First Case of Infective Endocarditis Caused by Vibrio metschnikovii: Clinico-Diagnostic Complexities and a Systematic Literature Review
Abstract
1. Introduction
2. Case Report
3. Systematic Literature Review
3.1. Methodology
3.2. Results
4. Discussion
5. Limitations
6. Recommendations for Culture-Negative Endocarditis
- Investigate further patient history (illicit drugs, medication, probiotics, travel, life and recreation habits).
- Perform a clinical examination to identify potential risk factors for specific microorganisms, serologies for specific microorganisms, or non-infectious miming causes.
- Verify proper management and performance of blood samples and tissue samples for culture after surgery.
- Prolonged incubation for up to 14 days may be beneficial for detecting certain organisms.
- Refer to specialised microbiology laboratories with advanced diagnostic methods, like MALDI-TOF, broad-range PCR, or targeted metagenomic sequencing.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCR | Polymerase chain reaction |
| IE | Infective endocarditis |
| TEE | Transoesophageal echocardiography |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| CARE Checklist | Case Report Checklist |
| HACEK | Haemophilus spp., Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae |
References
- Baker-Austin, C.; Lake, I.; Archer, E.; Hartnell, R.; Trinanes, J.; Martinez-Urtaza, J. Stemming the rising tide of Vibrio disease. Lancet Planet. Health 2024, 8, e515–e520. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Villaquirán-Muriel, M.Á.; Gallo Pérez, J.D.; Mondragón-Quiguanas, A.; Aranaga, C.; Correa, A. Identification of Vibrio metschnikovii and Vibrio injensis Isolated from Leachate Ponds: Characterization of Their Antibiotic Resistance and Virulence-Associated Genes. Antibiotics 2023, 12, 1571. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Zhukova, N.V.; Gorshkova, N.M.; Chaikina, E.L. Characterization of Aeromonas and Vibrio species isolated from a drinking water reservoir. J. Appl. Microbiol. 2001, 90, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jia, J.; Cao, J.; Chen, J.; Xu, Q.; Jiang, Y. Identification of Vibrio metschnikovii from Homarus americanus and its changes in membrane fatty acid composition in response to low temperature. Wei Sheng Wu Xue Bao 2013, 53, 628–634. [Google Scholar]
- Thompson, J.R.; Randa, M.A.; Marcelino, L.A.; Tomita-Mitchell, A.; Lim, E.; Polz, M.F. Diversity and dynamics of a North Atlantic coastal Vibrio community. Appl. Environ. Microbiol. 2004, 70, 4103–4110. [Google Scholar] [CrossRef]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, K.; Lan, R.; Glenn Morris, J.; Xiao, Y.; Ye, J.; Bai, X.; Luo, L.; Gao, H.; Wang, D.; et al. Vibrio metschnikovii as an emergent pathogen: Analyses of phylogeny and O-antigen and identification of possible virulence characteristics. Emerg. Microbes Infect. 2023, 12, 2252522. [Google Scholar] [CrossRef]
- Konechnyi, Y.; Khorkavyi, Y.; Ivanchuk, K.; Kobza, I.; Sękowska, A.; Korniychuk, O. Vibrio metschnikovii: Current state of knowledge and discussion of recently identified clinical case. Clin. Case Rep. 2021, 9, 2236–2244. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, Y.; Zhang, Y.; Zhang, J. Update on the epidemiology, diagnosis, and management of infective endocarditis: A review. Trends Cardiovasc. Med. 2024, 34, 499–506. [Google Scholar] [CrossRef]
- Li, M.; Kim, J.B.; Sastry, B.K.S.; Chen, M. Infective endocarditis. Lancet 2024, 404, 377–392. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; De Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Jaarsma, T.; Landmesser, U.; Køber, L.; James, S.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Salsano, A.; Santini, F.; Bassetti, M. Antibiotics and Missed Etiological Diagnosis of Infective Endocarditis: A Dangerous Duo. J. Clin. Med. 2022, 11, 4533. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J.; Saleh, O.A. Updates in Culture-Negative Endocarditis. Pathogens 2023, 12, 1027. [Google Scholar] [CrossRef]
- Tremoli, E.; Tripodi, A.; Mikus, E.; Savini, C.; Calvi, S.; Tenti, E.; Costantino, A.; Sangiorgi, D.; Fiorentino, M. Sex Differences and Pathogen Patterns in Surgically Treated Aortic Valve Endocarditis over 15 Years. Microbiol. Res. 2025, 16, 33. [Google Scholar] [CrossRef]
- Patel, S.K.; Ali Hassan, S.M.; Côté, M.; Leis, B.; Yanagawa, B. Current trends and challenges in infective endocarditis. Curr. Opin. Cardiol. 2024, 40, 75–84. [Google Scholar] [CrossRef]
- Mohananey, D.; Mohadjer, A.; Pettersson, G.; Navia, J.; Gordon, S.; Shrestha, N.; Grimm, R.A.; Rodriguez, L.L.; Griffin, B.P.; Desai, M.Y. Association of vegetation size with embolic risk in patients with infective endocarditis a systematic review and meta-analysis. JAMA Intern. Med. 2018, 178, 502–510. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Jensen, J.; Jellinge, M.E. Severe septic shock and cardiac arrest in a patient with Vibrio metschnikovii: A case report. J. Med. Case Rep. 2014, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Pariente Martín, M.; Escribano Garaizábal, E.; Liria Sánchez, P.J.; Crespo Sánchez, M.D. Vibrio metschnikovii from a human infected leg ulcer. Rev. Inst. Med. Trop. Sao Paulo 2008, 50, 311–312. [Google Scholar] [CrossRef]
- Prasad, R.; Kharidehal, N. Vibrio metschnikovii Sepsis in a Neonate. Internet J. Pediatr. Neonatol. 2005, 6, 1. [Google Scholar]
- Wallet, F.; Tachon, M.; Nseir, S.; Courcol, R.J.; Roussel-Delvallez, M. Vibrio metschnikovii pneumonia. Emerg. Infect. Dis. 2005, 11, 1641–1642. [Google Scholar] [CrossRef] [PubMed]
- Linde, H.J.; Kobuch, R.; Jayasinghe, S.; Reischl, U.; Lehn, N.; Kaulfuss, S.; Beutin, L. Vibrio metschnikovii, a rare cause of wound infection. J. Clin. Microbiol. 2004, 42, 4909–4911. [Google Scholar] [CrossRef]
- Ben Rejeb, A.; Ebdelli, N.; Bouali, M.R.; Goucha, A.; Bougrine, F.; Khediri, F.; Delsol, G. Primary digestive tract Kaposi sarcoma with idiopathic CD4+ lymphocytopenia, HIV negative, HHV8 positive. Gastroenterol. Clin. Biol. 2001, 25, 707–710. [Google Scholar] [PubMed]
- Dalsgaard, A.; Alarcon, A.; Lanata, C.F.; Jensen, T.; Hansen, H.J.; Delgado, F.; Taylor, D.; Gil, A.I.; Penny, M.E. Clinical manifestations and molecular epidemiology of five cases of diarrhoea in children associated with Vibrio metschnikovii in Arequipa, Peru. J. Med. Microbiol. 1996, 45, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, V.; Branco, A.; De Andrade Lima, R.; Magalhães, M. Vibrio metschnikovii among diarrheal patients during cholera epidemic in Recife Brazil. Rev. Inst. Med. Trop. Sao Paulo 1996, 38, 1–3. [Google Scholar] [CrossRef]
- Hardardottir, H.; Vikenes, K.; Digranes, A.; Lassen, J.; Halstensen, A. Mixed bacteremia with Vibrio metschnikovii in an 83-year-old female patient. Scand. J. Infect. Dis. 1994, 26, 493–494. [Google Scholar] [CrossRef]
- Hansen, W.; Freney, J.; Benyagoub, H.; Letouzey, M.N.; Gigi, J.; Wauters, G. Severe human infections caused by Vibrio metschnikovii. J. Clin. Microbiol. 1993, 31, 2529–2530. [Google Scholar] [CrossRef]
- Bitto, A.O.; Kale, O.O.; Oduntan, S.O. Epidemiological survey of an outbreak of gastroenteritis in a rural community in Oyo State. West Afr. J. Med. 1992, 11, 34–38. [Google Scholar]
- Jean-Jacques, W.; Rajashekaraiah, K.R.; Farmer, J.J.; Hickman, F.W.; Morris, J.G.; Kallick, C.A. Vibrio metschnikovii bacteremia in a patient with cholecystitis. J. Clin. Microbiol. 1981, 14, 711–712. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Drancourt, M.; Colson, P.; Rolain, J.-M.; Scola, B.L.; Raoult, D. Modern clinical microbiology: New challenges and solutions. Nat. Rev. Microbiol. 2013, 11, 574–585. [Google Scholar] [CrossRef]
- Blaschke, A.J.; Hersh, A.L.; Beekmann, S.E.; Ince, D.; Polgreen, P.M.; Hanson, K.E. Unmet diagnostic needs in infectious disease. Diagn. Microbiol. Infect. Dis. 2015, 81, 57–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Le Moing, V.; Bonnet, É.; Cattoir, V.; Chirouze, C.; Deconinck, L.; Duval, X.; Issa, N.; Vandenesch, F.; Hoen, B.; Tazi, A.; et al. Antibiotic therapy and prophylaxis of infective endocarditis—A SPILF-AEPEI position statement on the ESC 2023 guidelines. Infect. Dis. Now 2025, 55, 105011. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers 2018, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lagana, P.; Caruso, G.; Minutoli, E.; Zaccone, R.; Delia, S. Susceptibility to antibiotics of Vibrio spp. and Photobacterium damsela ssp. piscicida strains isolated from italian aquaculture farms. New Microbiol. 2011, 34, 53–63. [Google Scholar]
- Castello, A.; Alio, V.; Cammilleri, G.; Sciortino, S.; Macaluso, A.; Ferrantelli, V.; Ferrantelli, V.; Macaluso, A.; Servadei, I.; Costa, A.; et al. Microbiological and Toxicological Investigations on Bivalve Molluscs Farmed in Sicily. Foods 2024, 13, 552. [Google Scholar] [CrossRef]
- Basanisi, M.G.; Nobili, G.; La Bella, G.; D’Antuono, A.M.; Coppola, R.; Damato, A.M.; Scirocco, T.; La Salandra, G. One-year monitoring of potentially pathogenic microorganisms in the waters and sediments of the Lesina and Varano lagoons (South-Est Italy). Ital. J. Food Saf. 2024, 13, 12218. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Nonno, R.; Herman, L.; Guerra, B.; Messens, W.; et al. Public health aspects of Vibrio spp. related to the consumption of seafood in the EU. EFSA J. 2024, 22, e8896. [Google Scholar] [CrossRef]
- Santajit, S.; Kong-ngoen, T.; Tunyong, W.; Pumirat, P.; Ampawong, S.; Sookrung, N.; Indrawattana, N. Occurrence, antimicrobial resistance, virulence, and biofilm formation capacity of Vibrio spp. and Aeromonas spp. isolated from raw seafood marketed in Bangkok, Thailand. Vet World 2022, 15, 1887–1895. [Google Scholar] [CrossRef]
- Bolcato, V.; Bassetti, M.; Basile, G.; Bianco Prevot, L.; Speziale, G.; Tremoli, E.; Tronconi, L.P.; Maffessanti, F. The State-of-the-Art of Mycobacterium chimaera Infections and the Causal Link with Health Settings: A Systematic Review. Healthcare 2024, 12, 1788. [Google Scholar] [CrossRef]
- Desimone, D.C.; Garrigos, Z.E.; Marx, G.E.; Tattevin, P.; Hasse, B.; McCormick, D.W.; Hannan, M.M.; Zuhlke, L.J.; Radke, C.S.; Baddour, L.M. Blood Culture–Negative Endocarditis: A Scientific Statement from the American Heart Association Endorsed by the International Society for Cardiovascular Infectious Diseases. J. Am. Heart Assoc. 2025, 14, 40218. [Google Scholar] [CrossRef]
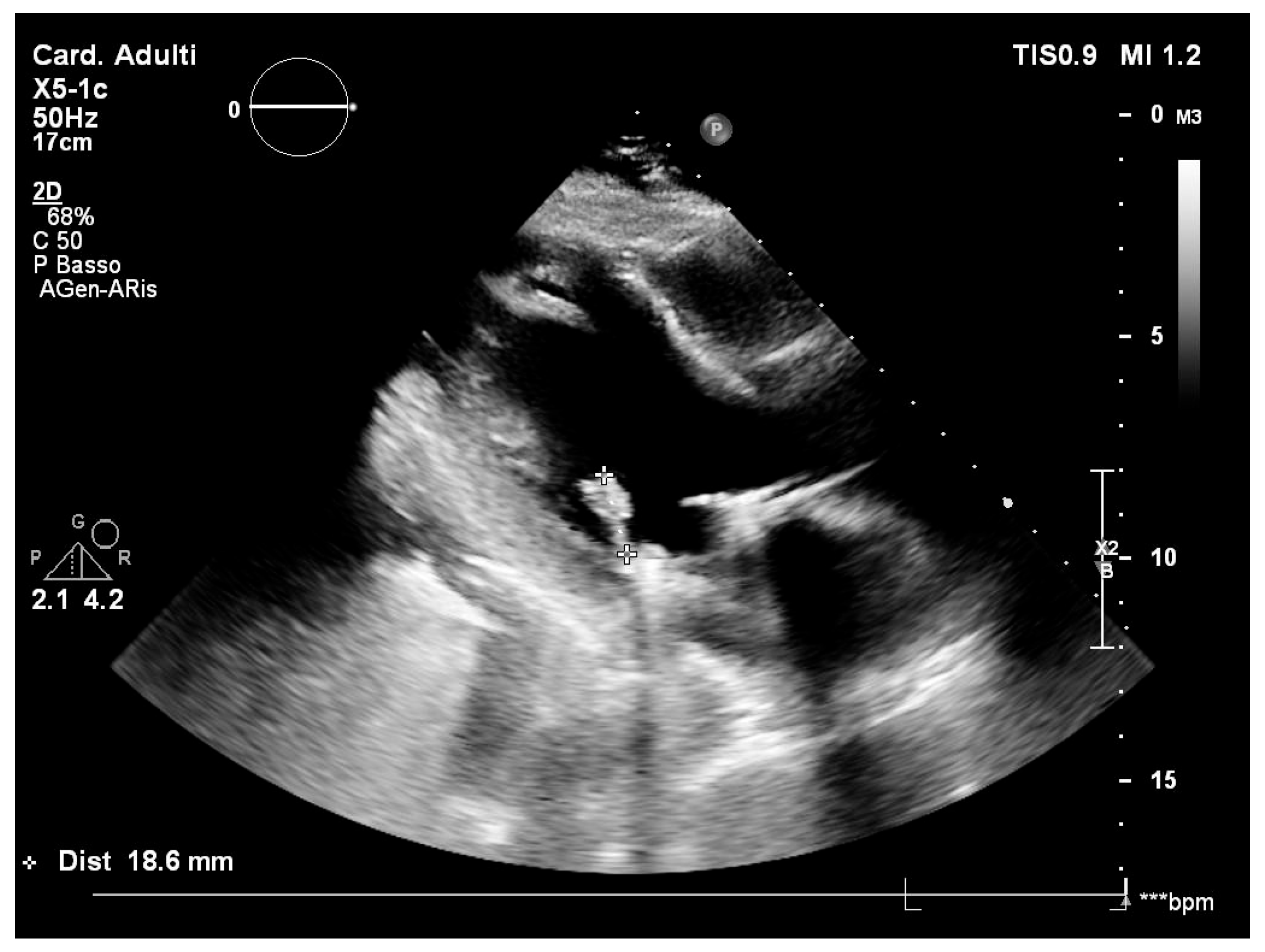
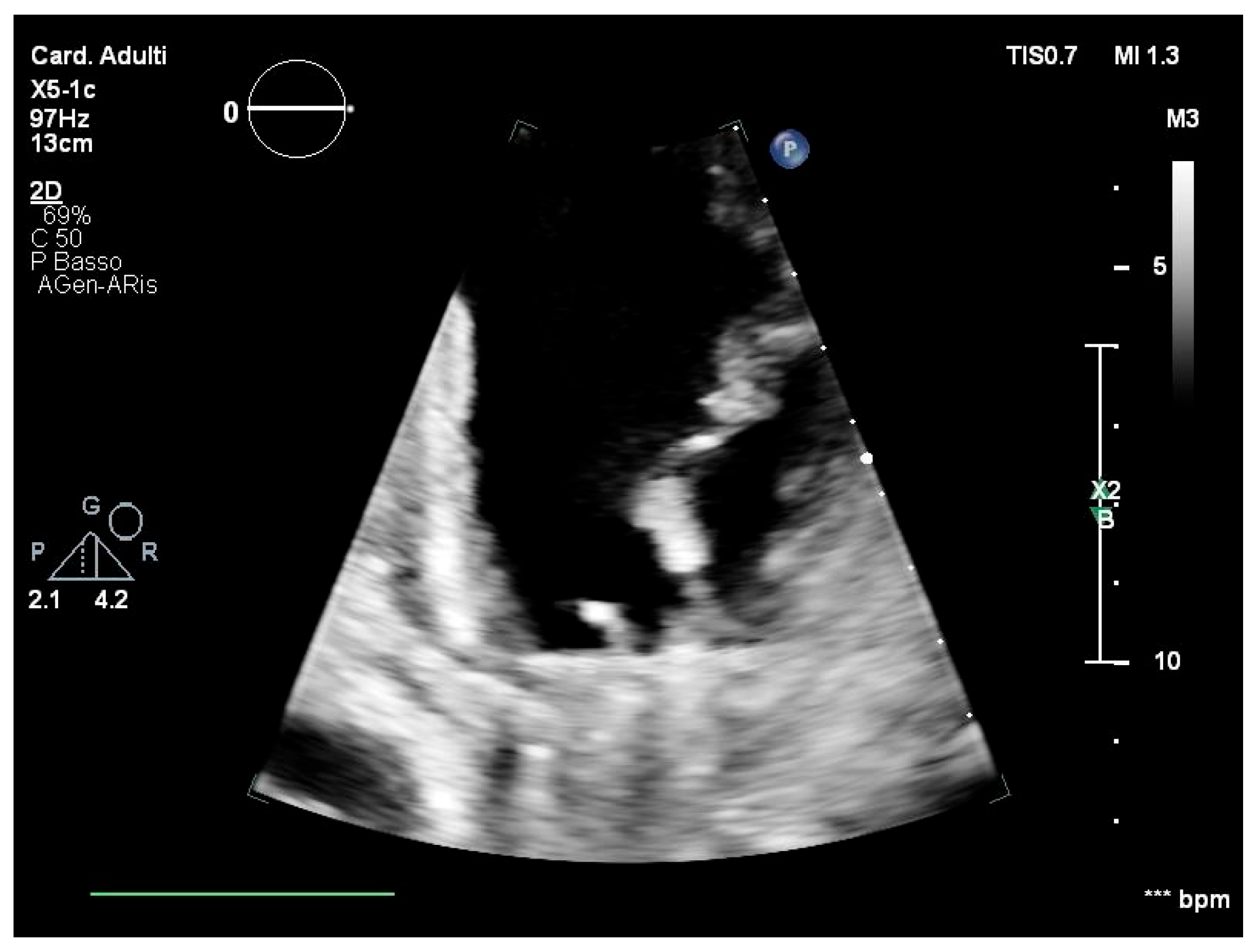

| Author and Year [Reference] | Number of Patients | Country | Age (Years) | Sex (M Male, F Female) | Source of Infection (If Available) | Clinical Diagnosis | Other Information |
|---|---|---|---|---|---|---|---|
| Konechnyi, 2021 [8] | 1 | Ukraine | 70 | M | Patient denied having contact with the sea or consuming marine products. Source not identified | Sepsis with aorto-bifemoral graft prosthesis infection due to aorto-small intestine fistula | Aorto-bifemoral graft pseudoaneurysm and aorto-small intestine fistula could be the cause for bacteriaemia |
| Jensen, 2014 [18] | 1 | Denmark | 78 | M | Probably food ingestion | Gastroenteritis and septic shock. Death | Negative echocardiography |
| Pariente Martìn, 2008 [19] | 1 | Spain | 49 | F | Moved five months prior from Uruguay, with a 7-year history of fibromyalgia and infected leg ulcers | Wound infection and ulcers in both legs, with chronic lymphoedema | _ |
| Prasad, 2005 [20] | 1 | India | 5 days | M | Possible perinatal transmission but not supported | Neonatal sepsis | _ |
| Wallet, 2005 [21] | 1 | France | 63 | M | Retired carpenter with no contact with domestic or wild animals and negative recent history of diarrhoea. Source not identified | Pneumonia | _ |
| Linde, 2004 [22] | 1 | Germany | 64 | M | Probably a zoonotic source; patient worked as a butler | Wound infection | _ |
| Ben Rejeb, 2001 [23] | 1 | Tunisia | 52 | M | Source not identified | Opportunistic pneumonia in primary digestive tract Kaposi sarcoma | _ |
| Dalsgaard, 1996 [24] | 5 | Peru | 15, 12, 12, 20, and 11 | 4 M, 1 F | Source not identified | Acute diarrhoea, with two cases showing moderate dehydration | Outbreak of diarrhoea associated with V. metschnikovii |
| Magalhães, 1996 [25] | 6 | Brazil | na | na | Three patients denied exposure to seafood. Source not identified | Diarrhoea and positivity in faecal specimens | Assessment of 4000 diarrheal faecal specimens between 1992 and 1993 with 73 Vibrio isolates and 6 of V. metschnikovii |
| Hardardottir, 1994 [26] | 1 | Sweden | 83 | F | Patient was not recently abroad and did not consume raw seafood. Source not identified | Sepsis with concomitant Staphylococcus hominis and Escherichia coli infections | Negative echocardiography |
| Hansen, 1993 [27] | 2 | Belgium and France | 80 and 70 | F, M | No travel or seafood consumption. Source not identified | Sepsis, one of which with wound infection. One patient dead | Negative echocardiography |
| Bitto, 1992 [28] | na | Nigeria | na | na | Probable water contamination after a festival with infected visitors | Outbreaks of gastroenteritis with the presence of V. metschnikovii in water and faecal specimens | _ |
| Jean-Jacques, 1981 [29] | 1 | US | 82 | F | Probably long-term gallbladder carriage after previous sea contact or eating seafood | Sepsis with cholecystitis and ascending cholangitis | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrozzo, A.; Bolcato, V.; Martinelli, L.; Dodi, F.; Vulcano, A.; Basile, G.; Tronconi, L.P. First Case of Infective Endocarditis Caused by Vibrio metschnikovii: Clinico-Diagnostic Complexities and a Systematic Literature Review. Clin. Pract. 2025, 15, 118. https://doi.org/10.3390/clinpract15070118
Carrozzo A, Bolcato V, Martinelli L, Dodi F, Vulcano A, Basile G, Tronconi LP. First Case of Infective Endocarditis Caused by Vibrio metschnikovii: Clinico-Diagnostic Complexities and a Systematic Literature Review. Clinics and Practice. 2025; 15(7):118. https://doi.org/10.3390/clinpract15070118
Chicago/Turabian StyleCarrozzo, Alessandro, Vittorio Bolcato, Luigi Martinelli, Ferdinando Dodi, Antonella Vulcano, Giuseppe Basile, and Livio P. Tronconi. 2025. "First Case of Infective Endocarditis Caused by Vibrio metschnikovii: Clinico-Diagnostic Complexities and a Systematic Literature Review" Clinics and Practice 15, no. 7: 118. https://doi.org/10.3390/clinpract15070118
APA StyleCarrozzo, A., Bolcato, V., Martinelli, L., Dodi, F., Vulcano, A., Basile, G., & Tronconi, L. P. (2025). First Case of Infective Endocarditis Caused by Vibrio metschnikovii: Clinico-Diagnostic Complexities and a Systematic Literature Review. Clinics and Practice, 15(7), 118. https://doi.org/10.3390/clinpract15070118






