Abstract
Background/Objectives: Telemedicine (TM) has emerged as a promising tool for improving heart failure (HF) management by allowing non-invasive, remote patient monitoring. However, patient adherence to TM plays a critical role in its effectiveness. This systematic review aims to assess adherence levels to non-invasive TM interventions and explore factors influencing compliance. Methods: This systematic review followed the PRISMA guidelines. A literature search was conducted across the PubMed, Medline, Web of Science, and Google Scholar databases to identify prospective randomized controlled trials published between January 2010 and June 2024. The inclusion criteria included studies focused on non-invasive TM in HF patients with a follow-up period longer than three months. Adherence rates were categorized as high (≥80%), moderate (60–79%), or low (<60%). Results: Of the 136 identified studies, 6 met the inclusion criteria. Three studies reported high adherence (>80%), and three moderate adherence (60–79%). Older patients (≥65 years) showed higher adherence, with two studies exceeding 85% adherence. Studies with higher female participation (>30%) reported better adherence, with two exceeding 88%. Across studies, a lack of racial diversity was especially notable, apart from a study that included a population with 69% black and 31% Hispanic participants, where adherence was 50% for ≥10 uploads over a 90-day period. Seasonal variations affected adherence, with December being the lowest (47–69%) and August the highest (>85%). Monitoring multiple health parameters correlated with better adherence (>85%) compared to single-parameter tracking (50–74%). Conclusions: TM is a promising tool for HF management, but adherence differs by age, sex, and the complexity of monitoring. To optimize TM use, standardized adherence measures and tailored strategies are needed.
1. Introduction
Heart failure (HF) is a significant global health problem, affecting over 64.3 million people worldwide [1,2,3,4]. In the United States, HF leads to approximately 1 million hospitalizations annually, representing 1% to 2% of all hospitalizations [1,2,3,4]. Several primary factors can be attributed to the increasing prevalence of heart failure. Firstly, there has been progress in the management of diseases that raise the probability of developing HF, such as hypertension, diabetes, and myocardial infarction. Furthermore, there are already many medications accessible that can prolong the lifespan of those who have already experienced heart failure. Moreover, the aging of the population in high- and middle-income countries has also played an important role in the increasing burden of HF. This trend is exacerbated by unhealthy lifestyle habits—including poor diet, physical inactivity, and obesity, which increase an individual’s risk for cardiovascular disease and heart failure [1,2]. Recent population data indicate that there has been an improvement in overall survival rates [4]. However, this improvement has been accompanied by a rise in healthcare costs. The main determinant responsible for the substantial costs and notable deterioration in the quality of life of HF patients is frequent hospitalizations. Therefore, there is a significant focus on reducing hospital admissions in the management and treatment of HF [5,6].
The role of telemedicine (TM) in HF management has received considerable attention in recent years, particularly in light of innovative solutions available to enhance patient care. Telehealth refers to technologies that allow for remote monitoring, consultations, and health metrics assessments between patients and healthcare professionals without an in-person visit. This model enables a complete patient assessment, allowing for timely changes to medical therapy. Importantly, studies showed that telemedicine may lead to reduced hospitalization rates for HF and significant clinical improvements [7,8,9,10,11,12,13,14,15,16,17,18]. TM serves as a valuable tool for ongoing patient education, promoting self-care and improving adherence to treatment [10]. This can leave patients feeling more in control and having closer ties to their healthcare team [11,12]. TM also increases access to healthcare, addressing geographic disparities [14,16,18]. These disparities are often due to the unequal distribution of healthcare resources, with rural areas generally having limited access to specialist care, diagnostic services, and timely follow-ups. That is where telemedicine comes in, as it can help bridge that gap by giving patients the ability to receive monitoring and expertise in a continuous fashion without excessive travel [19,20,21,22,23,24].
Non-invasive TM involves patient participation in a daily auto-evaluation routine, which is transmitted to a care facility [25,26,27]. The HF team regularly examines the transmitted data, looking for trends over extended periods of time, or alternatively received alerts if any variable falls outside a preset limit [28,29,30,31]. If early indicators of cardiac instability are detected, then a therapeutic response is triggered [32,33,34,35]. A meta-analysis showed that non-invasive TM significantly reduces the risk of all-cause mortality and HF hospitalization in HF patients [36].
However, non-invasive telemonitoring systems are limited by the fact that they need patients to strictly follow instructions. While wearables and Bluetooth technologies offer convenience, they do not eliminate the necessity of adherence to the technology itself [37,38,39,40].
This systematic review aims to assess adherence levels to non-invasive TM interventions and explore factors influencing compliance.
2. Materials and Methods
2.1. Protocol
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [41]. The protocol was prospectively registered with the International Prospective Register of Systematic Reviews and has been allocated the registration number CRD42024563922 (www.crd.york.ac.uk/prospero, accessed on 16 July 2025).
2.2. Search Strategy
A literature search was conducted to identify studies that reported compliance with telemonitoring in HF patients. Two reviewers independently screened titles and abstracts in the PubMed, Medline, Web of Science, and Google Scholar databases in August 2024 to identify eligible studies published between January 2010 and June 2024. Discrepancies between the two reviewers were resolved by discussion or through the involvement of up to two further reviewers. The initial reviewers were selected based on their clinical and research experience in cardiology and digital health, ensuring familiarity with both heart failure and telemedicine interventions. The additional reviewers were included to provide methodological oversight and maintain consistency with the predefined inclusion criteria. All the reviewers independently assessed the studies to minimize bias. Other limits included studies published in English. The keywords or MeSH (Medical Subject Headings) used during the search were ‘telemedicine’ OR ‘telehealth’ OR ‘telemonitoring’ OR ‘remote monitoring’ OR’ home monitoring’ AND ‘heart failure’ AND ‘compliance’ OR ‘adherence’ OR ‘acceptance’. The references of eligible studies and published systematic reviews were also searched to identify any additional studies.
2.3. Inclusion and Exclusion Criteria
Studies were eligible if they reported compliance or adherence outcomes with home non-invasive telemonitoring in HF patients. Only prospective, randomized control trials (RCTs) were considered. Furthermore, the included studies were required to employ home-based non-invasive TM service and to have an interventional study design. Heart failure was defined according to the criteria applied in each included study. The majority of studies used a combination of clinical parameters such as left ventricular ejection fraction (LVEF ≤ 45%), New York Heart Association (NYHA) functional classification (typically class II–IV), and/or a history of hospitalization due to HF within the preceding 12 months.
2.4. Data Extraction
Two authors independently extracted the data from the included studies, verified by a third author AT. The extracted data are study name, first author, year of publication, country of origin, study type, monocentric or multicentric study, number of subjects, percentage of male population, duration of follow-up, inclusion criteria and exclusion criteria, and description of TM intervention. This information, along with the compliance rates, was organized in tables to provide a summary of the studies.
The primary evaluated outcome was patient adherence to non-invasive telemonitoring protocols. Adherence was defined based on the criteria specified in each individual study. To standardize the analysis across studies, we categorized adherence rates into three groups: high adherence (≥80%), moderate adherence (60–79%), and low adherence (<60%), in alignment with the previous literature [42,43]. Definitions varied slightly among studies—for example, some measured adherence as the number of days with transmitted data over the expected monitoring period, while others used the number of uploads or compliance with specific parameters.
2.5. Risk of Bias Assessment
Two independent reviewers assessed the risk of bias using the revised Cochrane risk-of-bias tool for randomized controlled trials (ROB-2) [44]. RoB2 assessed 5 domains: randomization process, effect of assignment to intervention, missing outcome data, measurement of the outcome, and selection of the reported result. Overall quality was assessed as low risk (“low risk” in all domains), some concerns (at least 1 domain rated “some concern”), and high risk (at least 1 do-main rated “high risk” or 2 to 3 domains rated “some concerns”). The evaluation was independently evaluated by 2 reviewers. Discrepancies among the reviewers were resolved through discussion and consensus.
3. Results
The initial search produced 136 results. Following the elimination of duplicate articles, a total of 73 articles were included in the title and abstract screening process. Among these, 14 articles seemed relevant, and we performed a full-text review/evaluation, resulting in a total of 6 articles being eligible and included in this systematic review [45,46,47,48,49,50]. The detailed selection process is illustrated as a PRISMA flow diagram in Figure 1.
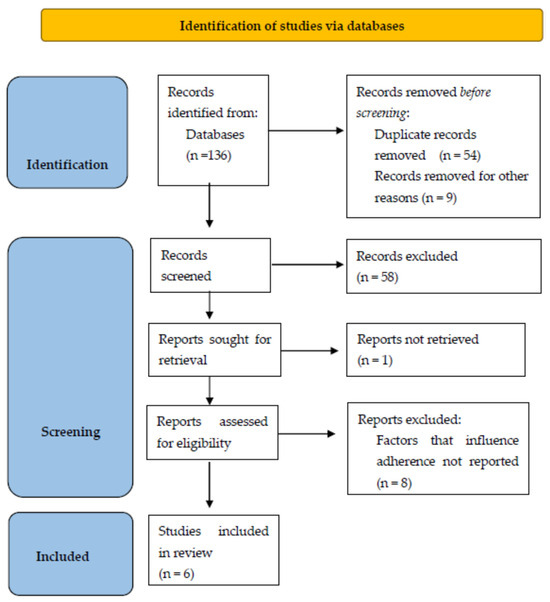
Figure 1.
PRISMA flow diagram of the literature screening process.
3.1. Risk of Bias
The quality of studies was found to be high. Details are provided in Table 1.

Table 1.
Results of quality appraisal.
3.2. Studies Characteristics
The included studies were conducted in a wide range of countries, including the United States (two studies), Europe (two studies), Asia (one study), and Australia (one study). An overview of the results is provided in Table 2 and Table 3. The largest study included 1571 participants, while the smallest study cohort comprised 104 participants. The shortest duration of follow-up was 3 months and the longest was 18 months. All six studies asked the patients to measure their weight. The BP was asked to be measured in three studies, the HR in three studies, the oxygen saturation rate in one, and the body composition in one. Finally, questions regarding health status were used in two studies.

Table 2.
Overview of the studies included in the systematic review, with a focus on the study protocols. RCT: randomized controlled trial; TM: telemedicine; UC: usual care; HFpEF: heart failure with preserved ejection fraction; HFmrEF: heart failure with mildly reduced ejection fraction; HFrEF: heart failure with reduced ejection fraction.

Table 3.
Overview of the studies included in the systematic review with a focus on the technical approaches. LVEF: left ventricular ejection fraction; HF: heart failure; BP: blood pressure; HR: heart rate; NYHA: New York Heart Association (NYHA).
In the OSICAT trial, the majority of the patients had a more advanced stage of the disease. Specifically, 71% of the patients were recruited while they were in the hospital, 2.6% of the patients received a heart transplant during the study, and 10.0% of the patients were classified as NYHA class IV. Similarly, 10% of the participants in the BEAT-HF study were NYHA class IV, while those with lower socioeconomic status represented 39.4% of the cohort. On the contrary, in the TIM-HF2 study, less than 1% of the patients were in NYHA class IV, while patients with serious depression were not included in the study. In the study by Pekmezaris et al., the participants were non-White, and in more detail: 31% were Hispanic and 69% were black. In the same study, the vast majority of the patients (72%) had low socioeconomic status. In the Homes-HF study, only patients with NYHA class II and III were included. Patients with severe depression or dementia and access to a telephone line were excluded. Finally, in the ITEC-CHF a high proportion of patients were suffering from chronic diseases, while patients with severe cognitive impairments were excluded.
3.3. Adherence
To summarize the reported compliance in this analysis, three studies were classified as reporting high compliance [45,49,50] and three studies as reporting medium compliance [46,47,48]. Nevertheless, differences were seen among the studies over the precise definition of ‘compliant’ and ‘noncompliant’. For example, Pekmezaris et al. defined as high adherence the transmission of 10 or more uploads over 90 days [50]. Conversely, Ding et al. adhered to the Australian clinical recommendations for managing HF, which describe good compliance as uploading weight measurements at least 6 days per week [48,51].
3.4. Age
In five out of the six included studies, the mean or median age of the HF patients was ≥65 years [45,46,47,48,49], whereas in just one study it was <65 years [50]. Within the trials that included individuals aged 65 years and older, two studies have shown a high level of adherence [45,49], whereas three studies indicated a moderate level of adherence [46,47,48]. In the study conducted with those below the age of 65, a high degree of adherence was seen [50].
3.5. Sex
Male participants represented the vast majority in all six studies. Out of the three trials where the female participants accounted for ≤30% of the entire group [45,46,48], compliance was rated as medium in two of them [46,48] and as high in one of them [45]. Conversely, in the three remaining trials where the female composition ranges from >30% to <40% of the group, compliance rates were high in two studies [49,50] and medium in one [47].
3.6. Race
There is only one study that looked exclusively at black and Hispanic patients, and it reported a high level of compliance [50]. But it is worth mentioning that in this study, adherence was defined as 10 or more uploads over 90 days. Another study revealed a high representation of these populations as well, but it did not elaborate on the compliance [47].
3.7. Place of Residence
Variations in telemonitoring compliance based on place of residence were examined in one study [45]. Patients living in rural areas showed higher compliance compared with patients living in urban areas.
3.8. Follow-Up Period
Throughout the research period, there were no observed changes in the degree of compliance (Table 4). Compliance was consistently high in two studies [45,49] and moderate in two others [46,47] throughout the length of the trials. In two trials, no relevant data were provided [48,50]. The participants showed a reduced probability of sending measurements on weekends as compared to weekdays [47]. Adherence varied by month, with lower levels observed in the winter months. December showed the lowest adherence across all months, while August had the highest amount [47].

Table 4.
Reported compliance results throughout the study period.
3.9. Number of Recorded Parameters
The number of sent parameters did not seem to hinder the level of compliance. Three studies that assessed the participants’ weight transmission demonstrated a modest level of compliance [46,47,48]. Conversely, three studies that required the participants to send several parameters demonstrated a high level of compliance [45,49,50].
4. Discussion
The present review highlights several challenges in patient compliance and adherence, which directly impact the effectiveness of TM interventions. One of the key takeaways from the review is the significant variation in adherence across different studies. Three studies reported high adherence, while three reported moderate adherence. The variability in adherence rates may be attributed to differences in how compliance was defined in the respective studies. For instance, in the study by Pekmezaris et al., high adherence was defined as 10 or more uploads over 90 days [50], while Ding et al. followed the Australian clinical guidelines [51], defining high adherence as uploading weight measurements at least six days per week [48]. However, such definitions are highly dependent on the specific characteristics of a given study population. This clearly highlights the need for standardized definitions that are clinically relevant and not designed only for the specific setting of a trial.
Patient characteristics, such as age and sex, were shown to affect adherence to TM. For example, most studies included older patients (≥65 years), and adherence was generally higher among older adults. This finding aligns with previous research suggesting that older patients may be more motivated to adhere to TM interventions as a means of managing their chronic condition and avoiding hospitalization [52,53,54]. Also, older persons frequently maintain stronger ties to caregivers which may bolster adherence by offering reminders, encouragement, or assistance with technology utilization. For those with cognitive impairment or reduced functional capacity, such assistance is essential for facilitating consistent engagement in telemonitoring practices.
Furthermore, gender disparities were observed, with female patients representing a smaller proportion of the study participants. The review indicates that in trials where female participation was low, adherence rates were typically moderate, whereas higher female representation correlated with higher adherence. This suggests that gender may play a role in TM compliance, possibly due to differences in health-seeking behaviors between men and women [55]. Moreover, many women might not be aware of telemedicine services, leading to reduced participation in studies. That can create a lack of gender diversity, leading to skewed findings that do not fully address the experience of female patients [56]. That, in turn, can perpetuate health disparities because research results may not generalize as well to women’s health problems [57]. Finally, the underrepresentation of women may explain some of the negative associations between female gender and telemedicine use in some studies [58].
A significant concern is the comparatively lower level of representation of persons of non-White race in the research. Among the six studies included in this review, only one study specifically examined the black and Hispanic populations with HF. This is an enduring issue. During their analysis, Granger et al. found that just four studies assessed the use of technology for self-management in the black population [59].
Interestingly, the review found no significant changes in adherence over time, suggesting that patients who initially engaged with TM interventions generally maintained their compliance throughout the study period. This contrasts with the trend in adherence to medication, which tends to decline over time for reasons such as treatment fatigue, adverse effects, or waning perceived benefit. One possible explanation is that telemonitoring—unlike medication intake—is a more interactive experience, offering patients immediate feedback, a greater amount of contact with their care teams, and a sense of empowerment and engagement that may help maintain motivation.
However, seasonal variations were observed, with adherence declining during winter months, particularly in December, and peaking in August. These seasonal trends may be related to changes in patients’ routines, such as holidays or weather conditions, which could affect their ability to engage with TM technology consistently. Moreover, caregiver and family support may be reduced during holiday time, resulting in less adherence for those who depend on others to perform monitoring activities.
Another notable factor influencing adherence was the complexity of the TM interventions. Studies that required patients to monitor multiple parameters generally reported higher adherence rates than those focusing solely on single measurements, such as body weight. This finding suggests that patients may be more engaged when they are asked to participate in more comprehensive monitoring activities, perhaps because they perceive the intervention as more robust and beneficial [60].
One study [45] evaluated differences in telemonitoring adherence by place of residence. Rural patients had better adherence compared to urban patients. Telemonitoring may be a unique opportunity for rural patients to regularly and in real-time connect with their healthcare providers. By contrast, patients who live in urban areas, despite having better access to health services, are likely to perceive less added value in telemonitoring, which may explain their lower engagement level with telemonitoring. These findings underscore the potential of telemedicine to close care gaps in underserved geographic areas and suggest that patient context should guide the design of remote care programs.
Another notable issue is the increased incidence of rejection during the recruitment phase. In the two major trials, TIM-HF2 and BEAT-HF, the proportion of subjects who declined the offer to participate in the trial accounts approximately for 50% and 33% of the cohort study, respectively. This is consistent with the findings published by Gorst et al. in their systematic study [61]. This is an existing problem and the proportion of patients who refuse TM is largely unknown. Research is needed to quantify the rates of patient uptake, refusal, and abandonment of telehealth, to understand the number of patients who are willing to accept and use it. Research also needs to explore patient beliefs and perceptions about telehealth to try and explain why patients decide to take up, refuse, abandon, or sustain their use of telehealth.
4.1. Standardizing Terminology
Defining and measuring compliance in-home telemedicine is highly important for improving patients’ healthcare efficiency, achieving system interoperability, and exploring research progress. The primary effort is to create a common language by ensuring that terms like “compliance” and “adherence” are well defined in order to avoid confusion. Also, a standardized compliance level can be a helpful approach (for example: compliant, partially compliant, or noncompliant). In order to carve out consistency, both the objective and subjective metrics should be standardized. Also, we can improve uniformity of assessments by defining compliance measurement intervals (e.g., daily, weekly, or monthly).
4.2. Improving Compliance
Patient engagement is the core of success for any healthcare model, and more so in telemedicine care. Many strategies have been proposed to improve patients’ adherence to telemedicine care [62,63]:
The Benefits Must be Clear: Acceptance and participation improve by providing information on how telemedicine can improve their health outcomes.
Patient-Centered Education: The designs should be patient-centered and consider the literacy levels of different populations and technological familiarity factors.
Make Technology User-Friendly: The availability of enough technical support by healthcare professionals can also improve the patients’ capability of using these services effectively.
Personalized Telemedicine Plans: Considering that adherence might differ among various populations, customized interventions should be introduced to address the requirements of different patient cohorts.
Strong Patient–Provider Relationships: Ongoing follow-ups, seamless availability of medical representatives to answer the patient questions, and encouragement for the involvement of caregivers or family members can drastically enhance adherence and patient experience.
5. Study Limitations
A major limitation of this review is the variability in adherence definitions and measurements in the studies that were included, which makes it difficult to directly compare. Also, no separate analysis based on HF categories was performed in any of the included studies. Furthermore, the review predominantly encompasses studies that are undertaken in high-income settings, which limits the applicability of its findings to low-resource contexts. Additional limitation arises from the underrepresentation of some demographic groups, especially non-White ethnic minorities and low socioeconomic individuals, which could affect the generalizability of the results.
6. Conclusions
In conclusion, while non-invasive TM has the potential to improve heart failure management, patient adherence remains a critical determinant of its success. The findings of this review underscore the importance of addressing barriers to compliance, including socioeconomic factors, patient education, and the complexity of TM interventions. Further research is needed to develop strategies for enhancing adherence and optimizing the effectiveness of TM programs in diverse patient populations. Standardizing adherence metrics across studies would also allow for more accurate comparisons and a clearer understanding of the factors that influence patient engagement with TM.
Author Contributions
Conceptualization, L.T., I.B. and P.T.; methodology, G.K. and D.C.; software, S.I. and O.G.; validation, S.I., O.G. and M.K.; formal analysis, I.B. and P.T.; investigation, S.I., O.G. and M.K.; resources, P.T.; data curation, S.I., O.G. and L.T.; writing—original draft preparation, O.G., L.T. and M.K.; writing—review and editing G.K., K.S.-Ż. and D.C.; visualization, K.S.-Ż.; supervision, G.K. and D.C.; project administration, P.T. and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was not required for this study as it is a systematic review of the previously published literature and did not involve direct human participants or primary data collection.
Data Availability Statement
No new data were generated or analyzed in this study. Data sharing is not applicable to this article as it is based on a systematic review of the publicly available literature.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. AHA statistical update: Heart disease and stroke statistics—2019 update. A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESCGuidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA)of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Feltner, C.; Jones, C.D.; Cene, C.W.; Zheng, Z.J.; Sueta, C.A.; Coker-Schwimmer, E.J.; Arvanitis, M.; Lohr, K.N.; Middleton, J.C.; Jonas, D.E. Transitional care interventions to prevent readmissions for persons with heart failure: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Sposato, B.; Volterrani, M. Telemonitoring for the Management of Patients with Heart Failure. Card. Fail. Rev. 2020, 6, e07. [Google Scholar] [CrossRef]
- Schmidt, S.; Schuchert, A.; Krieg, T.; Oeff, M. Home telemonitoring in patients with chronic heart failure: A chance to improve patient care? Dtsch. Arztebl. Int. 2010, 107, 131–138. [Google Scholar]
- Tedeschi, A.; Palazzini, M.; Trimarchi, G.; Conti, N.; Di Spigno, F.; Gentile, P.; D’Angelo, L.; Garascia, A.; Ammirati, E.; Morici, N.; et al. Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts. J. Clin. Med. 2024, 13, 2592. [Google Scholar] [CrossRef]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of heart failure patients: Practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 157–174. [Google Scholar] [CrossRef]
- Di Lenarda, A.; Casolo, G.; Gulizia, M.M.; Aspromonte, N.; Scalvini, S.; Mortara, A.; Alunni, G.; Ricci, R.P.; Mantovan, R.; Russo, G.; et al. The future of telemedicine for the management of heart failure patients: A Consensus Document of the Italian Association of Hospital Cardiologists (A.N.M.C.O), the Italian Society of Cardiology (S.I.C.) and the Italian Society for Telemedicine and eHealth (Digital S.I.T.). Eur. Heart J. Suppl. 2017, 19, D113–D129. [Google Scholar] [PubMed]
- Lopes, I.; Sousa, F.; Moreira, E.; Cardoso, J. Smartphone-Based Remote Monitoring Solution for Heart Failure Patients. Stud. Health Technol. Inform. 2019, 261, 109–114. [Google Scholar]
- Koehler, J.; Stengel, A.; Hofmann, T.; Wegscheider, K.; Koehler, K.; Sehner, S.; Rose, M.; Deckwart, O.; Anker, S.D.; Koehler, F.; et al. Telemonitoring in patients with chronic heart failure and moderate depressed symptoms: Results of the Telemedical Interventional Monitoring in Heart Failure (TIM-HF) study. Eur. J. Heart Fail. 2021, 23, 186–194. [Google Scholar] [CrossRef]
- Neubeck, L.; Hansen, T.; Jaarsma, T.; Klompstra, L.; Gallagher, R. Delivering healthcare remotely to cardiovascular patients during COVID-19: A rapid review of the evidence. Eur. J. Cardiovasc. Nurs. 2020, 19, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, G.; Marlicz, W.; Koulaouzidis, A. Telemedicine in the Time of COVID-19: Better Late Than Never. Am. J. Gastroenterol. 2021, 116, 1088–1089. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, G.; Charisopoulou, D.; Wojakowski, W.; Koulaouzidis, A.; Marlicz, W.; Jadczyk, T. Telemedicine in cardiology in the time of coronavirus disease 2019: A friend that everybody needs. Pol. Arch. Intern. Med. 2020, 130, 559–561. [Google Scholar] [CrossRef]
- Planinc, I.; Milicic, D.; Cikes, M. Telemonitoring in Heart Failure Management. Card. Fail. Rev. 2020, 6, e06. [Google Scholar] [CrossRef]
- Marlicz, W.; Koulaouzidis, A.; Charisopoulou, D.; Jankowski, J.; Marlicz, M.; Skonieczna-Zydecka, K.; Krynicka, P.; Loniewski, I.; Samochowiec, J.; Rydzewska, G.; et al. Burnout in healthcare—the Emperor’s New Clothes. Prz. Gastroenterol. 2023, 18, 274–280. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomized controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Abraham, W.T.; Maisel, A.; Zile, M.; Smart, F.; Costanzo, M.R.; Mehra, M.R.; Ducharme, A.; Sears, S.F.; Desai, A.S.; et al. Hemodynamic-GUIDEd management of Heart Failure (GUIDE-HF). Am. Heart J. 2019, 214, 18–27. [Google Scholar] [CrossRef]
- Angermann, C.E.; Assmus, B.; Anker, S.D.; Asselbergs, F.W.; Brachmann, J.; Brett, M.E.; Brugts, J.J.; Ertl, G.; Ginn, G.; Hilker, L.; et al. MEMS-HF Investigators. Pulmonary artery pressure-guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF). Eur. J. Heart Fail. 2020, 22, 1891–1901. [Google Scholar] [CrossRef]
- Veenis, J.F.; Brugts, J.J. Remote monitoring of chronic heart failure patients: Invasive versus non-invasive tools for optimising patient management. Neth. Heart J. 2020, 28, 3–13. [Google Scholar] [CrossRef]
- Halawa, A.; Enezate, T.; Flaker, G. Device monitoring in heart failure management: Outcomes based on a systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 2019, 9, 386–393. [Google Scholar] [CrossRef]
- Alotaibi, S.; Hernandez-Montfort, J.; Ali, O.E.; El-Chilali, K.; Perez, B.A. Remote monitoring of implantable cardiac devices in heart failure patients: A systematic review and meta-analysis of randomized controlled trials. Heart Fail. Rev. 2020, 25, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Ahmed, S.; Grapsa, J. Apps and Online Platforms for Patients with Heart Failure. Card. Fail. Rev. 2020, 6, e14. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cardoso, J.; Juanatey, J.R.G.; Comin-Colet, J.; Sousa, J.M.; Cavalheiro, A.; Moreira, E. The Future of Telemedicine in the Management of Heart Failure Patients. Card. Fail. Rev. 2021, 7, e11. [Google Scholar] [CrossRef] [PubMed]
- Koulaouzidis, G.; Barrett, D.; Mohee, K.; Clark, A.L. Telemonitoring in subjects with newly diagnosed heart failure with reduced ejection fraction: From clinical research to everyday practice. J. Telemed. Telecare 2019, 25, 167–171. [Google Scholar] [CrossRef]
- Stevenson, L.W.; Ross, H.J.; Rathman, L.D.; Boehmer, J.P. Remote Monitoring for Heart Failure Management at Home. J. Am. Coll. Cardiol. 2023, 81, 2272–2291. [Google Scholar] [CrossRef]
- Faragli, A.; Abawi, D.; Quinn, C.; Cvetkovic, M.; Schlabs, T.; Tahirovic, E.; Düngen, H.D.; Pieske, B.; Kelle, S.; Edelmann, F.; et al. The role of non-invasive devices for the telemonitoring of heart failure patients. Heart Fail. Rev. 2021, 26, 1063–1080. [Google Scholar] [CrossRef]
- Wańczura, P.; Aebisher, D.; Wiśniowski, M.; Kos, M.; Bukowski, H.; Hołownia-Voloskova, M.; Przybylski, A. Telemedical Intervention and Its Effect on Quality of Life in Chronic Heart Failure Patients: The Results from the Telemedicine and e-Health Solution Pilot Program. J. Clin. Med. 2024, 13, 2604. [Google Scholar] [CrossRef]
- Kinast, B.; Lutz, M.; Schreiweis, B. Telemonitoring of Real-World Health Data in Cardiology: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 9070. [Google Scholar] [CrossRef] [PubMed]
- Iakovidis, D.K.; Douska, D.; Barba, E.; Koulaouzidis, G. Wavelet-based signal analysis for heart failure hospitalization prediction. Stud. Health Technol. Inform. 2016, 224, 21–26. [Google Scholar] [PubMed]
- Joshi, R.; Gyllensten, I.C. Changes in Daily Measures of Blood Pressure and Heart Rate Improve Weight-Based Detection of Heart Failure Deterioration in Patients on Telemonitoring. IEEE J. Biomed. Health Inform. 2019, 23, 1041–1048. [Google Scholar] [CrossRef]
- Henriques, J.; Carvalho, P.; Paredes, S.; Rocha, T.; Habetha, J.; Antunes, M.; Morais, J. Prediction of Heart Failure Decompensation Events by Trend Analysis of Telemonitoring Data. IEEE J. Biomed. Health Inform. 2015, 19, 1757–1769. [Google Scholar] [CrossRef]
- Koulaouzidis, G.; Iakovidis, D.K.; Clark, A.L. Telemonitoring predicts in advance heart failure admissions. Int. J. Cardiol. 2016, 216, 78–84. [Google Scholar] [CrossRef]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. 2015, 2015, CD007228. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Wu, T.H.; Wu, Y.C.; Han, C.C. Bluetooth-Based Healthcare Information and Medical Resource Management System. Sensors 2023, 23, 5389. [Google Scholar] [CrossRef]
- Brahmbhatt, D.H.; Cowie, M.R. Remote Management of Heart Failure: An Overview of Telemonitoring Technologies. Card. Fail. Rev. 2019, 5, 86–92. [Google Scholar] [CrossRef]
- Sousa, C.; Leite, S.; Lagido, R.; Ferreira, L.; Silva-Cardoso, J.; Maciel, M.J. Telemonitoring in heart failure: A state-of-the-art review. Rev. Port. Cardiol. 2014, 33, 229–239. [Google Scholar] [CrossRef]
- Fedson, S.; Bozkurt, B. Telehealth in Heart Failure. Heart Fail. Clin. 2022, 18, 213–221. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Deka, P.; Pozehl, B.; Williams, M.A.; Yates, B. Adherence to recommended exercise guidelines in patients with heart failure. Heart Fail. Rev. 2017, 22, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Keeling, P. Compliance with Telemonitoring in Heart Failure. Are Study Findings Representative of Reality? A Narrative Literature Review. Telemed. E-Health J. 2022, 28, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Prescher, S.; Winkler, S.; Riehle, L.; Hiddemann, M.; Moeller, V.; Collins, C.; Deckwart, O.; Spethmann, S. Patient reported experience and adherence to remote patient management in chronic heart failure patients: A posthoc analysis of the TIM-HF2 trial. Eur. J. Cardiovasc. Nurs. 2023, 22, 245–253. [Google Scholar] [CrossRef]
- Galinier, M.; Roubille, F.; Berdague, P.; Brierre, G.; Cantie, P.; Dary, P.; Ferradou, J.; Fondard, O.; Labarre, J.P.; Mansourati, J.; et al. Telemonitoring versus standard care in heart failure: A randomized multicentre trial. Eur. J. Heart Fail. 2020, 22, 985–994. [Google Scholar] [CrossRef]
- Haynes, S.C.; Tancredi, D.J.; Tong, K.; Hoch, J.S.; Ong, M.K.; Ganiats, T.G.; Evangelista, L.S.; Black, J.T.; Auerbach, A.; Romano, P.S.; et al. Association of adherence to weight telemonitoring with health care use and death: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 2020, 3, e2010174. [Google Scholar] [CrossRef]
- Ding, H.; Jayasena, R.; Chen, S.H.; Maiorana, A.; Dowling, A.; Layland, J.; Good, N.; Karunanithi, M.; Edwards, I. The effects of telemonitoring on patient compliance with self-management recommendations and outcomes of the innovative telemonitoring enhanced care program for chronic heart failure: Randomized controlled trial. J. Med. Internet Res. 2020, 22, e17559. [Google Scholar] [CrossRef]
- On behalf of the HOMES-HF study investigators; Kotooka, N.; Kittaka, M.; Nagashima, K.; Asaka, M.; Kinugasa, Y.; Nochioka, K.; Mizuno, A.; Nagatomo, D.; Mine, D.; et al. The first multicenter, randomized, controlled trial of home telemonitoring for Japanese patients with heart failure: Home telemonitoring study for patients with heart failure (HOMES-HF). Heart Vessel. 2018, 33, 866–876. [Google Scholar] [CrossRef]
- Pekmezaris, R.; Nouryan, C.N.; Schwartz, R.; Castillo, S.; Makaryus, A.N.; Ahern, D.; Akerman, M.B.; Lesser, M.L.; Bauer, L.; Murray, L.; et al. A randomized controlled trial comparing telehealth self-management to standard outpatient management in underserved black and Hispanic patients living with heart failure. Telemed. J. E Health 2019, 25, 917–925. [Google Scholar] [CrossRef]
- NHFA CSANZ Heart Failure Guidelines Working Group; Atherton, J.J.; Sindone, A.; De Pasquale, C.G.; Driscoll, A.; MacDonald, P.S.; Hopper, I.; Kistler, P.M.; Briffa, T.; Wong, J.; et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Guidelines for the Prevention, Detection, and Management of Heart Failure in Australia 2018. Heart Lung Circ. 2018, 27, 1123–1208. [Google Scholar] [CrossRef] [PubMed]
- Şahin, E.; Yavuz Veizi, B.G.; Naharci, M.I. Telemedicine interventions for older adults: A systematic review. J. Telemed. Telecare 2024, 30, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, M.; Bujnowska-Fedak Grata-Borkowska, U. Use of telemedicine-based care for the aging and elderly: Promises and pitfalls. Smart Homecare Technol. TeleHealth 2015, 3, 91–105. [Google Scholar]
- Ware, P.; Dorai, M.; Ross, H.J.; Cafazzo, J.A.; Laporte, A.; Boodoo, C.; Seto, E. Patient Adherence to a Mobile Phone-Based Heart Failure Telemonitoring Program: A Longitudinal Mixed-Methods Study. JMIR Mhealth Uhealth 2019, 7, e13259. [Google Scholar] [CrossRef]
- Hay, K.; McDougal, L.; Percival, V.; Henry, S.; Klugman, J.; Wurie, H.; Raven, J.; Shabalala, F.; Fielding-Miller, R.; Dey, A.; et al. Gender Equality, Norms, and Health Steering Committee. Disrupting gender norms in health systems: Making the case for change. Lancet 2019, 393, 2535–2549. [Google Scholar] [CrossRef] [PubMed]
- Abd El Mawgod, M.M.; Alshutayli, A.A.; Alanazi, S.M.; Alqahtani, W.N.; Alqahtani, N.A.; Alamri, A.M.; Alshammari, N.Z. Awareness and Perception of Telemedicine Among the General Population in the Central, Northern, and Western Regions of Saudi Arabia. Cureus 2024, 16, e64895. [Google Scholar] [CrossRef]
- Muehlensiepen, F.; Hoffmann, M.J.; Nübel, J.; Ignatyev, Y.; Heinze, M.; Butter, C.; Haase-Fielitz, A. Acceptance of Telemedicine by Specialists and General Practitioners in Cardiology Care: Cross-Sectional Survey Study. JMIR Form. Res. 2024, 8, e49526. [Google Scholar] [CrossRef]
- Muehlensiepen, F.; Petit, P.; Knitza, J.; Welcker, M.; Vuillerme, N. Factors Associated with Telemedicine Use Among German General Practitioners and Rheumatologists: Secondary Analysis of Data from a Nationwide Survey. J. Med. Internet Res. 2022, 24, e40304. [Google Scholar] [CrossRef]
- Hughes, H.A.; Granger, B.B. Racial disparities and the use of technology for self-management in blacks with heart failure: A literature review. Curr. Heart Fail. Rep. 2014, 11, 281–289. [Google Scholar] [CrossRef]
- Franek, J. Self-management support interventions for persons with chronic disease: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2013, 13, 1–60. [Google Scholar]
- Gorst, S.L.; Armitage, C.J.; Brownsell, S.; Hawley, M.S. Home telehealth uptake and continued use among heart failure and chronic obstructive pulmonary disease patients: A systematic review. Ann. Behav. Med. 2014, 48, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Goorts, K.; Dizon, J.; Milanese, S. The effectiveness of implementation strategies for promoting evidence informed interventions in allied healthcare: A systematic review. BMC Health Serv. Res. 2021, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Edgman-Levitan, S.; Schoenbaum, S.C. Patient-centered care: Achieving higher quality by designing care through the patient’s eyes. Isr. J. Health Policy Res. 2021, 10, 21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).