Effects of Maturity and Processing on the Volatile Components, Phytochemical Profiles and Antioxidant Activity of Lotus (Nelumbo nucifera) Leaf
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Lotus-Leaf Tea and Aqueous Extract
2.3. Determination of Volatile Compounds
2.4. Determination of Total Phenolic and Flavonoid Content
2.5. Determination of Antioxidant Activity
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ABTS+ Radical Scavenging Activity
2.5.3. Ferric Reducing Power
2.6. Determination of Phenolic Compounds by UHPLC-MS/MS
2.7. Statistical Analysis
3. Results and Discussion
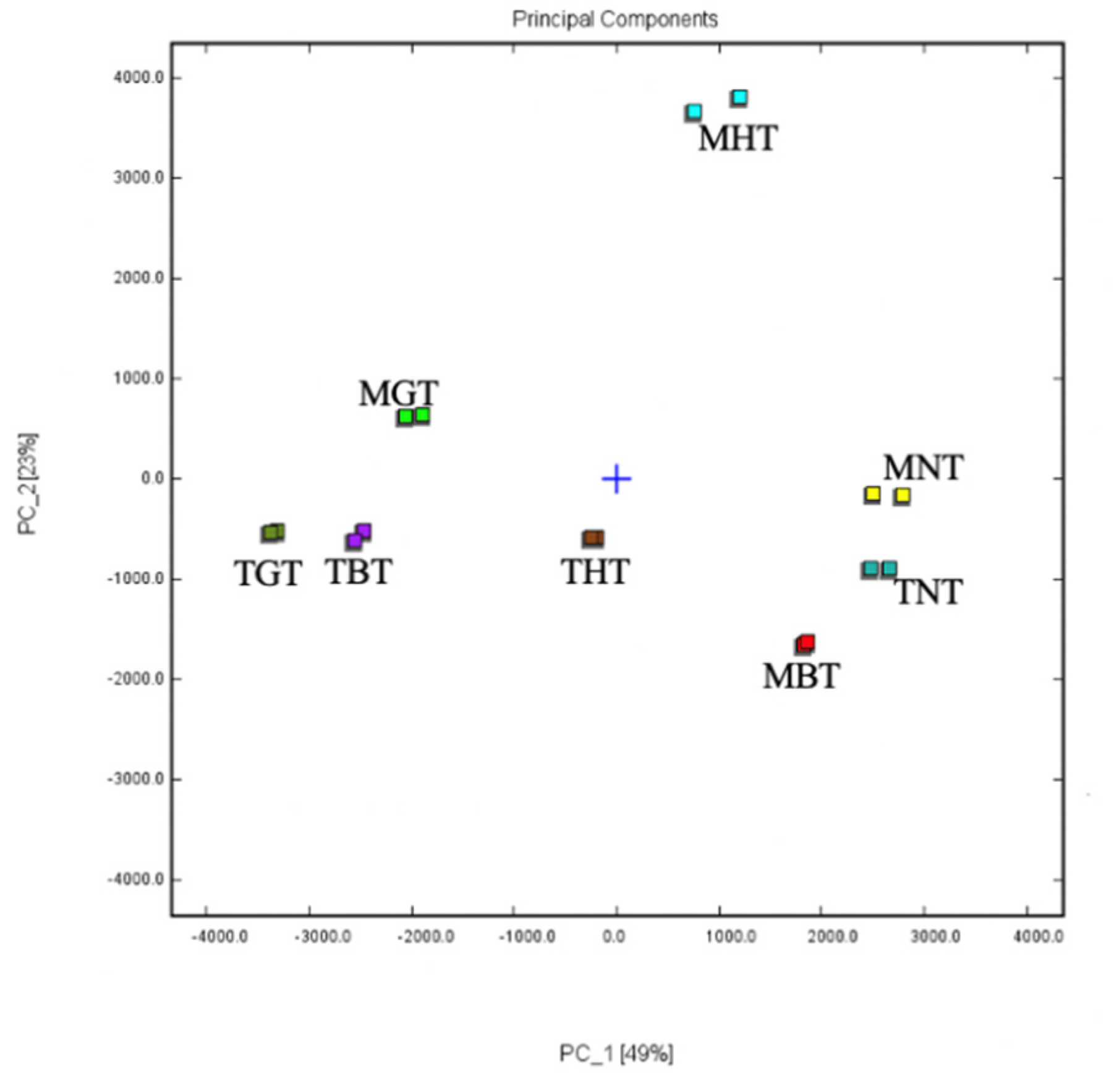
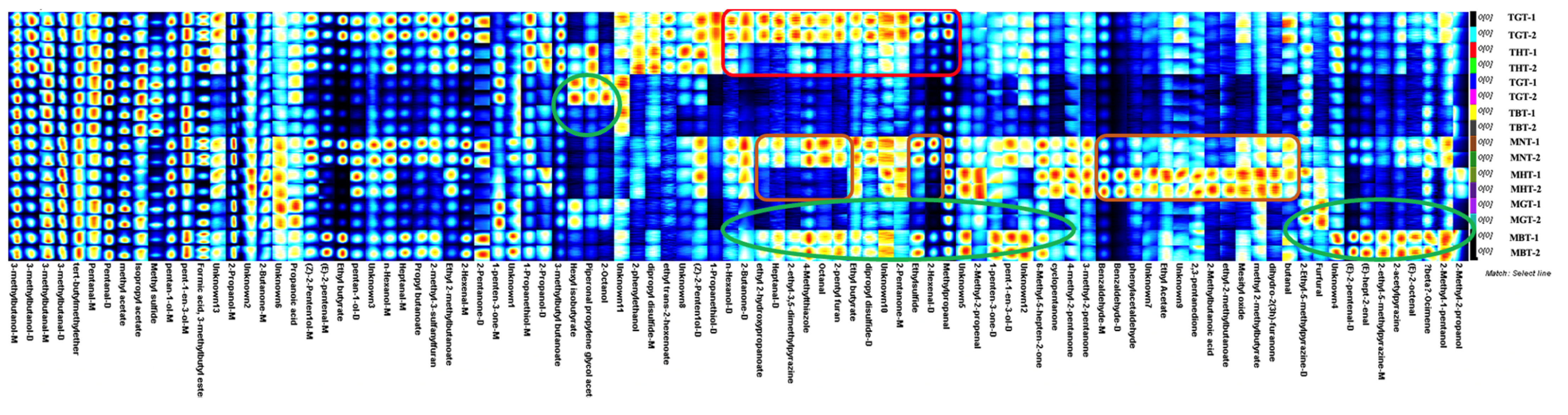
3.1. Volatile Compounds of Lotus-Leaf Tea by GC-IMS Analysis
3.2. Determination of TPC, TFC and Antioxidant Capacity
3.2.1. Analysis of TPC and TFC
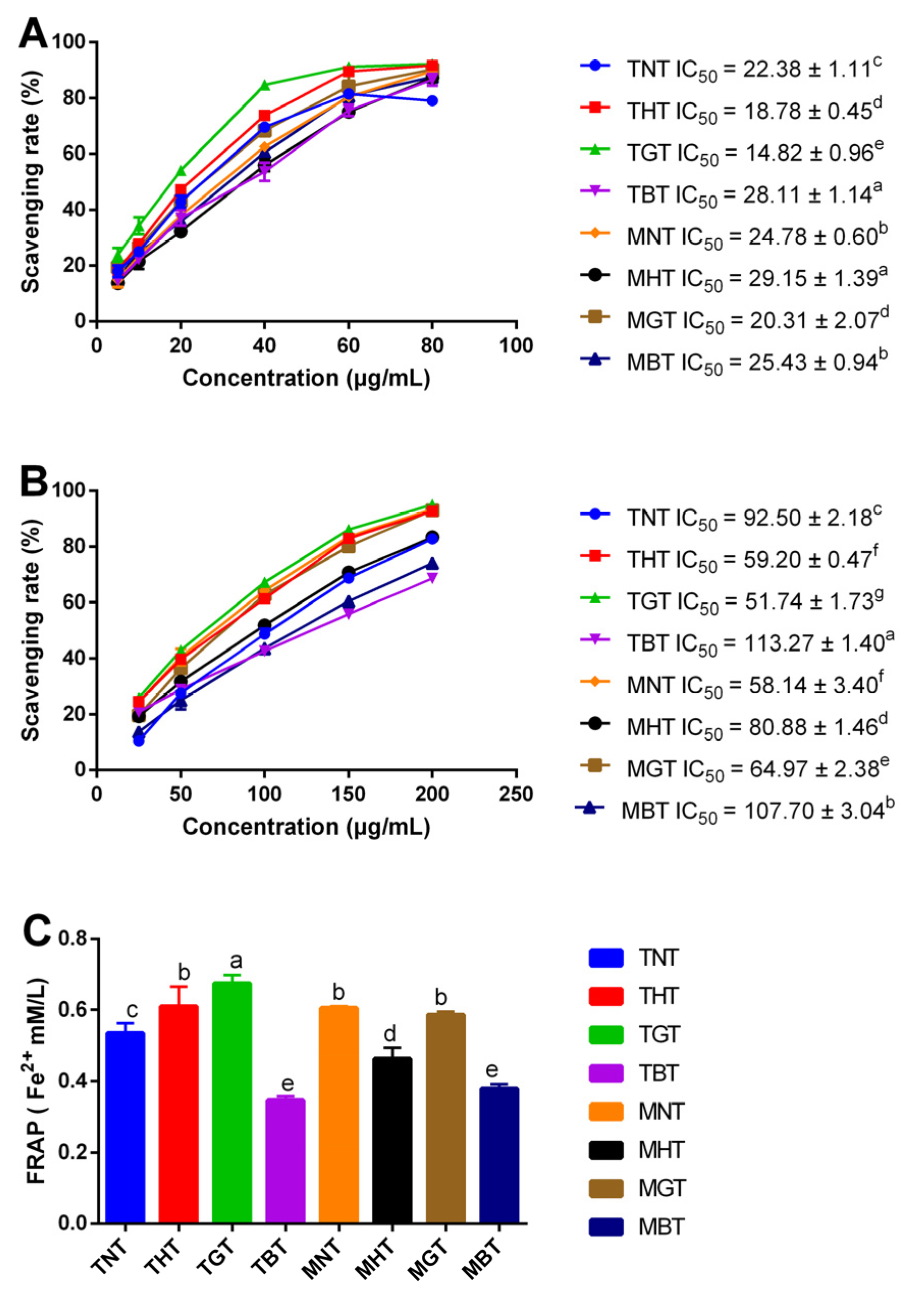
3.2.2. Analysis of DPPH, ABTS+ Radical Scavenging Activities and Ferric Reducing Power
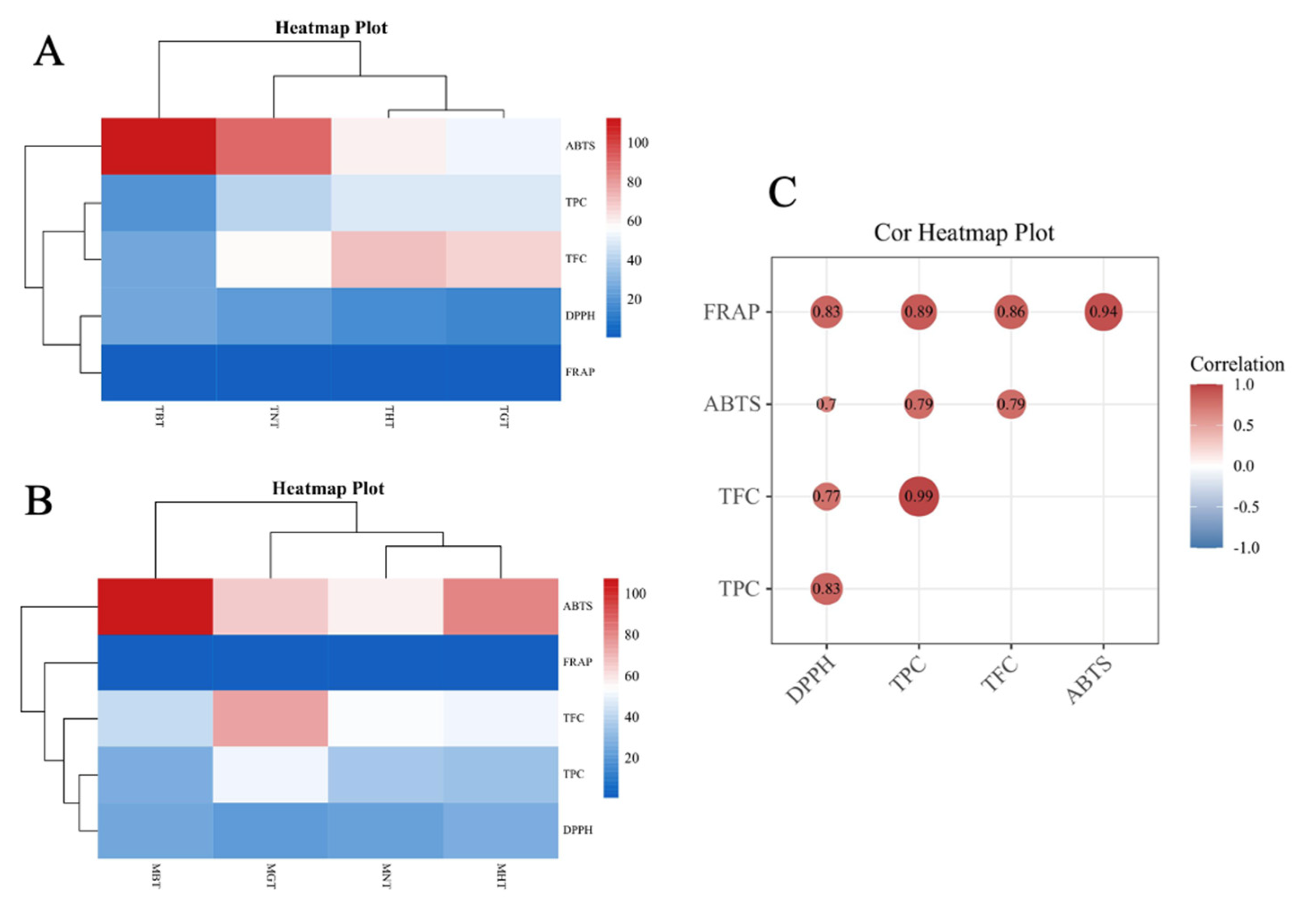
3.2.3. Cluster and Correlation Analysis between TPC, TFC and the Antioxidant Capacity
3.3. Identification of Phenolic Compounds in Lotus-Leaf Tea
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Qiu, L.; Deng, H.; Wang, J.; Yao, L.; Deng, L. Ultrahigh surface area carbon nanosheets derived from lotus leaf with super capacities for capacitive deionization and dye adsorption. Appl. Surf. Sci. 2020, 524, 146485. [Google Scholar] [CrossRef]
- Guo, H.B. Cultivation of lotus (Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China. Genet. Resour. Crop. Ev. 2009, 56, 323–330. [Google Scholar] [CrossRef]
- Liu, E.; Tsuboi, H.; Ikegami, S.; Kamiyama, T.; Asami, Y.; Ye, L.; Oda, M.; Ji, Z.-S. Effects of Nelumbo nucifera Leaf Extract on Obesity. Plant Foods Hum. Nut. 2021, 76, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Feng, X.; Tao, A. Explore the Lipid-Lowering and Weight-Reducing Mechanism of Lotus Leaf Based on Network Pharmacology and Molecular Docking. Evid. Based Complement Alternat. Med. 2021, 2021, 1464027. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Wang, X.; Sun, F.; Fan, Y.; Liu, X.; Li, H.; Deng, Z. Hemostatic action of lotus leaf charcoal is probably due to transformation of flavonol aglycons from flavonol glycosides in traditional Chinses medicine. J. Ethnopharmacol. 2020, 249, 112364. [Google Scholar] [CrossRef]
- Kim, S.; Hong, K.-B.; Jo, K.; Suh, H.J. Quercetin-3-O-glucuronide in the Ethanol Extract of Lotus Leaf (Nelumbo nucifera) Enhances Sleep Quantity and Quality in a Rodent Model via a GABAergic Mechanism. Molecules 2021, 26, 3023. [Google Scholar] [CrossRef]
- Ohara, K.; Wakabayashi, H.; Taniguchi, Y.; Shindo, K.; Yajima, H.; Yoshida, A. Quercetin-3-O-glucuronide induces ABCA1 expression by LXRα activation in murine macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 929–934. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Sritalahareuthai, V.; Promyos, N.; Thangsiri, S.; Pruesapan, K.; Srinuanchai, W.; Nuchuchua, O.; Siriwan, D.; On-nom, N.; Suttisansanee, U. The Effect of Sacred Lotus (Nelumbo nucifera) and Its Mixtures on Phenolic Profiles, Antioxidant Activities, and Inhibitions of the Key Enzymes Relevant to Alzheimer’s Disease. Molecules 2020, 25, 3713. [Google Scholar] [CrossRef]
- Jia, X.B.; Zhang, Q.; Xu, L.; Yao, W.J.; Wei, L. Lotus leaf flavonoids induce apoptosis of human lung cancer A549 cells through the ROS/p38 MAPK pathway. Biol. Res. 2021, 54, 7. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.-Y.; Li, X.-W.; Yang, T.; Qi, L.-W. Enrichment and separation of quercetin-3-O-β-d-glucuronide from lotus leaves (Nelumbo nucifera Gaertn.) and evaluation of its anti-inflammatory effect. J. Chromatogr. B 2017, 1040, 186–191. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, W.; Jin, Q.; Zhu, Y.; Chen, R.; Tian, Q.; Yan, N.; Guo, L. The effects of dietary supplementation with lotus leaf extract on the immune response and intestinal microbiota composition of broiler chickens. Poult. Sci. 2021, 100, 100925. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Li, N.; Chen, M.; Yuan, Y.; He, S.; Wang, Y.; Wu, Q.; Li, L.; Yang, H.; Zeng, Q. Effects of in vitro digestion on the composition of flavonoids and antioxidant activities of the lotus leaf at different growth stages. Int. J. Food Sci. Technol. 2018, 53, 1631–1639. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Dong, L.; Wu, Q.; Li, L.; Yang, H.; Zhang, M.; Su, D. Effects of thermal processing methods and simulated digestion on the phenolic content and antioxidant activity of lotus leaves. J. Food Process. Pres. 2019, 43, e13869. [Google Scholar] [CrossRef]
- Showkat, Q.A.; Rather, J.A.; Jabeen, A.; Dar, B.N.; Makroo, H.A.; Majid, D. Bioactive components, physicochemical and starch characteristics of different parts of lotus (Nelumbo nucifera Gaertn.) plant: A review. Int. J. Food Sci. Technol. 2021, 56, 2205–2214. [Google Scholar] [CrossRef]
- Choe, J.-H.; Jang, A.; Lee, E.-S.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Lee, M.-A.; Shim, S.-Y.; Kim, C.-J. Oxidative and color stability of cooked ground pork containing lotus leaf (Nelumbo nucifera) and barley leaf (Hordeum vulgare) powder during refrigerated storage. Meat Sci. 2011, 87, 12–18. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, Y.; Zeng, M.; Wang, Z.; Qin, F.; Wang, Y.; Chen, J.; He, Z. Lotus (Nelumbo nucifera Gaertn.) leaf: A narrative review of its Phytoconstituents, health benefits and food industry applications. Trends Food Sci. Technol. 2021, 112, 631–650. [Google Scholar] [CrossRef]
- Limwachiranon, J.; Huang, H.; Shi, Z.; Li, L.; Luo, Z. Lotus Flavonoids and Phenolic Acids: Health Promotion and Safe Consumption Dosages. Compr. Rev. Food Sci. Food Saf. 2018, 17, 458–471. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yao, W.; Li, B.; Qian, S.; Wei, B.; Gong, S.; Wang, J.; Liu, M.; Wei, M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020, 52, 1959–1975. [Google Scholar] [CrossRef]
- Ho, C.-T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Hum. Well 2015, 4, 9–27. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.-X.; Rana, M.M.; Liu, G.-F.; Gao, M.-J.; Li, D.-X.; Wu, F.-G.; Li, X.-B.; Wan, X.-C.; Wei, S. Green tea flavour determinants and their changes over manufacturing processes. Food Chem. 2016, 212, 739–748. [Google Scholar] [CrossRef]
- Wei, Y.; Yin, X.; Wu, H.; Zhao, M.; Huang, J.; Zhang, J.; Li, T.; Ning, J. Improving the flavor of summer green tea (Camellia sinensis L.) using the yellowing process. Food Chem. 2022, 388, 132982. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qu, F.; Wang, P.; Zhao, L.; Wang, Z.; Han, Y.; Zhang, X. Characterization analysis of flavor compounds in green teas at different drying temperature. LWT-Food Sci. Technol. 2022, 161, 113394. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Liu, D.; Yang, L.; Hu, W.; Kuang, L.; Huang, Y.; Teng, J.; Liu, Y. Multi-omics and enzyme activity analysis of flavour substances formation: Major metabolic pathways alteration during Congou black tea processing. Food Chem. 2023, 403, 134263. [Google Scholar] [CrossRef] [PubMed]
- Tsurunaga, Y.; Kanou, M.; Ikeura, H.; Makino, M.; Oowatari, Y.; Tsuchiya, I. Effect of different tea manufacturing methods on the antioxidant activity, functional components, and aroma compounds of Ocimum gratissimum. LWT-Food Sci. Technol. 2022, 169, 114058. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef]
- Li, Y.; Ran, W.; He, C.; Zhou, J.; Chen, Y.; Yu, Z.; Ni, D. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chem. X 2022, 14, 100289. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, X.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes. Foods 2021, 10, 2836. [Google Scholar] [CrossRef]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 7979. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, M.C.; Deng, Y.; Yuan, H.; Jiang, Y. Insight into aroma dynamic changes during the whole manufacturing process of chestnut-like aroma green tea by combining GC-E-Nose, GC-IMS, and GC × GC-TOFMS. Food Chem. 2022, 387, 132813. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Chen, J.; Xie, J.; Shen, S.; Deng, Y.; Zhu, J.; Yuan, H.; Jiang, Y. Characterization of the key aroma compounds in black teas with different aroma types by using gas chromatography electronic nose, gas chromatography-ion mobility spectrometry, and odor activity value analysis. LWT-Food Sci. Technol. 2022, 163, 113492. [Google Scholar] [CrossRef]
- Chen, X.-M.; Ma, Z.; Kitts, D.D. Effects of processing method and age of leaves on phytochemical profiles and bioactivity of coffee leaves. Food Chem. 2018, 249, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Jo, H.; Cho, A.R.; Kim, S.-J.; Han, J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control 2013, 31, 403–409. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Y.; Huang, W.; Feng, X.; Yang, F.; Li, D. Separation, Identification, and Antioxidant Activity of Polyphenols from Lotus Seed Epicarp. Molecules 2019, 24, 4007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Wang, M.; Dong, Z.; Zhu, Y.; Shi, J.; Ma, W.; Lin, Z.; Lv, H. Volatile components and key odorants of Chinese yellow tea (Camellia sinensis). LWT-Food Sci. Technol. 2021, 146, 111512. [Google Scholar] [CrossRef]
- Shi, J.; Wu, W.; Zhang, Y.; Baldermann, S.; Peng, Q.; Wang, J.; Xu, L.; Yang, G.; Fu, J.; Lv, H.; et al. Comprehensive analysis of carotenoids constituents in purple-coloured leaves and carotenoid-derived aroma differences after processing into green, black, and white tea. LWT-Food Sci. Technol. 2023, 173, 114286. [Google Scholar] [CrossRef]
- Zuo, H.; Si, X.; Li, P.; Li, J.; Chen, Z.; Li, P.; Chen, C.; Liu, Z.; Zhao, J. Dynamic change of tea (Camellia sinensis) leaf cuticular wax in white tea processing for contribution to tea flavor formation. Food Res. Int. 2023, 163, 112182. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, Y.; Shi, J.; Wang, J.; Wang, M.; Shao, C.; Yan, H.; Lin, Z.; Lv, H. Insight into the volatile profiles of four types of dark teas obtained from the same dark raw tea material. Food Chem. 2021, 346, 128906. [Google Scholar] [CrossRef]
- Wang, H.; Hua, J.; Jiang, Y.; Yang, Y.; Wang, J.; Yuan, H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef]
- Wu, W.; Li, K.; Zhao, C.; Ran, X.; Zhang, Y.; Zhang, T. A rapid HPLC–MS/MS method for the simultaneous determination of luteolin, resveratrol and their metabolites in rat plasma and its application to pharmacokinetic interaction studies. J. Chromatogr. B 2022, 1191, 123118. [Google Scholar] [CrossRef]
- Dutschke, J.; Suchowski, M.; Pietsch, J. Simultaneous determination of selected catechins and pyrogallol in deer intoxications by HPLC-MS/MS. J. Chromatogr. B 2021, 1180, 122886. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Kuo, Y.-H.; Lin, Y.-L.; Chiang, W. Antioxidative Effect and Active Components from Leaves of Lotus (Nelumbo nucifera). J. Agric. Food Chem. 2009, 57, 6623–6629. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Yang, M.-Y.; Chan, K.-C.; Chung, P.-J.; Ou, T.-T.; Wang, C.-J. Improvement in High-Fat Diet-Induced Obesity and Body Fat Accumulation by a Nelumbo nucifera Leaf Flavonoid-Rich Extract in Mice. J. Agric. Food Chem. 2010, 58, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, H.; Simal-Gandara, J.; Cheng, K.-W.; Wang, M.; Cao, H.; Xiao, J. Investigation of new products of quercetin formed in boiling water via UPLC-Q-TOF-MS-MS analysis. Food Chem. 2022, 386, 132747. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Ban, X.; He, J.; Tong, J.; Tian, J.; Wang, Y. Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 2010, 120, 873–878. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R. Cistus incanus a promising herbal tea rich in bioactive compounds: LC–MS/MS determination of catechins, flavonols, phenolic acids and alkaloids—A comparison with Camellia sinensis, Rooibos and Hoan Ngoc herbal tea. J. Food Compos. Anal. 2018, 74, 71–81. [Google Scholar] [CrossRef]
- Goo, H.R.; Choi, J.S.; Na, D.H. Simultaneous determination of quercetin and its glycosides from the leaves of Nelumbo nucifera by reversed-phase high-performance liquid chromatography. Arch. Pharm. Res. 2009, 32, 201–206. [Google Scholar] [CrossRef]
- Ren, M.; Xu, W.; Zhang, Y.; Ni, L.; Lin, Y.; Zhang, X.; Huang, M. Qualitative and quantitative analysis of phenolic compounds by UPLC-MS/MS and biological activities of Pholidota chinensis Lindl. J. Pharm. Biomed. Anal. 2020, 187, 113350. [Google Scholar] [CrossRef]

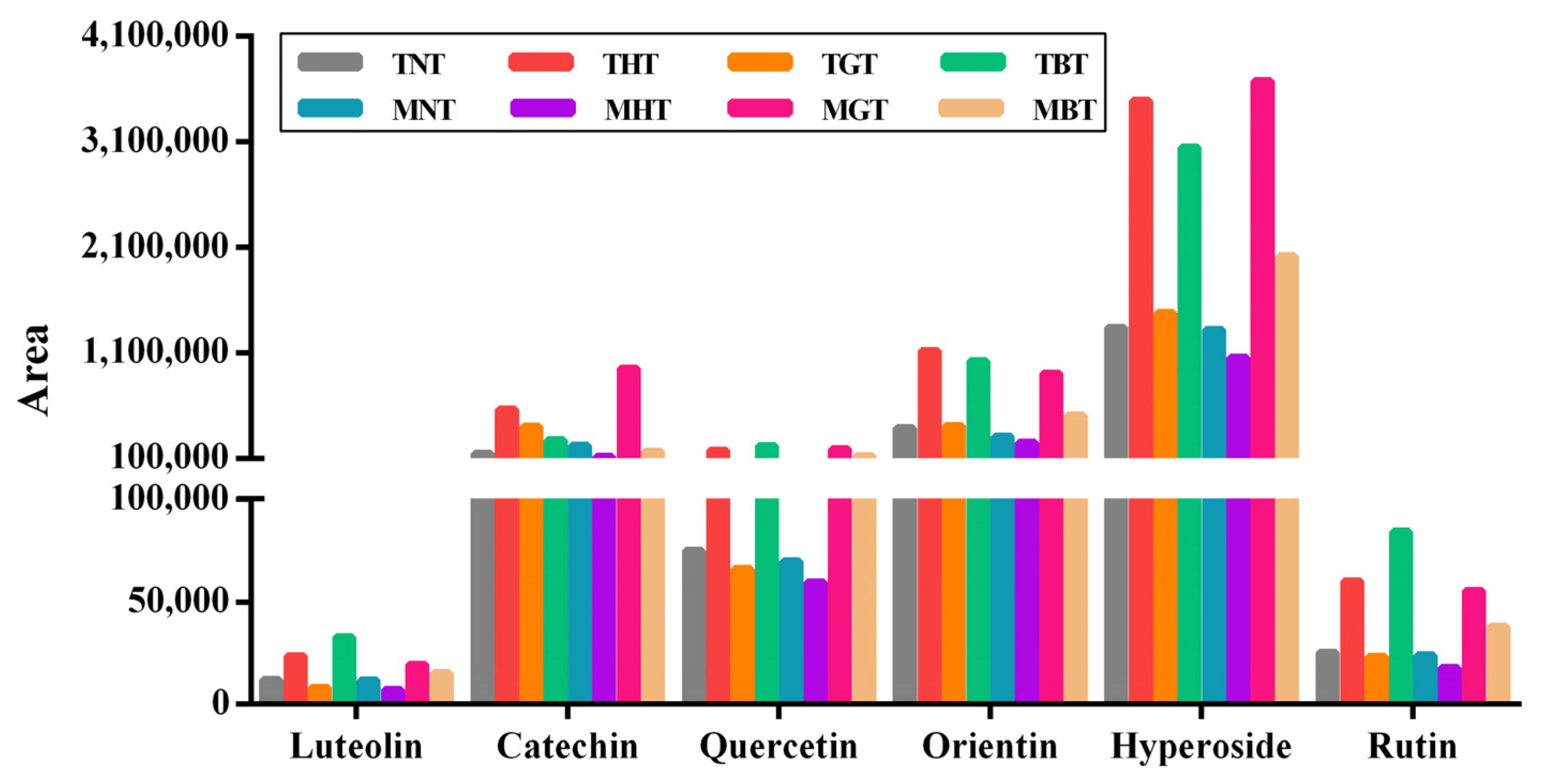
| TNT | THT | TGT | TBT | MNT | MHT | MGT | MBT | |
|---|---|---|---|---|---|---|---|---|
| TPC (mg gallic acid/g lotus tea) | 41.59 ± 0.50 A | 48.62 ± 0.40 B | 47.45 ± 0.24 C | 18.65 ± 0.13 D | 35.42 ± 0.29 E | 32.77 ± 0.61 F | 49.97 ± 0.37 G | 27.51 ± 0.40 H |
| TFC (mg rutin/g lotus tea) | 57.25 ± 0.51 A | 70.09 ± 0.86 B | 65.78 ± 2.71 C | 26.02 ± 0.46 D | 52.67 ± 0.82 E | 49.87 ± 0.89 F | 73.43 ± 1.30 G | 43.02 ± 1.12 H |
| No. | Formula | Molecular Weight (g/mol) | Retention Time (min) | Typical MS/MS Ions (m/z) | Identification |
|---|---|---|---|---|---|
| 1 | C15H10O6 | 286.04 | 4.33 | 151.00, 133.06, 107.01 | Luteolin |
| 2 | C15H14O6 | 290.07 | 3.01 | 245.89 | Catechin |
| 3 | C15H10O7 | 302.03 | 4.08 | 273.06, 257.04, 179.03, 164.03 | Quercetin |
| 4 | C21H20O11 | 448.09 | 4.31 | 357.07, 327.08, 299.05 | Orientin |
| 5 | C21H20O12 | 464.09 | 4.07 | 301.05, 300.05, 271.02, 255.82 | Hyperoside |
| 6 | C27H30O16 | 609.14 | 3.95 | 301.05, 300.05 | Rutin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Ma, Y.; Liu, Y.; Zhou, B.; Zhao, Y.; Wu, P.; Zhang, D.; Li, D. Effects of Maturity and Processing on the Volatile Components, Phytochemical Profiles and Antioxidant Activity of Lotus (Nelumbo nucifera) Leaf. Foods 2023, 12, 198. https://doi.org/10.3390/foods12010198
Ma Z, Ma Y, Liu Y, Zhou B, Zhao Y, Wu P, Zhang D, Li D. Effects of Maturity and Processing on the Volatile Components, Phytochemical Profiles and Antioxidant Activity of Lotus (Nelumbo nucifera) Leaf. Foods. 2023; 12(1):198. https://doi.org/10.3390/foods12010198
Chicago/Turabian StyleMa, Zhili, Yu Ma, Yin Liu, Bei Zhou, Yalin Zhao, Ping Wu, Dexin Zhang, and Deyuan Li. 2023. "Effects of Maturity and Processing on the Volatile Components, Phytochemical Profiles and Antioxidant Activity of Lotus (Nelumbo nucifera) Leaf" Foods 12, no. 1: 198. https://doi.org/10.3390/foods12010198
APA StyleMa, Z., Ma, Y., Liu, Y., Zhou, B., Zhao, Y., Wu, P., Zhang, D., & Li, D. (2023). Effects of Maturity and Processing on the Volatile Components, Phytochemical Profiles and Antioxidant Activity of Lotus (Nelumbo nucifera) Leaf. Foods, 12(1), 198. https://doi.org/10.3390/foods12010198





