Efficiency and Applicability of Virtual Surgical Planning in Maxillofacial and Mandibular Bone Reconstruction: A Narrative Review
Abstract
1. Introduction
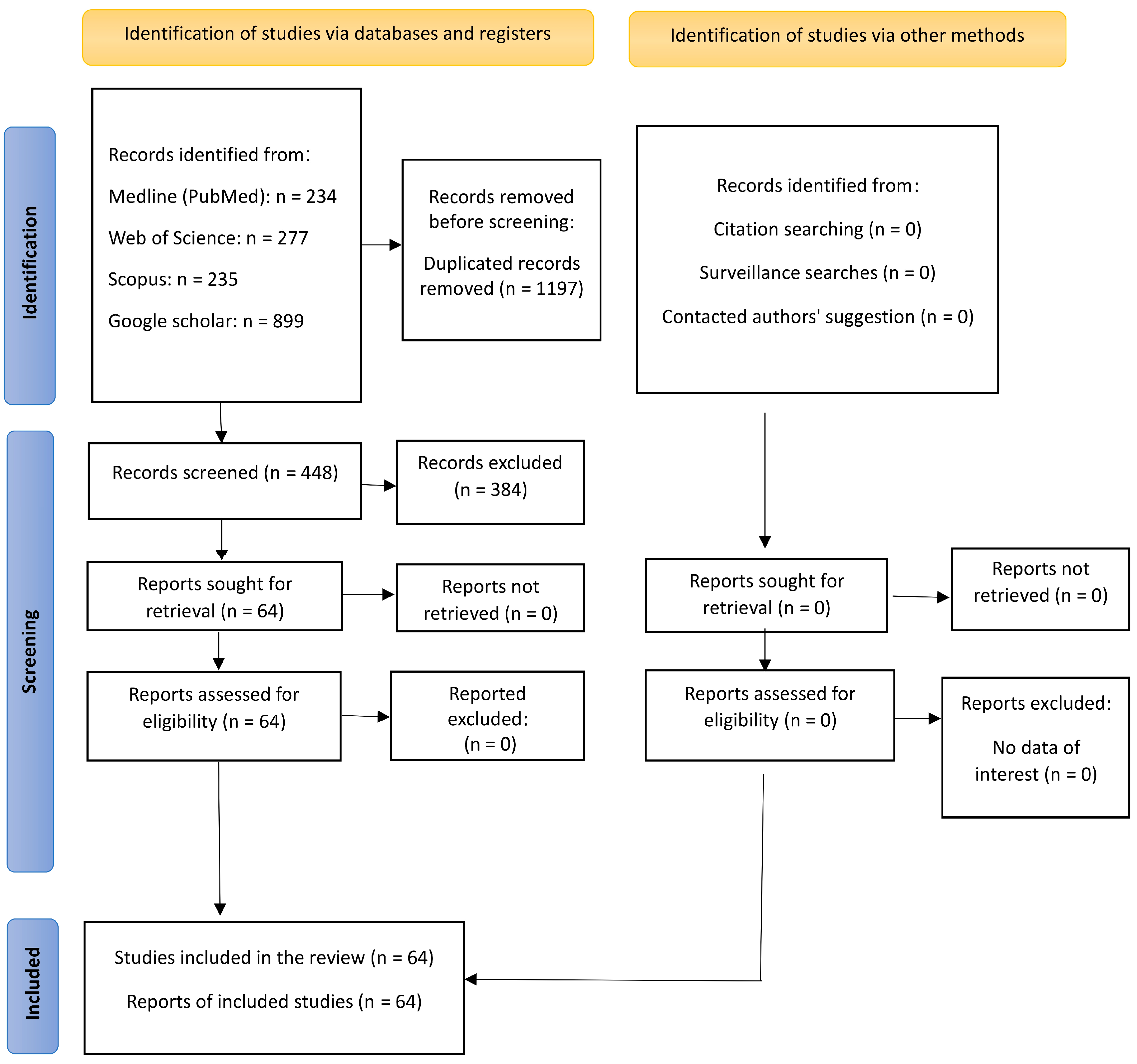
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. First Theme: Evolution from 2D Freehand Surgical Planning to 3D Virtual Surgical Planning
3.1.1. Quality and Precision of Digital Planning in Facial Reconstruction
3.1.2. CT vs. CBCT Accuracy
3.1.3. Printing Technology and Quality
3.2. Second Theme: Advances in Facial Reconstructive Surgery Using VSP
3.3. Third Theme: Examples of Some VSP Clinical Applications
3.3.1. Zygomaticomaxillary Complex Surgeries
3.3.2. For Tripod Fractures of the Orbito-Zygomaticomaxillary Complex Management There Were a Few Methods
Virtual Reduction Method
Mirroring Method
Traditional Method
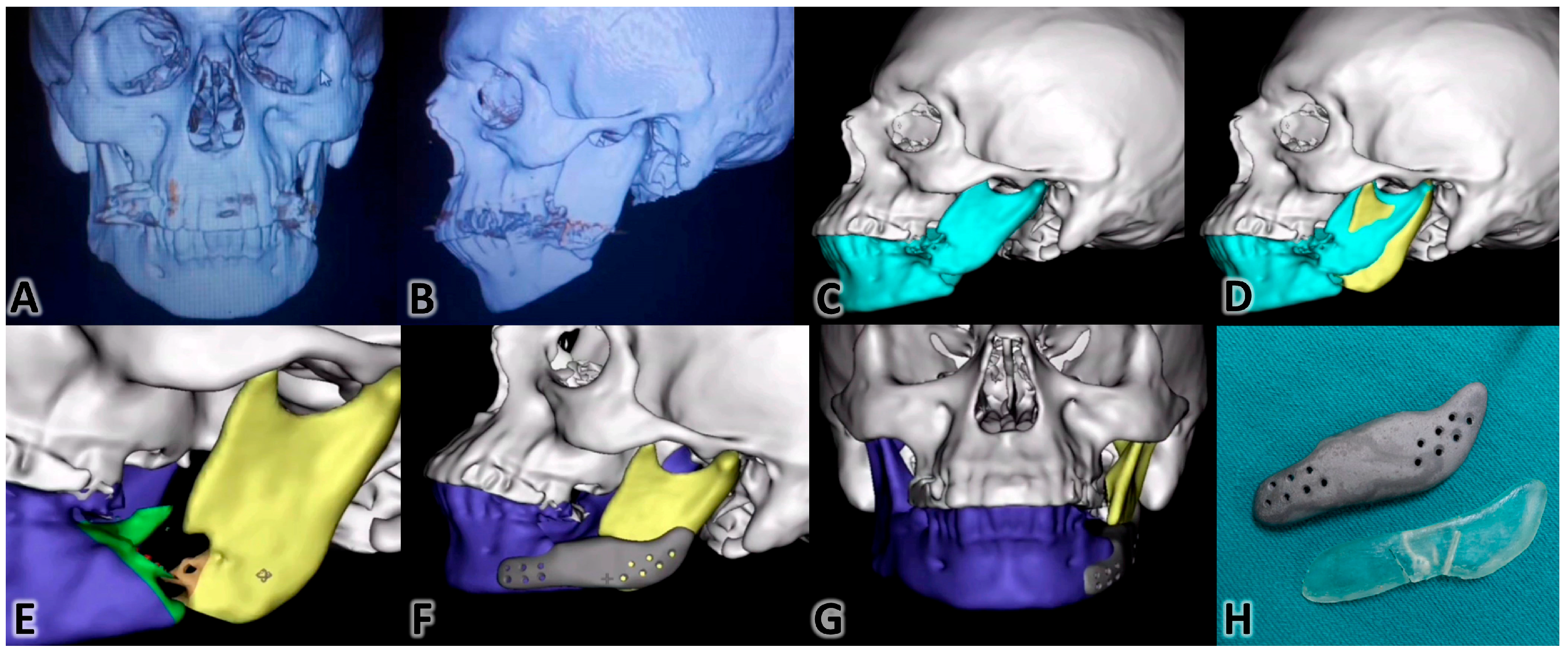
3.3.3. Mandibular Reconstruction
3.3.4. Hemifacial Microsomia
3.3.5. Soft Tissue Correction
3.3.6. Fabrication of Intraoperative Guidance
4. Discussion
4.1. Efficiency VSP in Facial Reconstructive Surgery
4.2. Applicability of VSP in Facial Reconstructive Surgery
4.3. Future Research
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VSP | virtual surgical planning |
| TSP | traditional surgical planning |
| 2D | two-dimensional |
| 3D | three-dimensional |
| CT | computed tomography |
References
- Fisher, J. Microvascular Reconstruction in the Head and Neck. Mayo Clin. Proc. 1986, 61, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Epsteen, C.M. Psychological impact of facial deformities. Am. J. Surg. 1958, 96, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Swendseid, B.P.; Roden, D.F.; Vimawala, S.; Richa, T.; Sweeny, L.; Goldman, R.A.; Luginbuhl, A.; Heffelfinger, R.N.; Khanna, S.; Curry, J.M. Virtual Surgical Planning in Subscapular System Free Flap Reconstruction of Midface Defects. Oral Oncol. 2020, 101, 104508. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, D.; Filippi, A.; Canzi, G.; De Ponti, E.; Bozzetti, A.; Novelli, G. Surgical Navigation in Mandibular Reconstruction: Accuracy Evaluation of an Innovative Protocol. J. Clin. Med. 2022, 11, 2060. [Google Scholar] [CrossRef]
- Pucci, R.; Weyh, A.; Smotherman, C.; Valentini, V.; Bunnell, A.; Fernandes, R. Accuracy of virtual planned surgery versus conventional free-hand surgery for reconstruction of the mandible with osteocutaneous free flaps. Int. J. Oral Maxillofac. Surg. 2020, 49, 1153–1161. [Google Scholar] [CrossRef]
- Yetman, D. What Is Facial Reconstructive Surgery? Available online: https://www.healthline.com/health/facial-reconstruction-surgery (accessed on 28 November 2024).
- Navarro Cuéllar, C.; Martínez, E.B.; Navarro Cuéllar, I.; López López, A.M.; Rial, M.T.; Pérez, A.S.; Salmerón Escobar, J.I. Primary Maxillary Reconstruction with Fibula Flap and Dental Implants: A Comparative Study Between Virtual Surgical Planning and Standard Surgery in Class IIC Defects. J. Oral Maxillofac. Surg. 2021, 79, 237–248. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Shield, M.E.; Allen, R.S.; Jessop, Z.M.; Dobbs, T.D.; Whitaker, I.S. Facial Reconstruction: A Systematic Review of Current Image Acquisition and Processing Techniques. Front. Surg. 2020, 7, 537616. [Google Scholar] [CrossRef]
- Patel, S.A.; Chang, E.I. Principles and practice of reconstructive surgery for head and neck cancer. Surg. Oncol. Clin. N. Am. 2015, 24, 473–489. [Google Scholar] [CrossRef]
- Lee, Y.J.; Oh, J.H.; Kim, S.G. Virtual surgical plan with custom surgical guide for orthognathic surgery: Systematic review and meta-analysis. Maxillofac. Plast. Reconstr. Surg. 2024, 46, 39. [Google Scholar] [CrossRef]
- Stella, F.; Dolci, G.; Dell’Amore, A.; Badiali, G.; De Matteis, M.; Asadi, N.; Marchetti, C.; Bini, A. Three-dimensional surgical simulation-guided navigation in thoracic surgery: A new approach to improve results in chest wall resection and reconstruction for malignant diseases. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 7–12. [Google Scholar] [CrossRef]
- Puel, U.; Beysang, A.; Hossu, G.; Eliezer, M.; Assabah, B.; Ambarki, K.; Gondim Teixeira, P.A.; Blum, A.; Parietti-Winkler, C.; Gillet, R. Comparison of CT-like MRI sequences for preoperative planning of cochlear implantation using super-high-resolution CT as a reference. Eur. Radiol. Exp. 2025, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Lemon, J.C.; Kiat-amnuay, S.; Gettleman, L.; Martin, J.W.; Chambers, M.S. Facial prosthetic rehabilitation: Preprosthetic surgical techniques and biomaterials. Curr. Opin. Otolaryngol. Head Neck Surg. 2005, 13, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Bachelet, J.T.; Jouan, R.; Prade, V.; Francisco, C.; Jaby, P.; Gleizal, A. Place of 3D printing in facial epithesis. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 224–227. [Google Scholar] [CrossRef]
- Understanding Implants: Different Types and Their Surgical Applications. Available online: https://highsurgery.com/understanding-implants-different-types-and-their-surgical-applications/ (accessed on 28 November 2024).
- Papel, I.D.; Jiannetto, D.F. Advances in computer imaging/applications in facial plastic surgery. Facial Plast. Surg. 1999, 15, 119–125. [Google Scholar] [CrossRef]
- Tzou, C.H.; Artner, N.M.; Pona, I.; Hold, A.; Placheta, E.; Kropatsch, W.G.; Frey, M. Comparison of three-dimensional surface-imaging systems. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 489–497. [Google Scholar] [CrossRef]
- Pratt, R.; Deprest, J.; Vercauteren, T.; Ourselin, S.; David, A.L. Computer-assisted surgical planning and intraoperative guidance in fetal surgery: A systematic review. Prenat. Diagn. 2015, 35, 1159–1166. [Google Scholar] [CrossRef]
- Paterick, T.E.; Patel, N.; Tajik, A.J.; Chandrasekaran, K. Improving health outcomes through patient education and partnerships with patients. Bayl. Univ. Med. Cent. Proc. 2017, 30, 112–113. [Google Scholar] [CrossRef]
- Fu, K.; Liu, Y.; Gao, N.; Cai, J.; He, W.; Qiu, W. Reconstruction of Maxillary and Orbital Floor Defect with Free Fibula Flap and Whole Individualized Titanium Mesh Assisted by Computer Techniques. J. Oral Maxillofac. Surg. 2017, 75, 1791.e1791–1791.e1799. [Google Scholar] [CrossRef]
- Numajiri, T.; Morita, D.; Nakamura, H.; Tsujiko, S.; Yamochi, R.; Sowa, Y.; Toyoda, K.; Tsujikawa, T.; Arai, A.; Yasuda, M.; et al. Using an In-House Approach to Computer-Assisted Design and Computer-Aided Manufacturing Reconstruction of the Maxilla. J. Oral Maxillofac. Surg. 2018, 76, 1361–1369. [Google Scholar] [CrossRef]
- Schepers, R.H.; Kraeima, J.; Vissink, A.; Lahoda, L.U.; Roodenburg, J.L.N.; Reintsema, H.; Raghoebar, G.M.; Witjes, M.J. Accuracy of secondary maxillofacial reconstruction with prefabricated fibula grafts using 3D planning and guided reconstruction. J. Cranio-Maxillofac. Surg. 2016, 44, 392–399. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ciocca, L.; Scotti, R.; Cipriani, R.; Marchetti, C. Surgical reconstruction of maxillary defects using a computer-assisted design/computer-assisted manufacturing-produced titanium mesh supporting a free flap. J. Cranio-Maxillofac. Surg. 2016, 44, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Kim, S.G. Redefining precision and efficiency in orthognathic surgery through virtual surgical planning and 3D printing: A narrative review. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 42. [Google Scholar] [CrossRef] [PubMed]
- McGoldrick, D.; Praveen, P.; Parmar, S. Modern Day Reconstruction of the Facial Bones. In Critical Issues in Head and Neck Oncology; Springer: Cham, Switzerland, 2023; pp. 293–303. [Google Scholar]
- Efanov, J.I.; Roy, A.A.; Huang, K.N.; Borsuk, D.E. Virtual Surgical Planning: The Pearls and Pitfalls. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1443. [Google Scholar] [CrossRef]
- Parisi, F.R.; Zampogna, B.; Zampoli, A.; Ferrini, A.; Albimonti, G.; Del Monaco, A.; Za, P.; Papalia, G.F.; Papalia, R. Planning Accuracy and Stem Offset Assessment in Digital Two-Dimensional Versus Three-Dimensional Planning in Cementless Hip Arthroplasty: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 6566. [Google Scholar] [CrossRef]
- Kamel, M.E.; Alsayed, A.A.E.; ElKhashab, M.A.; Nader, N.; Radi, I.A. Passive fit and time efficiency for prefabricated versus conventionally constructed cobalt chromium CAD\CAM 3-unit implant supported frameworks in free end saddle models: A pilot invitro study. BMC Oral Health 2024, 24, 1225. [Google Scholar] [CrossRef]
- Biswas, G. Midface Reconstruction: Planning and Outcome. Indian J. Plast. Surg. 2020, 53, 324–334. [Google Scholar] [CrossRef]
- Saad, A.; Winters, R.; Wise, M.W.; Dupin, C.L.; St Hilaire, H. Virtual surgical planning in complex composite maxillofacial reconstruction. Plast. Reconstr. Surg. 2013, 132, 626–633. [Google Scholar] [CrossRef]
- Jacobs, C.A.; Lin, A.Y. A New Classification of Three-Dimensional Printing Technologies: Systematic Review of Three-Dimensional Printing for Patient-Specific Craniomaxillofacial Surgery. Plast. Reconstr. Surg. 2017, 139, 1211–1220. [Google Scholar] [CrossRef]
- Jang, W.H.; Lee, J.M.; Jang, S.; Kim, H.D.; Ahn, K.M.; Lee, J.H. Mirror Image Based Three-Dimensional Virtual Surgical Planning and Three-Dimensional Printing Guide System for the Reconstruction of Wide Maxilla Defect Using the Deep Circumflex Iliac Artery Free Flap. J. Craniofac. Surg. 2019, 30, 1829–1832. [Google Scholar] [CrossRef]
- Seth, I.; Lim, B.; Lu, P.Y.J.; Xie, Y.; Cuomo, R.; Ng, S.K.-H.; Rozen, W.M.; Sofiadellis, F. Digital Twins Use in Plastic Surgery: A Systematic Review. J. Clin. Med. 2024, 13, 7861. [Google Scholar] [CrossRef]
- Wilkinson, C.; Rynn, C.; Peters, H.; Taister, M.; Kau, C.H.; Richmond, S. A blind accuracy assessment of computer-modeled forensic facial reconstruction using computed tomography data from live subjects. Forensic Sci. Med. Pathol. 2006, 2, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Assari, A. Usability of Three-dimensional Printing in Maxillofacial Surgery: A Narrative Review. Open Dent. J. 2023, 17, e187421062304190. [Google Scholar] [CrossRef]
- Melek, L.N.; Noureldin, M.G. Zygomaticomaxillary complex fractures: Finding the least complicated surgical approach (A Randomized Clinical Trial). BMC Oral Health 2023, 23, 539. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.; Barnes, C.; Wong, B.J.F. Overview of Facial Plastic Surgery and Current Developments. Surg. J. 2016, 2, e17–e28. [Google Scholar] [CrossRef]
- De Oliveira Santos, A.B.; Cernea, C.R. Open Access Atlas of Otolaryngology, Head & Neck Operative Surgery; University of Cape Town: Cape Town, South Africa, 2014. [Google Scholar]
- Gómez Roselló, E.; Quiles Granado, A.M.; Artajona Garcia, M.; Juanpere Martí, S.; Laguillo Sala, G.; Beltrán Mármol, B.; Pedraza Gutiérrez, S. Facial fractures: Classification and highlights for a useful report. Insights Imaging 2020, 11, 49. [Google Scholar] [CrossRef]
- Petrova, I.; Dzhongova, E.; Georgieva, V. Applications of 3D printing in oral and maxillofacial surgery. Scr. Sci. Med. Dent. 2022, 8, 14–21. [Google Scholar] [CrossRef]
- Wilde, F.; Schramm, A. Computer-aided reconstruction of the facial skeleton: Planning and implementation in clinical routine. HNO 2016, 64, 641–649. [Google Scholar] [CrossRef]
- Ursan, I.D.; Chiu, L.; Pierce, A. Three-dimensional drug printing: A structured review. J. Am. Pharm. Assoc. 2013, 53, 136–144. [Google Scholar] [CrossRef]
- Antony, A.K.; Chen, W.F.; Kolokythas, A.; Weimer, K.A.; Cohen, M.N. Use of virtual surgery and stereolithography-guided osteotomy for mandibular reconstruction with the free fibula. Plast. Reconstr. Surg. 2011, 128, 1080–1084. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, J.; Li, J.; Li, M.M.; Huang, W.; Ow, A. Special considerations in virtual surgical planning for secondary accurate maxillary reconstruction with vascularised fibula osteomyocutaneous flap. J. Plast. Reconstr. Aesthet. Surg. 2012, 65, 893–902. [Google Scholar] [CrossRef]
- Tonin, R.H.; Iwaki Filho, L.; Yamashita, A.L.; Ferraz, F.; Tolentino, E.S.; Previdelli, I.; Brum, B.; Iwaki, L.C.V. Accuracy of 3D virtual surgical planning for maxillary positioning and orientation in orthognathic surgery. Orthod. Craniofac. Res. 2020, 23, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Martelli, N.; Serrano, C.; van den Brink, H.; Pineau, J.; Prognon, P.; Borget, I.; El Batti, S. Advantages and disadvantages of 3-dimensional printing in surgery: A systematic review. Surgery 2016, 159, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Otterburn, D.; Saadeh, P.; Levine, J.; Hirsch, D.L. 3D volume assessment techniques and computer-aided design and manufacturing for preoperative fabrication of implants in head and neck reconstruction. Facial Plast. Surg. Clin. N. Am. 2011, 19, 683–709. [Google Scholar] [CrossRef] [PubMed]
- Rodby, K.A.; Turin, S.; Jacobs, R.J.; Cruz, J.F.; Hassid, V.J.; Kolokythas, A.; Antony, A.K. Advances in oncologic head and neck reconstruction: Systematic review and future considerations of virtual surgical planning and computer aided design/computer aided modeling. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1171–1185. [Google Scholar] [CrossRef]
- Hou, J.S.; Chen, M.; Pan, C.B.; Tao, Q.; Wang, J.G.; Wang, C.; Zhang, B.; Huang, H.Z. Immediate reconstruction of bilateral mandible defects: Management based on computer-aided design/computer-aided manufacturing rapid prototyping technology in combination with vascularized fibular osteomyocutaneous flap. J. Oral Maxillofac. Surg. 2011, 69, 1792–1797. [Google Scholar] [CrossRef]
- Hanken, H.; Schablowsky, C.; Smeets, R.; Heiland, M.; Sehner, S.; Riecke, B.; Nourwali, I.; Vorwig, O.; Gröbe, A.; Al-Dam, A. Virtual planning of complex head and neck reconstruction results in satisfactory match between real outcomes and virtual models. Clin. Oral Investig. 2015, 19, 647–656. [Google Scholar] [CrossRef]
- Nilsson, J.; Hindocha, N.; Thor, A. Time matters-Differences between computer-assisted surgery and conventional planning in cranio-maxillofacial surgery: A systematic review and meta-analysis. J. Cranio-Maxillofac. Surg. 2020, 48, 132–140. [Google Scholar] [CrossRef]
- Seruya, M.; Fisher, M.; Rodriguez, E.D. Computer-assisted versus conventional free fibula flap technique for craniofacial reconstruction: An outcomes comparison. Plast. Reconstr. Surg. 2013, 132, 1219–1228. [Google Scholar] [CrossRef]
- Smithers, F.A.E.; Cheng, K.; Jayaram, R.; Mukherjee, P.; Clark, J.R. Maxillofacial reconstruction using in-house virtual surgical planning. ANZ J. Surg. 2018, 88, 907–912. [Google Scholar] [CrossRef]
- Maglitto, F.; Dell’Aversana Orabona, G.; Committeri, U.; Salzano, G.; De Fazio, G.R.; Vaira, L.A.; Abbate, V.; Bonavolontà, P.; Piombino, P.; Califano, L. Virtual surgical planning and the “in-house” rapid prototyping technique in maxillofacial surgery: The current situation and future perspectives. Appl. Sci. 2021, 11, 1009. [Google Scholar] [CrossRef]
- Ciocca, L.; Mazzoni, S.; Fantini, M.; Marchetti, C.; Scotti, R. The design and rapid prototyping of surgical guides and bone plates to support iliac free flaps for mandible reconstruction. Plast. Reconstr. Surg. 2012, 129, 859e–861e. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.E.; Mullin, D.P.; Patel, A.K. Reconstruction of the segmental mandibular defect: Current state of the art. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Modabber, A.; Gerressen, M.; Stiller, M.B.; Noroozi, N.; Füglein, A.; Hölzle, F.; Riediger, D.; Ghassemi, A. Computer-assisted mandibular reconstruction with vascularized iliac crest bone graft. Aesthetic Plast. Surg. 2012, 36, 653–659. [Google Scholar] [CrossRef]
- Ayoub, N.; Ghassemi, A.; Rana, M.; Gerressen, M.; Riediger, D.; Hölzle, F.; Modabber, A. Evaluation of computer-assisted mandibular reconstruction with vascularized iliac crest bone graft compared to conventional surgery: A randomized prospective clinical trial. Trials 2014, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Sorice, S.; Hershman, G.N.; Saadeh, P.; Levine, J.P.; Hirsch, D. Use of virtual 3-dimensional surgery in post-traumatic craniomaxillofacial reconstruction. J. Oral Maxillofac. Surg. 2011, 69, 733–741. [Google Scholar] [CrossRef]
- Chopra, K.; Gastman, B.R.; Manson, P.N. Stereolithographic modeling in reconstructive surgery of the craniofacial skeleton after tumor resection. Plast. Reconstr. Surg. 2012, 129, 743e–745e. [Google Scholar] [CrossRef]
- Naros, A.; Weise, H.; Tilsen, F.; Hoefert, S.; Naros, G.; Krimmel, M.; Reinert, S.; Polligkeit, J. Three-dimensional accuracy of mandibular reconstruction by patient-specific pre-bent reconstruction plates using an “in-house” 3D-printer. J. Cranio-Maxillofac. Surg. 2018, 46, 1645–1651. [Google Scholar] [CrossRef]
- Yang, W.F.; Choi, W.S.; Wong, M.C.; Powcharoen, W.; Zhu, W.Y.; Tsoi, J.K.; Chow, M.; Kwok, K.W.; Su, Y.X. Three-Dimensionally Printed Patient-Specific Surgical Plates Increase Accuracy of Oncologic Head and Neck Reconstruction Versus Conventional Surgical Plates: A Comparative Study. Ann. Surg. Oncol. 2021, 28, 363–375. [Google Scholar] [CrossRef]
- Zhang, W.B.; Yu, Y.; Wang, Y.; Mao, C.; Liu, X.J.; Guo, C.B.; Yu, G.Y.; Peng, X. Improving the accuracy of mandibular reconstruction with vascularized iliac crest flap: Role of computer-assisted techniques. J. Cranio-Maxillofac. Surg. 2016, 44, 1819–1827. [Google Scholar] [CrossRef]
- Bartier, S.; Mazzaschi, O.; Benichou, L.; Sauvaget, E. Computer-assisted versus traditional technique in fibular free-flap mandibular reconstruction: A CT symmetry study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 23–27. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Skoracki, R.J. Computer-assisted design and rapid prototype modeling in microvascular mandible reconstruction. Laryngoscope 2013, 123, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Jenkins, M.P.; Patel, S.A.; Topham, N.S. Long-Term Operative Outcomes of Preoperative Computed Tomography-Guided Virtual Surgical Planning for Osteocutaneous Free Flap Mandible Reconstruction. Plast. Reconstr. Surg. 2016, 137, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Resnick, C.M.; Inverso, G.; Wrzosek, M.; Padwa, B.L.; Kaban, L.B.; Peacock, Z.S. Is There a Difference in Cost Between Standard and Virtual Surgical Planning for Orthognathic Surgery? J. Oral Maxillofac. Surg. 2016, 74, 1827–1833. [Google Scholar] [CrossRef]
- Patel, N.; Kim, B.; Zaid, W. Use of Virtual Surgical Planning for Simultaneous Maxillofacial Osteotomies and Custom Polyetheretherketone Implant in Secondary Orbito-Frontal Reconstruction: Importance of Restoring Orbital Volume. J. Craniofac. Surg. 2017, 28, 387–390. [Google Scholar] [CrossRef]
- Longeac, M.; Depeyre, A.; Pereira, B.; Barthelemy, I.; Pham Dang, N. Virtual surgical planning and three-dimensional printing for the treatment of comminuted zygomaticomaxillary complex fracture. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Aziz, S.; Shum, J.W. Virtual Surgical Planning in Oral and Maxillofacial Surgery. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 519–530. [Google Scholar] [CrossRef]
- Ho, J.; Schreurs, R.; Milstein, D.M.J.; Dubois, L.; Maal, T.J.J.; de Lange, J.; Becking, A.G. Measuring zygomaticomaxillary complex symmetry three-dimensionally with the use of mirroring and surface based matching techniques. J. Cranio-Maxillofac. Surg. 2016, 44, 1706–1712. [Google Scholar] [CrossRef]
- Shukla, D.; Bhola, N. Management of Ectropion Associated with a Malunited Zygomaticomaxillary Complex Fracture: A Case Report. Cureus 2024, 16, e52909. [Google Scholar] [CrossRef]
- Committeri, U.; Magliulo, R.; Carraturo, E.; Arena, A.; Abbate, V.; Salzano, G.; Troise, S.; Barone, S.; Germano, C.; Vaira, L.A.; et al. Virtual surgical planning in tripod zygomatico-maxillary complex fractures: A prospective comparison between two different strategies. J. Cranio-Maxillofac. Surg. 2024, 52, 1497–1504. [Google Scholar] [CrossRef]
- Dessoky, N.Y.; El-Mahallawy, A.S.; Fahmy, M.H.; Khalil, M.M. Use of custom made peek plates for treatment of mandibular fracture. Alex. Dent. J. 2020, 45, 125–128. [Google Scholar] [CrossRef]
- Möllmann, H.L.; Apeltrath, L.; Karnatz, N.; Wilkat, M.; Riedel, E.; Singh, D.D.; Rana, M. Comparison of the Accuracy and Clinical Parameters of Patient-Specific and Conventionally Bended Plates for Mandibular Reconstruction. Front. Oncol. 2021, 11, 719028. [Google Scholar] [CrossRef] [PubMed]
- Probst, F.A.; Liokatis, P.; Mast, G.; Ehrenfeld, M. Virtual planning for mandible resection and reconstruction. Innov. Surg. Sci. 2023, 8, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.; Thomas, J.; Saha, M.; Srikanth, G.; Kamath, A.T.; Abhijith, S.M. Mandibular Reconstruction Modalities Using Virtual Surgical Planning and 3D Printing Technology: A Tertiary Care Centre Experience. J. Maxillofac. Oral Surg. 2025, 24, 246–254. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Zhang, Z.; Li, X.; Ye, B.; Li, J. Comprehensive consideration and design with the virtual surgical planning-assisted treatment for hemifacial microsomia in adult patients. J. Cranio-Maxillofac. Surg. 2018, 46, 1268–1274. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Z.; Wang, Y.; Li, X.; Ye, B.; Li, J. The accuracy of virtual-surgical-planning-assisted treatment of hemifacial microsomia in adult patients: Distraction osteogenesis vs. orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2019, 48, 341–346. [Google Scholar] [CrossRef]
- Shalabi, M.M.; Darwich, K.M.A.; Kheshfeh, M.N.; Hajeer, M.Y. Accuracy of 3D Virtual Surgical Planning Compared to the Traditional Two-Dimensional Method in Orthognathic Surgery: A Literature Review. Cureus 2024, 16, e73477. [Google Scholar] [CrossRef]
- Singh, P.; Wang, C.; Ajmera, D.H.; Xiao, S.S.; Song, J.; Lin, Z. Biomechanical Effects of Novel Osteotomy Approaches on Mandibular Expansion: A Three-Dimensional Finite Element Analysis. J. Oral Maxillofac. Surg. 2016, 74, 1658.e1–1658.e15. [Google Scholar] [CrossRef]
- Lo, L.J.; Yamaguchi, K.; Niu, L.S.; Liao, C.H.; Lin, H.H. Fat Grafting in Patients with Extensive Unilateral Facial Deficiency: Three-Dimensional Computer-Assisted Planning, Implementation, and Outcome Assessment. Ann. Plast. Surg. 2020, 84, S94–S99. [Google Scholar] [CrossRef]
- Marschall, J.S.; Dutra, V.; Flint, R.L.; Kushner, G.M.; Alpert, B.; Scarfe, W.; Azevedo, B. In-House Digital Workflow for the Management of Acute Mandible Fractures. J. Oral Maxillofac. Surg. 2019, 77, 2084.e2081–2084.e2089. [Google Scholar] [CrossRef]
- Ramanathan, M.; Panneerselvam, E.; Krishna Kumar Raja, V.B. 3D planning in mandibular fractures using CAD/CAM surgical splints-A prospective randomized controlled clinical trial. J. Cranio-Maxillofac. Surg. 2020, 48, 405–412. [Google Scholar] [CrossRef]
- Demian, N.; Pearl, C.; Woernley, T.C., 3rd; Wilson, J.; Seaman, J. Surgical Navigation for Oral and Maxillofacial Surgery. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Azarmehr, I.; Stokbro, K.; Bell, R.B.; Thygesen, T. Surgical Navigation: A Systematic Review of Indications, Treatments, and Outcomes in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2017, 75, 1987–2005. [Google Scholar] [CrossRef] [PubMed]
- Oyama, A.; Kumagai, S.; Arai, N.; Takata, T.; Saikawa, Y.; Shiraishi, K.; Kobayashi, T.; Kotoku, J.i. Image quality improvement in cone-beam CT using the super-resolution technique. J. Radiat. Res. 2018, 59, 501–510. [Google Scholar] [CrossRef] [PubMed]

| Treatment plan | facial bone reconstruction, facial fractures, hemifacial microsomia, facial bone grafting, osteotomies, augmentation, replacement, grafting, breakages, and jaw replacement. |
| Surgical planning tools | virtual surgical planning, computer-assisted methods, free-hand methods, traditional surgical planning, 3D digital planning, 2D methods, |
| Outcomes | accuracy, satisfaction, operation time, cost, discomfort, stabilization, harms, recurrence, untoward effects. |
| PubMed Publication date: Up to 8 December 2024 Search builder: All fields | #1 (facial bone reconstruction “OR” facial fractures “OR” hemifacial microsomia “OR” facial bone grafting “OR” osteotomies “OR” augmentation “OR” replacement “OR” grafting “OR” breakages “OR” jaw replacement) #2 (virtual surgical planning “OR” computer-assisted methods “OR” free-hand methods “OR” traditional surgical planning “OR” 3D digital planning “OR” 2D methods) #3 (accuracy “OR” satisfaction “OR” operation time “OR” cost “OR” discomfort “OR” stabilization “OR” harms “OR” recurrence “OR” untoward effects) #4 #1 AND #2 AND #3 |
| Web of Science | #1TS= (facial bone reconstruction OR facial fractures OR hemifacial microsomia OR facial bone grafting OR osteotomies OR augmentation OR replacement OR grafting OR breakages OR jaw replacement) #2TS= (virtual surgical planning OR computer-assisted methods OR free-hand methods OR traditional surgical planning OR 3D digital planning OR 2D methods) #3TS= (accuracy OR satisfaction OR operation time OR cost OR discomfort “OR” stabilization OR harms OR recurrence OR untoward effects) #4 #1 AND #2 AND #3 |
| Scopus Publication date: Up to 8 December 2024 | #1 TITLE ABS-KEY 1 (facial bone reconstruction “OR” facial fractures “OR” hemifacial microsomia “OR” facial bone grafting “OR” osteotomies “OR” augmentation “OR” replacement “OR” grafting “OR” breakages “OR” jaw replacement) #2 TITLE ABS-KEY (virtual surgical planning “OR” computer-assisted methods “OR” free-hand methods “OR” traditional surgical planning “OR” 3D digital planning “OR” 2D methods) #3 TITLE ABS-KEY (accuracy “OR” satisfaction “OR” operation time “OR” cost “OR” discomfort “OR” stabilization “OR” harms “OR” recurrence “OR” untoward effects) #4 #1 AND #2 AND #3 |
| Google Scholar | (facial bone reconstruction “OR” facial fractures “OR” hemifacial microsomia “OR” facial bone grafting “OR” osteotomies “OR” augmentation “OR” replacement “OR” grafting “OR” breakages “OR” jaw replacement) AND (virtual surgical planning “OR” computer-assisted methods “OR” free-hand methods “OR” traditional surgical planning “OR” 3D digital planning “OR” 2D methods) AND (accuracy “OR” satisfaction “OR” operation time “OR” cost “OR” discomfort “OR” stabilization “OR” harms “OR” recurrence “OR” untoward effects) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalabi, M.M.; Darwich, K.M.A.; Kheshfeh, M.N.; Hajeer, M.Y. Efficiency and Applicability of Virtual Surgical Planning in Maxillofacial and Mandibular Bone Reconstruction: A Narrative Review. Clin. Pract. 2025, 15, 62. https://doi.org/10.3390/clinpract15030062
Shalabi MM, Darwich KMA, Kheshfeh MN, Hajeer MY. Efficiency and Applicability of Virtual Surgical Planning in Maxillofacial and Mandibular Bone Reconstruction: A Narrative Review. Clinics and Practice. 2025; 15(3):62. https://doi.org/10.3390/clinpract15030062
Chicago/Turabian StyleShalabi, Mohammed Mahmoud, Khaldoun M. A. Darwich, Mohammad Naem Kheshfeh, and Mohammad Younis Hajeer. 2025. "Efficiency and Applicability of Virtual Surgical Planning in Maxillofacial and Mandibular Bone Reconstruction: A Narrative Review" Clinics and Practice 15, no. 3: 62. https://doi.org/10.3390/clinpract15030062
APA StyleShalabi, M. M., Darwich, K. M. A., Kheshfeh, M. N., & Hajeer, M. Y. (2025). Efficiency and Applicability of Virtual Surgical Planning in Maxillofacial and Mandibular Bone Reconstruction: A Narrative Review. Clinics and Practice, 15(3), 62. https://doi.org/10.3390/clinpract15030062








