Suboptimal LDL-C Goal Attainment After Ischemic Stroke and TIA: Prevalence, Determinants, and Clinical Implications
Abstract
1. Introduction
2. Methods
2.1. Study Setting and Participants
2.2. Variables and Data Collection
2.3. Statistical Analyses
2.4. Ethical Considerations
3. Results
3.1. Baseline Characteristics of Participants
3.2. Prevalence of LDL-C Goal Attainment
3.3. Determinants of LDL-C Goal Attainment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katan, M.; Luft, A. Global burden of stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Authors/Task Force Members; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A., III; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.A.; et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef]
- Pan, Y.; Wangqin, R.; Li, H.; Jin, A.; Li, J.; Lin, J.; Meng, X.; Xian, Y.; Laskowitz, D.T.; Wang, Y. LDL-C levels, lipid-lowering treatment and recurrent stroke in minor ischaemic stroke or TIA. Stroke Vasc. Neurol. 2022, 7, 276–284. [Google Scholar] [CrossRef]
- Amarenco, P.; Kim, J.S.; Labreuche, J.; Charles, H.; Abtan, J.; Béjot, Y.; Cabrejo, L.; Cha, J.-K.; Ducrocq, G.; Giroud, M.; et al. A comparison of two LDL cholesterol targets after ischemic stroke. N. Engl. J. Med. 2020, 382, 9. [Google Scholar] [CrossRef]
- Amarenco, P.; Lavallée, P.C.; Kim, J.S.; Labreuche, J.; Charles, H.; Giroud, M.; Lee, B.-C.; Mahagne, M.-H.; Meseguer, E.; Nighoghossian, N.; et al. More than 50 percent reduction in LDL cholesterol in patients with target LDL < 70 mg/dL after a stroke. Stroke 2023, 54, 1993–2001. [Google Scholar] [CrossRef]
- Chen, K.N.; He, L.; Zhong, L.M.; Ran, Y.Q.; Liu, Y. Meta-analysis of dyslipidemia management for the prevention of ischemic stroke recurrence in China. Front. Neurol. 2020, 11, 483570. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, Y.L.; Li, Z.X.; Wang, Y.J. The management of LDL cholesterol and predictors of goal achievement in stroke patients in China: A cross-sectional study. CNS Neurosci. Ther. 2016, 22, 577–583. [Google Scholar] [CrossRef]
- Zafrir, B.; Aker, A.; Naoum, I.; Saliba, W. Guideline-directed low-density lipoprotein cholesterol management after acute ischemic stroke: Findings from a national health care service. Am. J. Cardiol. 2023, 203, 332–338. [Google Scholar] [CrossRef]
- Barrios, V.; Pintó, X.; Escobar, C.; Varona, J.F.; Gámez, J.M. Real-world attainment of low-density lipoprotein cholesterol goals in patients at high risk of cardiovascular disease treated with high-intensity statins: The TERESA Study. J. Clin. Med. 2023, 12, 3187. [Google Scholar] [CrossRef]
- Wongsalap, Y.; Jedsadayanmata, A. Trends and predictors of high-intensity statin therapy and LDL-C goal achievement among Thai patients with acute coronary syndrome. J. Cardiol. 2020, 75, 275–281. [Google Scholar] [CrossRef]
- Mostaza, J.M.; García-Ortiz, L.; Suárez Tembra, M.A.; Calle, P.T.; García, J.C.; Pérez, V.E.; Díaz-Díaz, J.; Manzano-Espinosa, L.; Catapano, A.; Ray, K.; et al. Failure of LDL-C goals achievement and underuse of lipid-lowering therapies in patients at high and very high cardiovascular risk: Spanish subset from the European SANTORINI study. Rev. Clin. Esp. 2025, 225, 78–84. [Google Scholar] [CrossRef]
- Navar, A.M.; Electricwala, B.; Multani, J.K.; Zhou, Z.; Chen, C.-C.; Agatep, B.C.; Petrilla, A.A.; Schwartz, T.T.; N’DRi, L.; Cristino, J.; et al. Lipid management in United States commercial and Medicare enrollees with atherosclerotic cardiovascular disease: Treatment patterns and low-density lipoprotein cholesterol control. Am. J. Cardiol. 2025, 242, 1–9. [Google Scholar] [CrossRef]
- Ersbøll, A.K.; Kristensen, M.S.; Nybo, M.; Hede, S.M.; Mikkelsen, K.H.; Gislason, G.; Larsen, M.L.; Green, A. Trends in low-density lipoprotein cholesterol goal achievement and changes in lipid-lowering therapy after incident atherosclerotic cardiovascular disease: Danish cohort study. PLoS ONE 2023, 18, e0286376. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Phrommintikul, A.; Boonyaratvej, S.; Na Ayudhya, R.K.; Tatsanavivat, P.; Komoltri, C.; Sritara, P.; CORE Investigators. The rate of patients at high risk for cardiovascular disease with an optimal low-density cholesterol level: A multicenter study from Thailand. J. Geriatr. Cardiol. 2019, 16, 344–353. [Google Scholar] [CrossRef]
- Lee, M.; Cheng, C.Y.; Wu, Y.L.; Lee, J.D.; Hsu, C.Y.; Ovbiagele, B. Association between intensity of low-density lipoprotein cholesterol reduction with statin-based therapies and secondary stroke prevention: A meta-analysis of randomized clinical trials. JAMA Neurol. 2022, 79, 349–358. [Google Scholar] [CrossRef]
- Chen, P.S.; Cheng, C.L.; Kao Yang, Y.H.; Li, Y.H. Statin adherence after ischemic stroke or transient ischemic attack is associated with clinical outcome. Circ. J. 2016, 80, 731–737. [Google Scholar] [CrossRef]
- Colivicchi, F.; Bassi, A.; Santini, M.; Caltagirone, C. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke 2007, 38, 2652–2657. [Google Scholar] [CrossRef]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef]
- Ray, K.K.; Aguiar, C.; Arca, M.; Connolly, D.L.; Eriksson, M.; Ferrières, J.; Laufs, U.; Mostaza, J.M.; Nanchen, D.; Bardet, A.; et al. Use of combination therapy is associated with improved LDL cholesterol management: 1-year follow-up results from the European observational SANTORINI study. Eur. J. Prev. Cardiol. 2024, 31, 1792–1803. [Google Scholar] [CrossRef]
- Adams, S.P.; Tsang, M.; Wright, J.M. Lipid-lowering efficacy of atorvastatin. Cochrane Database Syst. Rev. 2015, 2015, CD008226. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Safety and efficacy of statins in Asians. Am. J. Cardiol. 2007, 99, 410–414. [Google Scholar] [CrossRef]
- Tomlinson, B.; Chan, P.; Liu, Z.M. Statin intolerance-an Asian perspective. J. Atheroscler. Thromb. 2020, 27, 485–488. [Google Scholar] [CrossRef]
- Li, Y.F.; Feng, Q.Z.; Gao, W.Q.; Zhang, X.J.; Huang, Y.; Chen, Y.D. The difference between Asian and Western in the effect of LDL-C lowering therapy on coronary atherosclerotic plaque: A meta-analysis report. BMC Cardiovasc. Disord. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Saely, C.H.; Eber, B.; Pfeiffer, K.P.; Drexel, H.; LIIFE-IN-LIFE study group. Low serum LDL cholesterol in patients with type 2 diabetes: An analysis on two different patient populations. Int. J. Cardiol. 2010, 144, 394–398. [Google Scholar] [CrossRef]
- Saely, C.H.; Sternbauer, S.; Vonbank, A.; Heinzle, C.; Zanolin-Purin, D.; Larcher, B.; Mader, A.; Leiherer, A.; Muendlein, A.; Drexel, H. Type 2 diabetes mellitus is a strong predictor of LDL cholesterol target achievement in patients with peripheral artery disease. J. Diabetes Complicat. 2020, 34, 107692. [Google Scholar] [CrossRef]
- Aparisi, Á.; Martín-Fernández, M.; Ybarra-Falcón, C.; Gil, J.F.; Carrasco-Moraleja, M.; Martínez-Paz, P.; Cusácovich, I.; Gonzalo-Benito, H.; Fuertes, R.; Marcos-Mangas, M.; et al. Dyslipidemia and inflammation as hallmarks of oxidative stress in COVID-19: A follow-up study. Int. J. Mol. Sci. 2022, 23, 15350. [Google Scholar] [CrossRef]
- Kowalska, K.; Sabatowska, Z.; Forycka, J.; Młynarska, E.; Franczyk, B.; Rysz, J. The influence of SARS-CoV-2 infection on lipid metabolism-the potential use of lipid-lowering agents in COVID-19 management. Biomedicines 2022, 10, 2320. [Google Scholar] [CrossRef]
- Govori, V.; Budinčević, H.; Morović, S.; Đerke, F.; Demarin, V. Updated perspectives on lifestyle interventions as secondary stroke prevention measures: A narrative review. Medicina 2024, 60, 504. [Google Scholar] [CrossRef] [PubMed]
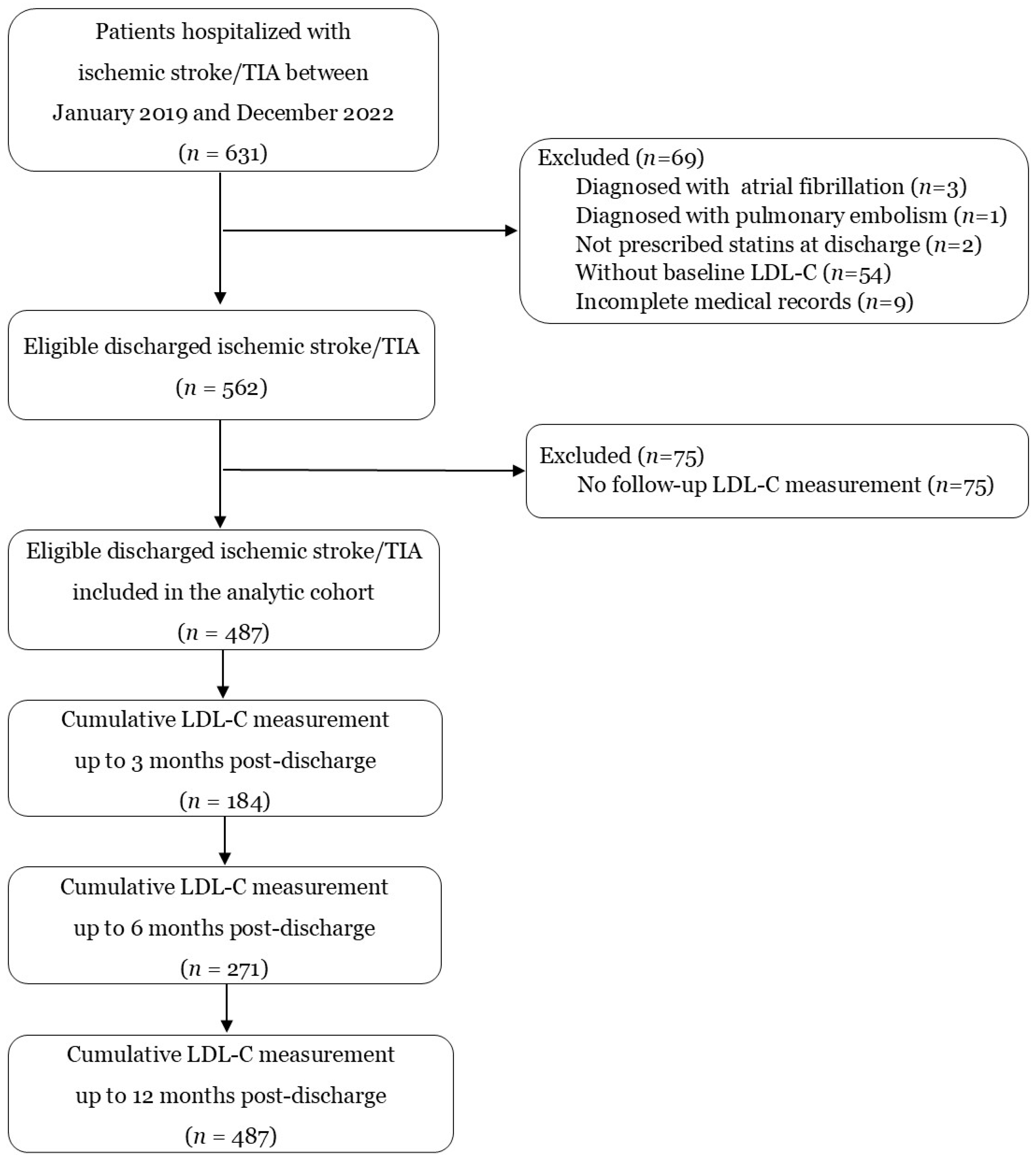
| Characteristics | Values |
|---|---|
| Age (years)—median (IQR) | 64 (54–73) |
| Age 60 years—no. (%) | 295 (60.6) |
| Male—no. (%) | 241 (49.5) |
| Body mass index (kg/m2)—median (IQR) | 24.1 (21.3–27.1) |
| Principal Diagnosis—no. (%) | |
| • Ischemic stroke | 418 (85.8) |
| • Transient ischemic attack | 69 (14.2) |
| NIHSS score—median (IQR) (n = 388) | 3 (2–5) |
| ABCD2 score—median (IQR) (n = 63) | 4 (3–5) |
| Current smoking—no. (%) | 123 (25.3) |
| Comorbidities—no. (%) | |
| • Hypertension | 368 (75.6) |
| • Diabetes mellitus | 166 (34.1) |
| • Chronic kidney disease | 38 (7.8) |
| • Coronary artery disease | 18 (3.7) |
| Glycated hemoglobin (mg/dL)—median (IQR) | 7.2 (5.9–7.2) |
| Serum creatinine (mg/dL)—median (IQR) | 0.9 (0.7–1.1) |
| Baseline lipid profile (mg/dL)—median (IQR) | |
| • Total cholesterol | 197 (163–235) |
| • LDL-C | 122 (92–155) |
| • HDL-C | 45 (36–53) |
| • Triglyceride | 113 (78–170) |
| Discharged statins—no. (%) | |
| • Low–intensity statins (simvastatin 10 mg daily) | 14 (2.9) |
| • Moderate–intensity statins | 108 (22.2) |
| • Atorvastatin 20 mg daily | 6 (1.2) |
| • Simvastatin 40 mg daily | 3 (0.6) |
| • Simvastatin 20 mg daily | 99 (20.4) |
| • High–intensity statins (atorvastatin 40 mg daily) | 365 (74.9) |
| Other discharge medications—no. (%) | |
| • Aspirin | 477 (97.9) |
| • Clopidogrel | 188 (38.6) |
| • Beta-blockers | 26 (5.3) |
| • ACEIs or ARBs | 113 (23.2) |
| Time of LDL-C measurement post-discharge—no. (%) | |
| • 1 to 3 months | 184 (37.8) |
| • 4 to 6 months | 87 (17.9) |
| • 7 to 12 months | 216 (44.3) |
| Time to first LDL-C measurement—mean (SD) | 6.4 (4.1) |
| Time to first LDL-C measurement—median (IQR) | 6 (3–11) |
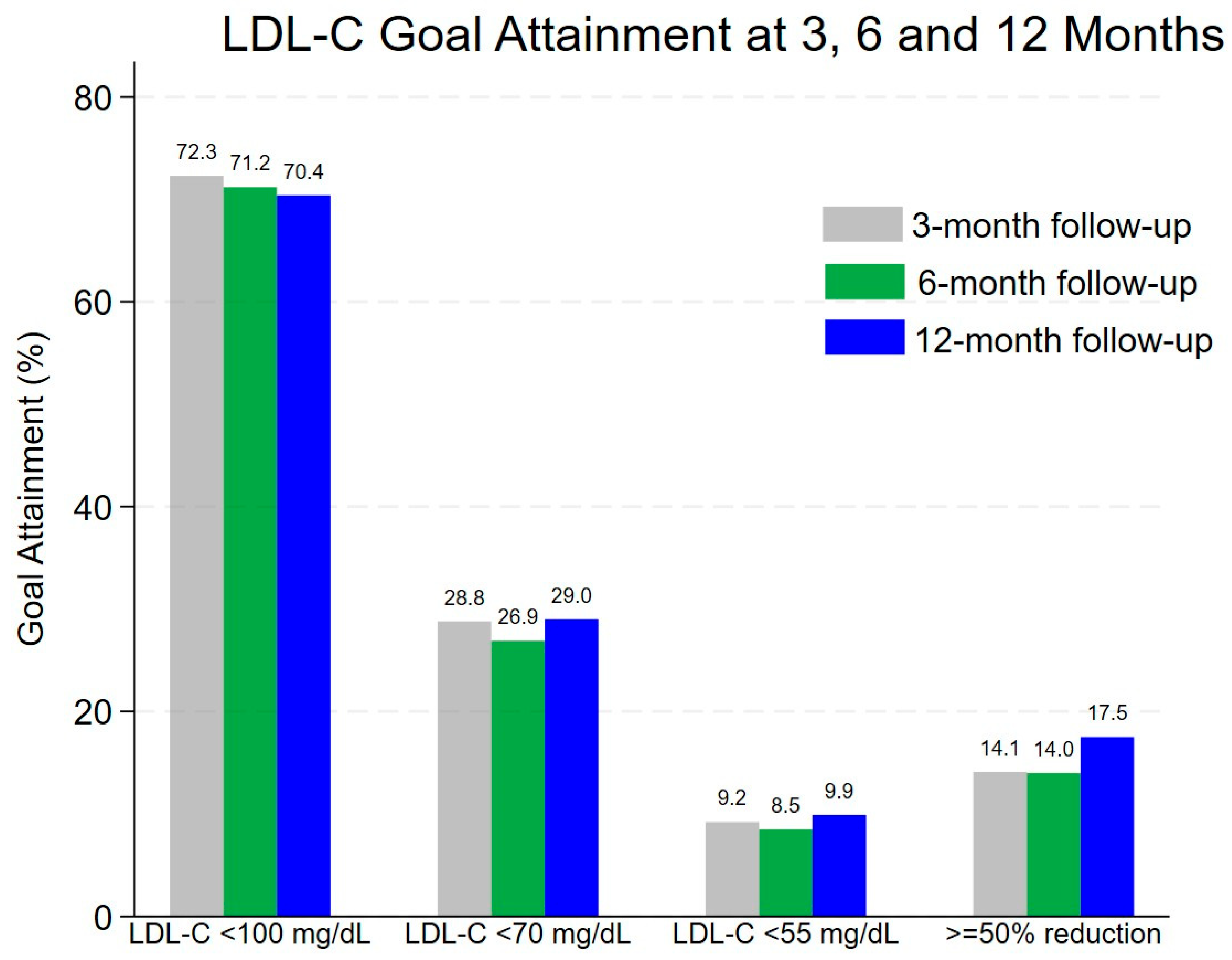
| LDL-C Goal | LDL-C Goal Attainment After Discharge * n (%, 95%CI) ** | ||
|---|---|---|---|
| Within 3 Months (N = 184) | Within 6 Months (N = 271) | Within 12 Months (N = 487) | |
| LDL-C 100 mg/dL | 133 (72.3, 65.2–78.6) | 193 (71.2, 65.4–76.5) | 343 (70.4, 66.2–74.5) |
| LDL-C 70 mg/dL | 53 (28.8, 22.4–35.9) | 73 (26.9, 21.7–32.6) | 141 (29.0, 25.0–33.2) |
| LDL-C 55 mg/dL | 17 (9.2, 5.5–14.4) | 23 (8.5, 5.5–12.5) | 48 (9.9, 7.4–12.9) |
| 50% LDL-C reduction from baseline | 26 (14.1, 9.4–20.0) | 38 (14.0, 10.1–18.7) | 85 (17.5, 14.2–21.1) |
| Variables * | LDL-C Goal Attainment Within 12 Months | |||||||
|---|---|---|---|---|---|---|---|---|
| <100 mg/dL | <70 mg/dL | <55 mg/dL | ≥50% Reduction from Baseline | |||||
| Adjusted OR (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value | |
| High-intensity statin | 1.64 (1.01–2.67) | 0.045 | 1.91 (1.09–3.34) | 0.023 | 1.65 (0.72–3.79) | 0.237 | 2.32 (1.14–4.73) | 0.020 |
| Age 60 years | 1.25 (0.82–1.90) | 0.305 | 1.22 (0.78–1.92) | 0.388 | 1.17 (0.59–2.32) | 0.656 | 1.11 (0.66–1.88) | 0.683 |
| Male | 1.24 (0.79–1.96) | 0.345 | 0.70 (0.44–1.13) | 0.144 | 0.81 (0.41–1.61) | 0.542 | 0.90 (0.52–1.57) | 0.719 |
| Current smokers | 0.60 (0.36–1.01) | 0.057 | 1.41 (0.82–2.45) | 0.219 | 1.04 (0.45–2.43) | 0.922 | 1.10 (0.58–2.07) | 0.775 |
| DM | 0.75 (0.48–1.16) | 0.192 | 0.89 (0.56–1.42) | 0.630 | 1.26 (0.65–2.45) | 0.489 | 1.75 (1.03–2.98) | 0.040 |
| CKD | 1.48 (0.63–3.45) | 0.364 | 0.94 (0.38–2.35) | 0.898 | 2.04 (0.65–6.41) | 0.225 | 0.50 (0.13–1.89) | 0.310 |
| CAD | 0.41 (0.14–1.17) | 0.094 | 0.52 (0.15–1.76) | 0.293 | 0.69 (0.14–3.41) | 0.646 | 0.69 (0.14–3.46) | 0.650 |
| LDL-C ≥ 100 mg/dL | 0.31 (0.19–0.52) | <0.001 | 0.17 (0.11–0.27) | <0.001 | 0.13 (0.07–0.26) | <0.001 | 12.13 (4.31–34.15) | <0.001 |
| Variables * | Coefficients ** (% LDL-C Reduction) | 95%CIs | z | p-Value |
|---|---|---|---|---|
| High-intensity statin | 6.27 | (1.51, 11.05) | 2.14 | 0.010 |
| Age 60 years | −3.45 | (−7.91, 1.00) | −1.52 | 0.128 |
| Male | −2.59 | (−7.36, 2.19) | −1.06 | 0.288 |
| Current smokers | −3.07 | (−9.01, 2.87) | −1.01 | 0.311 |
| DM | −5.32 | (−10.80, 0.15) | −1.90 | 0.057 |
| CKD | −1.07 | (−12.62, 10.48) | −0.18 | 0.856 |
| CAD | −8.73 | (−22.98, 5.51) | −1.20 | 0.230 |
| Baseline LDL-C > 100 mg/dL | 30.37 | (24.50, 36.23) | 10.15 | <0.001 |
| Intercept | 5.12 | (−1.48, 11.72) | 1.52 | 0.129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabob, P.; Jedsadayanmata, A. Suboptimal LDL-C Goal Attainment After Ischemic Stroke and TIA: Prevalence, Determinants, and Clinical Implications. Clin. Pract. 2025, 15, 193. https://doi.org/10.3390/clinpract15110193
Rabob P, Jedsadayanmata A. Suboptimal LDL-C Goal Attainment After Ischemic Stroke and TIA: Prevalence, Determinants, and Clinical Implications. Clinics and Practice. 2025; 15(11):193. https://doi.org/10.3390/clinpract15110193
Chicago/Turabian StyleRabob, Pawonrath, and Arom Jedsadayanmata. 2025. "Suboptimal LDL-C Goal Attainment After Ischemic Stroke and TIA: Prevalence, Determinants, and Clinical Implications" Clinics and Practice 15, no. 11: 193. https://doi.org/10.3390/clinpract15110193
APA StyleRabob, P., & Jedsadayanmata, A. (2025). Suboptimal LDL-C Goal Attainment After Ischemic Stroke and TIA: Prevalence, Determinants, and Clinical Implications. Clinics and Practice, 15(11), 193. https://doi.org/10.3390/clinpract15110193







