Efficacy of Non-Surgical Periodontal Therapy with Adjunctive Methylene Blue and Toluidine Blue O Mediated Photodynamic in Treatment of Periodontitis: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Settings, and Recruitment
2.2. Sample Size Calculation
2.3. Inclusion and Exclusion Criteria
2.4. Periodontal Parameters
2.5. Randomization and Treatment Protocol
2.6. Primary and Secondary Outcomes
2.7. Statistical Analysis
3. Results
3.1. Study Population
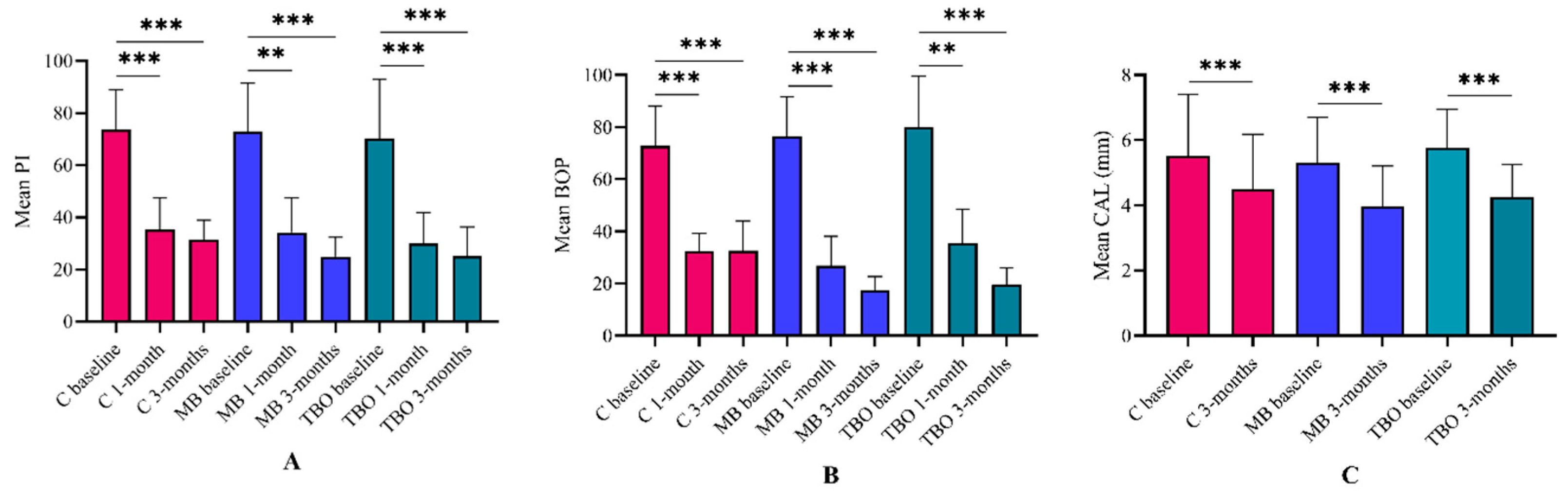
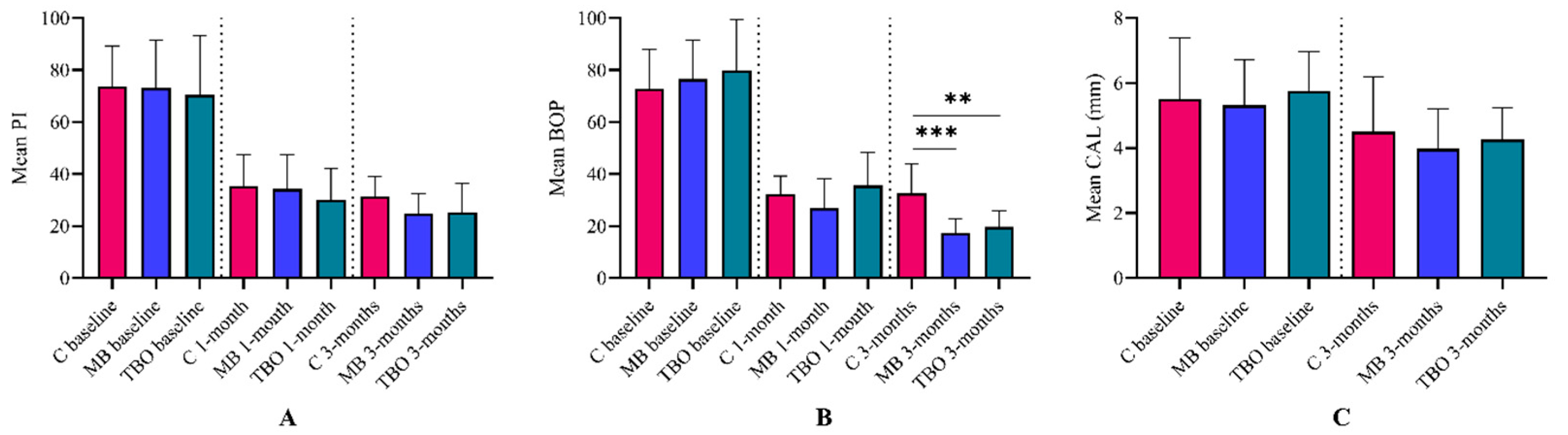
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Vieira Colombo, A.P.; Magalhães, C.B.; Hartenbach, F.A.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Hartenbach, F.; Silva-Boghossian, C.M.; Colombo, A.P.V. The effect of supragingival biofilm re-development on the subgingival microbiota in chronic periodontitis. Arch. Oral Biol. 2018, 85, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Iqbal, Z.; Ali, J.; Baboota, S.; Talegaonkar, S.; Ahmad, Z.; Sahni, J.K. Treatment modalities and evaluation models for periodontitis. Int. J. Pharm. Investig. 2012, 2, 106–122. [Google Scholar] [CrossRef]
- Van der Weijden, G.A.F.; Dekkers, G.J.; Slot, D.E. Success of non-surgical periodontal therapy in adult periodontitis patients: A retrospective analysis. Int. J. Dent. Hyg. 2019, 17, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Barca, E.; Cifcibasi, E.; Cintan, S. Adjunctive use of antibiotics in periodontal therapy. J. Istanb. Univ. Fac. Dent. 2015, 49, 55–62. [Google Scholar] [CrossRef]
- Heta, S.; Robo, I. The Side Effects of the Most Commonly Used Group of Antibiotics in Periodontal Treatments. Med. Sci. 2018, 6, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [PubMed]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage. Front. Physiol. 2017, 8, 439. [Google Scholar] [CrossRef]
- Moro, M.G.; de Carvalho, V.F.; Godoy-Miranda, B.A.; Kassa, C.T.; Horliana, A.; Prates, R.A. Efficacy of antimicrobial photodynamic therapy (aPDT) for nonsurgical treatment of periodontal disease: A systematic review. Lasers Med. Sci. 2021, 36, 1573–1590. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Butera, A.; Giordano, A.; Gallo, S.; Pascadopoli, M.; Scribante, A.; Albanese, M. Photodynamic Therapy in Non-Surgical Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 1086. [Google Scholar] [CrossRef]
- Atarchi, A.R.; Atarchi, Z.R. Efficacy of a single antimicrobial photodynamic therapy session as an adjunct to non-surgical periodontal therapy on clinical outcomes for periodontitis patients. A systematic review. J. Bagh. Coll. Dent. 2023, 35, 76–87. [Google Scholar] [CrossRef]
- Bundidpun, P.; Srisuwantha, R.; Laosrisin, N. Clinical effects of photodynamic therapy as an adjunct to full-mouth ultrasonic scaling and root planing in treatment of chronic periodontitis. Laser Ther. 2018, 27, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Ide, M.; Bissett, S.M.; Holliday, R.; Panagakos, F.S.; Trombelli, L.; Lansdowne, N.; Pickering, K.; Taylor, J.A.; Levonian, A.M.; et al. No benefit of an adjunctive phototherapy protocol in treatment of periodontitis: A split-mouth randomized controlled trial. J. Clin. Periodontol. 2021, 48, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I–III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.S.; Douglas, C.W.; Griffiths, G.S.; Rawlinson, A. A pilot study of active enzyme levels in gingival crevicular fluid of patients with chronic periodontal disease. J. Clin. Periodontol. 2016, 43, 629–636. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Ahad, A.; Lamba, A.K.; Faraz, F.; Tandon, S.; Chawla, K.; Yadav, N. Effect of antimicrobial photodynamic therapy as an adjunct to nonsurgical treatment of deep periodontal pockets: A clinical study. J. Lasers Med. Sci. 2016, 7, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Galgut, P. A comparison of different indices used in the clinical assessment of plaque and gingival bleeding. Clin. Oral Investig. 1999, 3, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29, 22–32. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front. Microbiol. 2016, 7, 175020. [Google Scholar] [CrossRef]
- Kwon, T.; Salem, D.M.; Levin, L. Nonsurgical periodontal therapy based on the principles of cause-related therapy: Rationale and case series. Quintessence Int. 2019, 50, 370–376. [Google Scholar] [CrossRef]
- Mittal, A.; Nichani, A.S.; Venugopal, R.; Rajani, V. The effect of various ultrasonic and hand instruments on the root surfaces of human single rooted teeth: A Planimetric and Profilometric study. J. Indian Soc. Periodontol. 2014, 18, 710–717. [Google Scholar] [CrossRef]
- Riaz, S.; Ahmed, S.; Shabbir, S.; Khan, Z.R.; Zaidi, S.J.A.; Naeem, M.M.; Farooqui, W.A. Analysing root roughness and smear layer relationship by comparing contemporary dental curettes with conventional dental curettes: A randomised controlled trial. BMC Oral Health 2022, 22, 237. [Google Scholar] [CrossRef]
- Qin, Y.L.; Luan, X.L.; Bi, L.J.; Sheng, Y.Q.; Zhou, C.N.; Zhang, Z.G. Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J. Periodontal Res. 2008, 43, 162–167. [Google Scholar] [CrossRef]
- Maisch, T. Anti-microbial photodynamic therapy: Useful in the future? Lasers Med. Sci. 2007, 22, 83–91. [Google Scholar] [CrossRef]
- Braham, P.; Herron, C.; Street, C.; Darveau, R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J. Periodontol. 2009, 80, 1790–1798. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, W.; Wang, C.; Qin, W.; Ming, J.; Zhang, M.; Qian, H.; Jiao, T. Methylene Blue-Mediated Photodynamic Therapy Induces Macrophage Apoptosis via ROS and Reduces Bone Resorption in Periodontitis. Oxid. Med. Cell Longev. 2019, 2019, 1529520. [Google Scholar] [CrossRef]
- Park, D.; Choi, E.J.; Weon, K.Y.; Lee, W.; Lee, S.H.; Choi, J.S.; Park, G.H.; Lee, B.; Byun, M.R.; Baek, K.; et al. Non-Invasive Photodynamic Therapy against -Periodontitis-causing Bacteria. Sci. Rep. 2019, 9, 8248. [Google Scholar] [CrossRef] [PubMed]
- Oruba, Z.; Łabuz, P.; Macyk, W.; Chomyszyn-Gajewska, M. Periopathogens differ in terms of the susceptibility to toluidine blue O-mediated photodynamic inactivation. Photodiagnosis Photodyn. Ther. 2017, 20, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Dehn, C.; Krause, F.; Jepsen, S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2008, 35, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Shu, R.; Li, Y.; Li, C.; Luo, L.; Song, Z.; Xie, Y.; Liu, D. Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed. Laser Surg. 2011, 29, 33–37. [Google Scholar] [CrossRef]
- Berakdar, M.; Callaway, A.; Eddin, M.F.; Ross, A.; Willershausen, B. Comparison between scaling-root-planing (SRP) and SRP/photodynamic therapy: Six-month study. Head Face Med. 2012, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Betsy, J.; Prasanth, C.S.; Baiju, K.V.; Prasanthila, J.; Subhash, N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2014, 41, 573–581. [Google Scholar] [CrossRef]
- Berezow, A.B.; Darveau, R.P. Microbial shift and periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef]
- Claffey, N.; Egelberg, J. Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. J. Clin. Periodontol. 1995, 22, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Joss, A.; Orsanic, T.; Gusberti, F.A.; Siegrist, B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986, 13, 590–596. [Google Scholar] [CrossRef]
- Yilmaz, S.; Kuru, B.; Kuru, L.; Noyan, U.; Argun, D.; Kadir, T. Effect of gallium arsenide diode laser on human periodontal disease: A microbiological and clinical study. Lasers Surg. Med. 2002, 30, 60–66. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.R.; Schwartz-Filho, H.O.; Novaes, A.B.; Garlet, G.P.; de Souza, R.F.; Taba, M.; Scombatti de Souza, S.L.; Ribeiro, F.J. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Cytokine profile in gingival crevicular fluid, preliminary results. J. Periodontol. 2009, 80, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, A.; Canakci, V.; Aydin, T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013, 84, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Niazi, F.H.; Noushad, M.; Tanvir, S.B.; Ali, S.; Al-Khalifa, K.S.; Qamar, Z.; Al-Sheikh, R. Antimicrobial efficacy of indocyanine green-mediated photodynamic therapy compared with Salvadora persica gel application in the treatment of moderate and deep pockets in periodontitis. Photodiagnosis Photodyn. Ther. 2020, 29, 101665. [Google Scholar] [CrossRef]
- Petelin, M.; Perkič, K.; Seme, K.; Gašpirc, B. Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med. Sci. 2015, 30, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Vidal, M.; Guerra-Ojeda, S.; Vallés, L.S.; López-Roldán, A.; Mauricio, M.D.; Aldasoro, M.; Alpiste-Illueca, F.; Vila, J.M. Effects of photodynamic therapy in periodontal treatment: A randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 915–925. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Silva, S.P.; Pires, J.R.; Soares, G.H.; Pontes, A.E.; Zuza, E.P.; Spolidório, D.M.; de Toledo, B.E.; Garcia, V.G. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med. Sci. 2012, 27, 687–693. [Google Scholar] [CrossRef]
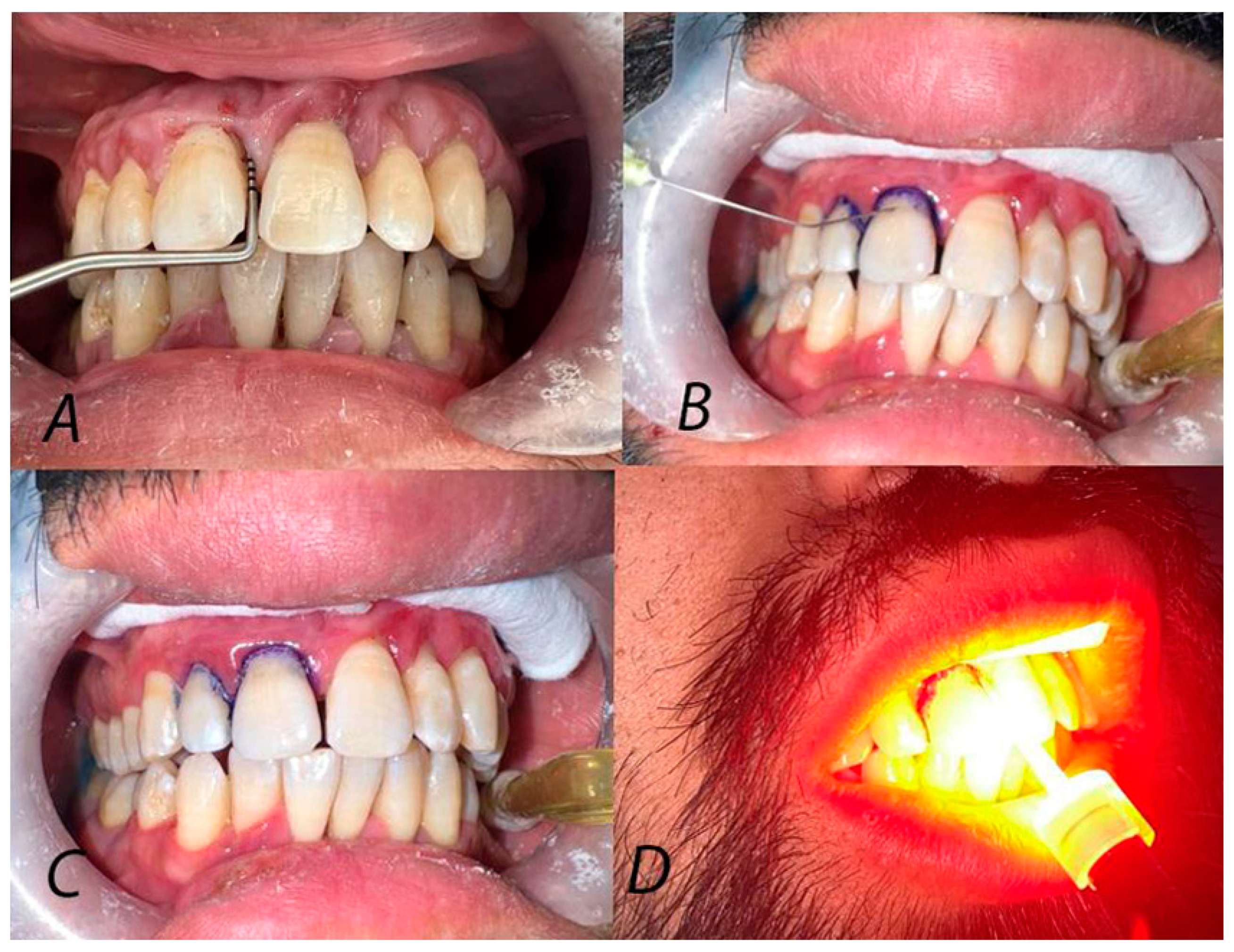
| Sex (n, %) | Age (Mean ± SD) | ||
| Male | 9, 50% | 44.0 ± 9.1 | |
| Female | 9, 50% | 42.9 ± 12.9 | |
| Total | 18, 100% | 43.4 ± 10.9 | |
| Sites (n, %) | All sites | 4 to 6 mm | >6 mm |
| Control | 102, 30.7% | 73, 32.7% | 29, 26.6% |
| MB | 124, 37.3% | 77, 34.5% | 33, 30.3% |
| TBO | 106, 31.9% | 73, 32.7% | 47, 43.1% |
| Total | 332, 100% | 223, 100% | 109, 100% |
| PPD | Control † | MB † | TBO † |
|---|---|---|---|
| Baseline | 5.8 ± 1.7 | 5.9 ± 1.6 | 6.2 ± 1.4 |
| 3 months | 4.0 ± 1.2 | 3.1 ± 1.0 | 3.4 ± 1.1 |
| p value * | <0.001 | <0.001 | <0.001 |
| Effect size ‡ | 0.7 | 0.5 | |
| ΔPPD (3-month baseline) | −1.8 ± 0.8 | −2.8 ± 0.9 ** | −2.8 ± 1.1 ** |
| 4 to 6 mm | |||
| Baseline | 4.8 ± 0.6 | 5.0 ± 0.7 | 5.1 ± 0.5 |
| 3 months | 3.3 ± 0.9 | 2.7 ± 0.9 | 2.9 ± 0.8 |
| p value * | <0.001 | <0.001 | <0.001 |
| Effect size ‡ | 0.3 | 0.6 | |
| ΔPPD (3-month baseline) | −1.5 ± 0.5 | −2.3 ± 0.8 ** | −2.1 ± 0.7 ** |
| >6 mm | |||
| Baseline | 8.1 ± 1.1 | 7.8 ± 0.8 | 8.0 ± 1.0 |
| 3 months | 5.4 ± 1.2 | 4.2 ± 0.8 | 4.4 ± 0.8 |
| p value * | <0.001 | <0.001 | <0.001 |
| Effect size ‡ | 0.9 | 0.7 | |
| ΔPPD (3-month-Baseline) | −2.6 ± 0.7 | −3.5 ± 1.0 ** | −3.6 ± 1.1 ** |
| Pockets Closure | Endpoint Achieved † | p Value * | Odds Ratio | 95% CI | |
|---|---|---|---|---|---|
| PPD § | Yes | No | |||
| Control | 60, 18.1% | 42, 12.7% | 1 | ||
| MB | 99, 29.8% | 25, 7.5% | <0.001 | 3.422 | 1.950 to 5.669 |
| TBO | 88, 26.5% | 18, 5.4% | <0.001 | 2.772 | 1.704 to 4.451 |
| Total | 247, 74.4% | 85, 25.6% | |||
| 4 to 6 mm § | |||||
| Control | 44, 19.7% | 29, 13.0% | 1 | ||
| MB | 61, 26.5% | 12, 5.4% | 0.003 | 3.350 | 1.722 to 6.618 |
| TBO | 59, 27.4% | 18, 8.1% | 0.04 | 2.160 | 1.222 to 3.948 |
| Total | 164, 73.5% | 59, 26.5% | |||
| >6 mm § | |||||
| Control | 16, 14.7% | 13, 11.9% | 1 | ||
| MB | 27, 24.8% | 6, 5.5% | 0.03 | 3.656 | 1.395 to 9.198 |
| TBO | 40, 36.7% | 7, 6.4% | 0.007 | 4.643 | 1.929 to 10.270 |
| Total | 83, 76.1% | 26, 23.9% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najm, K.K.; Gul, S.S.; Abdulkareem, A.A. Efficacy of Non-Surgical Periodontal Therapy with Adjunctive Methylene Blue and Toluidine Blue O Mediated Photodynamic in Treatment of Periodontitis: A Randomized Clinical Trial. Clin. Pract. 2024, 14, 954-964. https://doi.org/10.3390/clinpract14030076
Najm KK, Gul SS, Abdulkareem AA. Efficacy of Non-Surgical Periodontal Therapy with Adjunctive Methylene Blue and Toluidine Blue O Mediated Photodynamic in Treatment of Periodontitis: A Randomized Clinical Trial. Clinics and Practice. 2024; 14(3):954-964. https://doi.org/10.3390/clinpract14030076
Chicago/Turabian StyleNajm, Kashan Kamal, Sarhang Sarwat Gul, and Ali Abbas Abdulkareem. 2024. "Efficacy of Non-Surgical Periodontal Therapy with Adjunctive Methylene Blue and Toluidine Blue O Mediated Photodynamic in Treatment of Periodontitis: A Randomized Clinical Trial" Clinics and Practice 14, no. 3: 954-964. https://doi.org/10.3390/clinpract14030076
APA StyleNajm, K. K., Gul, S. S., & Abdulkareem, A. A. (2024). Efficacy of Non-Surgical Periodontal Therapy with Adjunctive Methylene Blue and Toluidine Blue O Mediated Photodynamic in Treatment of Periodontitis: A Randomized Clinical Trial. Clinics and Practice, 14(3), 954-964. https://doi.org/10.3390/clinpract14030076








