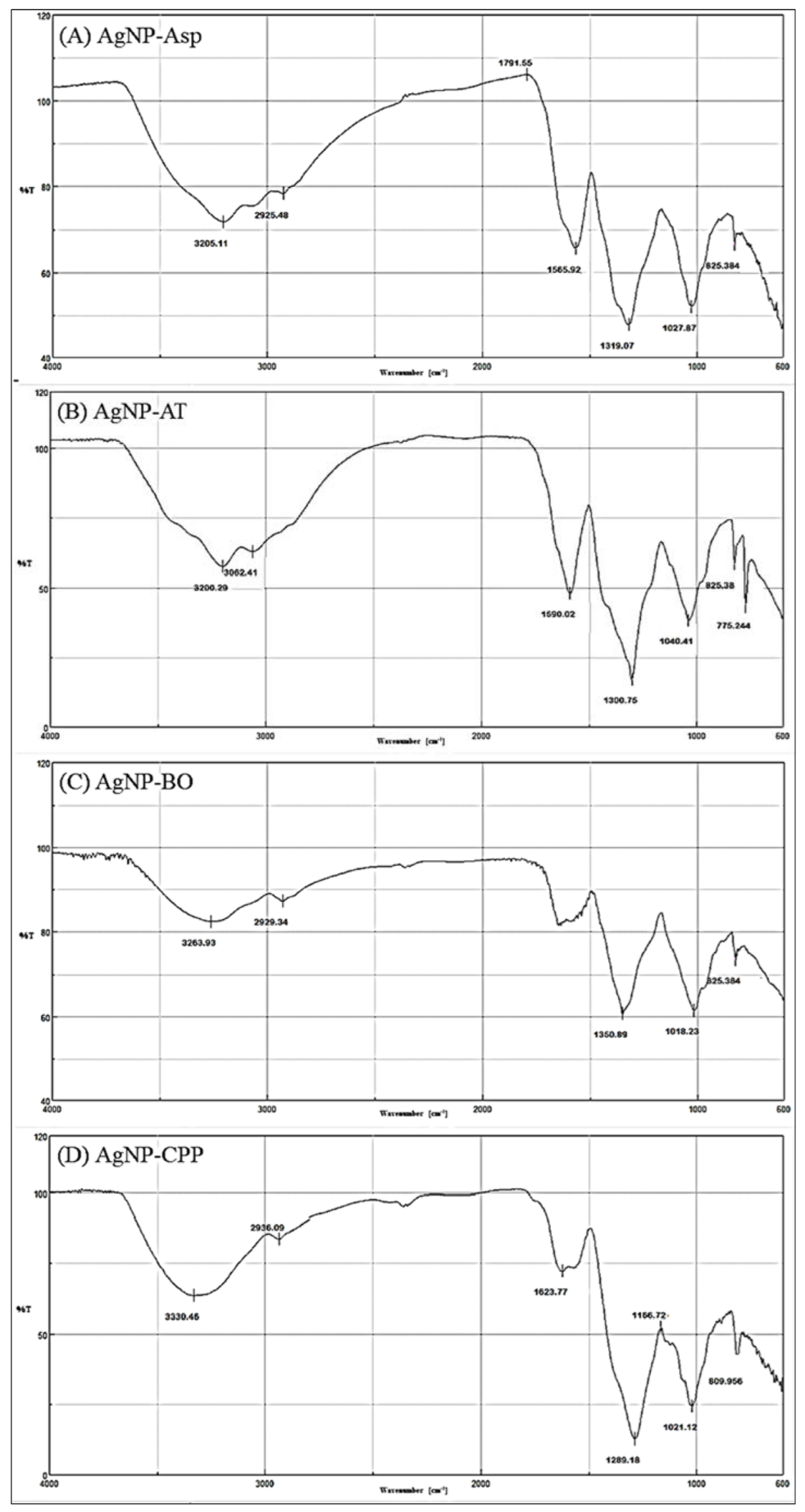
| AgNPs | ≈Vibrational Frequencies (cm−1) | Functional Groups |
|---|---|---|
| AgNP-Asp | 825–860 | Aromatic ring (2 adjacent H) |
| 1020–1170 | C-O (ether) | |
| 1320 | C-N (aromatic) | |
| 1560–1490 | N-H | |
| 1790 | C=O of acyl chloride | |
| 2925 | Aliphatic C-H | |
| 3200 | O-H (chelate) | |
| AgNP-AT | 840–775 | R2C=CHR (C-H out of plane) |
| 1165–1040 | C-O (ether) | |
| 1300 | C-O (carboxyl) | |
| 1590–1500 2927 | C=C (aromatic) Aliphatic C-H | |
| 3062 | C-H (aromatic) | |
| 3200 | O-H (chelate) | |
| AgNP-BO | 825 | Aromatic ring (2 adjacent H) |
| 1030–1018 | C-O (ether) | |
| 1350–1300 | C-O (ester) | |
| 1648 | C=O (amides) | |
| 2929–2900 | Aliphatic C-H | |
| 3400–3263 | O-H (chelate) | |
| AgNP-CPP | 840–790 | R2C=CHR (C-H out of plane) |
| 1160–1021 | C-O (ether) | |
| 1289 | C-N (aromatic) | |
| 1490 | N-H | |
| 1625 | C=C (aromatic) | |
| 2936 | O-H (chelate) | |
| 3330 | Free NH (secondary amine) | |
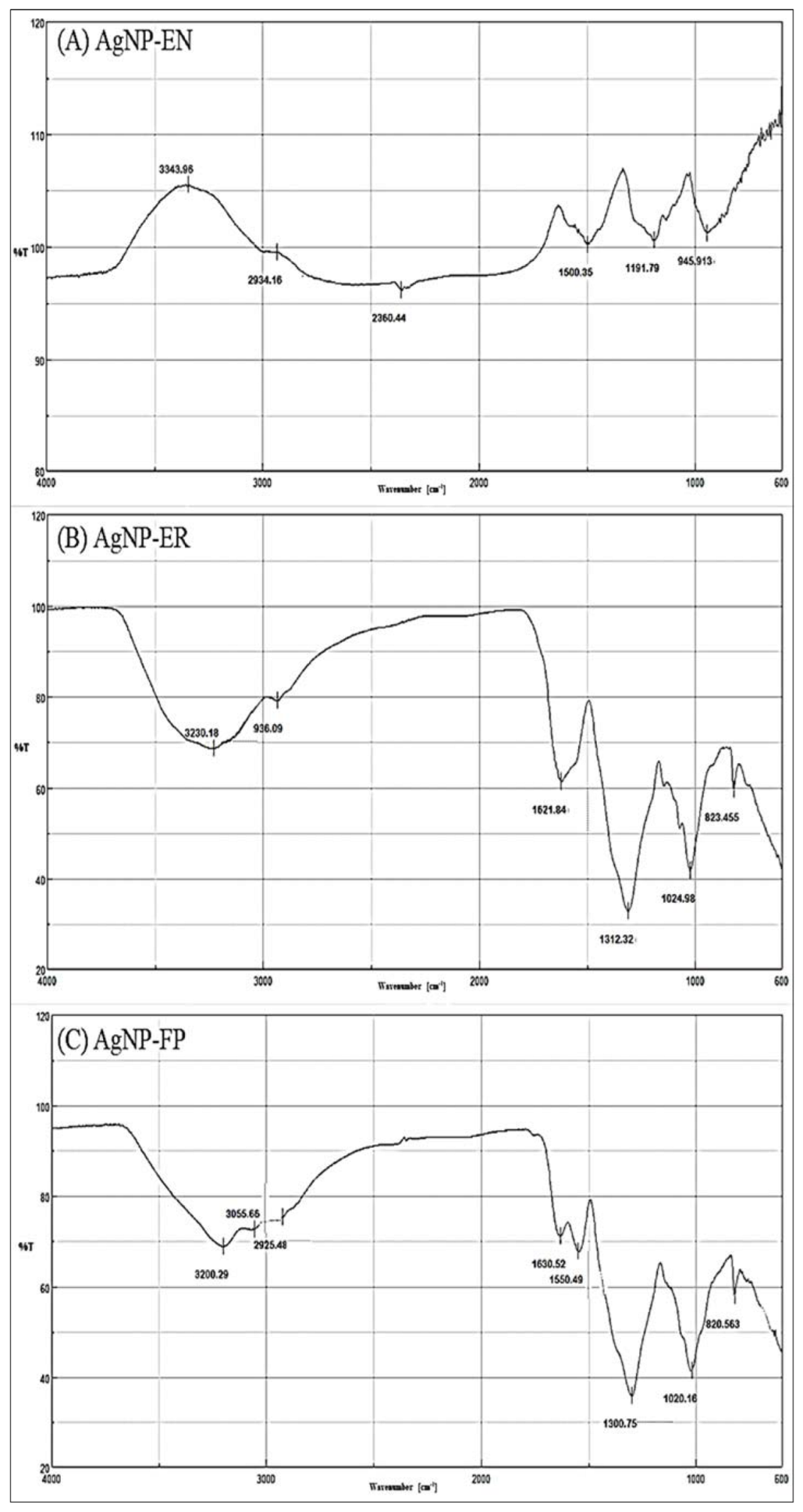
| AgNP-EN | 945 | RCH=CH2 |
| 1190–1026 | C-O (ethers) | |
| 1336 | SO2 (sulfone) | |
| 1500 | C=C (aromatic) | |
| 2360 | CO2 | |
| 2934 | O-H (chelate) | |
| 3343 | Free NH (secondary amines) | |
| AgNP-ER | 820–798 | R2C=CHR |
| 1170–1025 | C-O (ether) | |
| 1312 | SO2 (sulfone) | |
| 1493 | N-H | |
| 1621 | C=C (aromatic) | |
| 2936 | Aliphatic C-H | |
| 3230 | O-H (chelate) | |
| AgNP-FP | 835–820 | Aromatic ring |
| 1168–1020 | C-O (ether) | |
| 1300 | C-O (ester) | |
| 1490 | N-H | |
| 1550 | NH2 | |
| 1630 | C=O (amides) | |
| 1790 | C=O (acyl chloride) | |
| 2925 | C-H (aliphatic) | |
| 3055 | C-H (alkene) | |
| 3200 | O-H (chelate) |
| Yeasts | AgNP-Asp | AgNP-AT | AgNP-BO | AgNP-CPP | AgNP-EN | AgNP-ER | AgNP-FP | AMB |
|---|---|---|---|---|---|---|---|---|
| C. albicans ATCC 36802 | 2.5 | 20 | 5 | >40 | 1.25 | 20 | 10 | 32 |
| C. albicans IOC 4556 | 10 | 40 | 20 | >40 | 20 | 20 | 20 | >32 |
| C. albicans IOC 4525 | 2.5 | 20 | 20 | >40 | 10 | 20 | 20 | >32 |
| C. albicans IOC 4558 | 10 | 40 | 20 | >40 | 20 | 10 | 10 | >32 |
| C. glabrata IOC 4565 | 5 | 5 | 2.5 | 1.25 | 2.5 | 5 | >40 | >32 |
| C. krusei IOC 4559 | 1.25 | 2.5 | 1.25 | 1.25 | 1.25 | 5 | 1.25 | >32 |
| C. guillermondii IOC 4557 | 1.25 | 2.5 | 1.25 | 1.25 | 1.25 | 2.5 | 1.25 | 32 |
| C. parapsilosis IOC 4564 | 40 | 20 | 10 | 2.5 | 10 | 10 | >40 | >32 |
| C. tropicalis IOC 4560 | >40 | 20 | 20 | 5 | 20 | 10 | 5 | >32 |
| Phytopathogens | AgNP-Asp | AgNP-AT | AgNP-BO | AgNP-CPP | AgNP-EN | AgNP-FP | AgNP-ER | AMB | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | FC | MIC | FC | MIC | FC | MIC | FC | MIC | FC | MIC | FC | MIC | FC | MIC | FC | |
| C. lunata | 8 | 8 | 4 | 4 | 16 | 16 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| F. sacchari | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 | 16 | 16 |
| F. subglutinans | 60 | 60 | 60 | 60 | >250 | >250 | 120 | 120 | 120 | 120 | 60 | 60 | 120 | 120 | 16 | 16 |
| F. oxysporum (WLA-FP07) | 120 | 120 | 120 | 120 | >250 | >250 | 250 | 250 | 120 | 120 | 120 | 120 | 120 | 120 | >32 | >32 |
| F. oxysporum (WLA-FP25) | 30 | 30 | 30 | 30 | 30 | 30 | 120 | 120 | 60 | 60 | 30 | 30 | 30 | 30 | 16 | 16 |
| F. verticillioides | 16 | 16 | 8 | 8 | 16 | 16 | 8 | 8 | 60 | 60 | 16 | 16 | 8 | 8 | 16 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, L.G.; Roque, G.S.C.; Conrado, R.; De Souza, A.O. Antifungal Activity of Mycogenic Silver Nanoparticles on Clinical Yeasts and Phytopathogens. Antibiotics 2023, 12, 91. https://doi.org/10.3390/antibiotics12010091
Ribeiro LG, Roque GSC, Conrado R, De Souza AO. Antifungal Activity of Mycogenic Silver Nanoparticles on Clinical Yeasts and Phytopathogens. Antibiotics. 2023; 12(1):91. https://doi.org/10.3390/antibiotics12010091
Chicago/Turabian StyleRibeiro, Luiz Gustavo, Gabriella Sales Calaço Roque, Rafael Conrado, and Ana Olívia De Souza. 2023. "Antifungal Activity of Mycogenic Silver Nanoparticles on Clinical Yeasts and Phytopathogens" Antibiotics 12, no. 1: 91. https://doi.org/10.3390/antibiotics12010091







