Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance
Abstract
1. Introduction
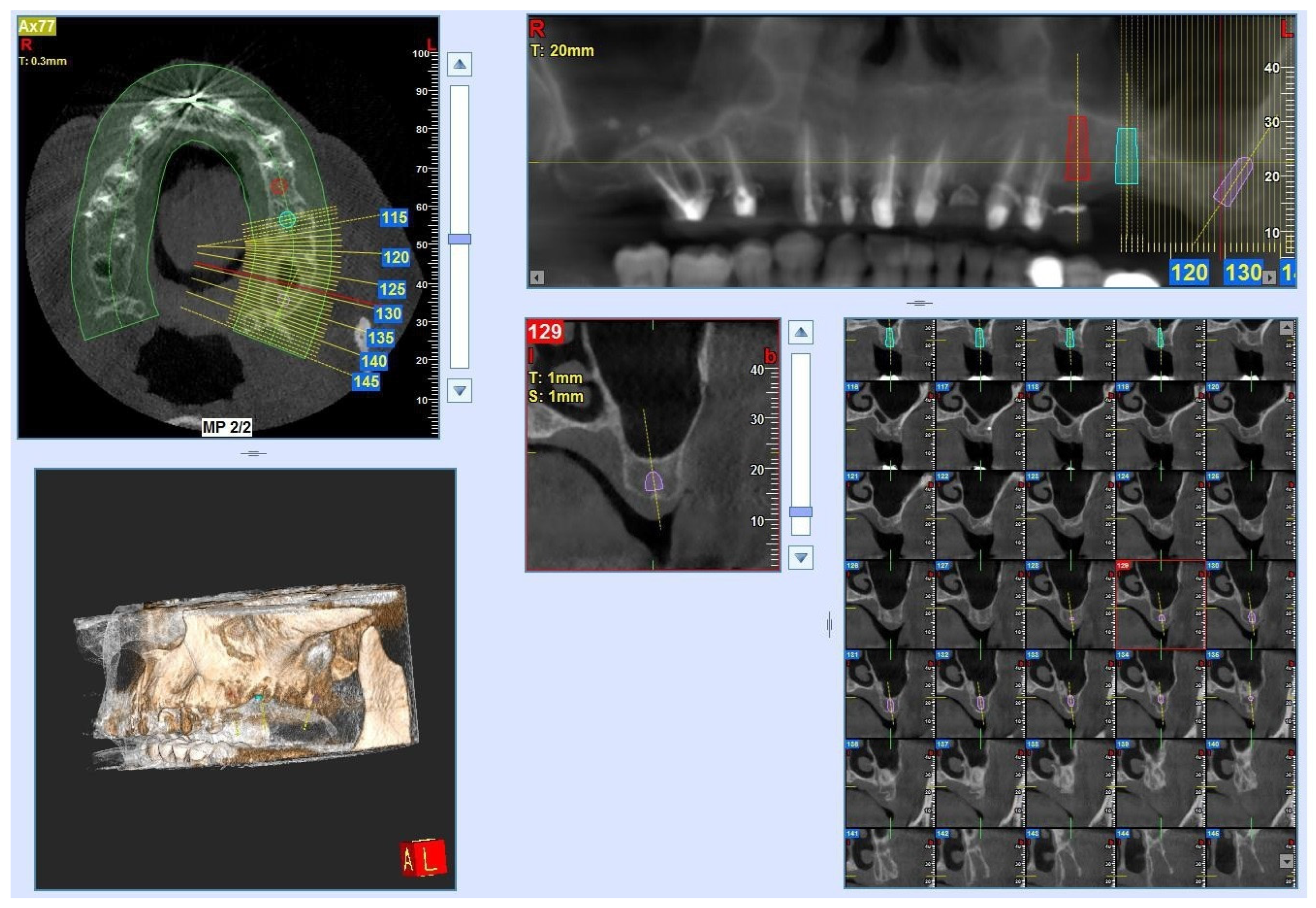
2. Materials and Methods
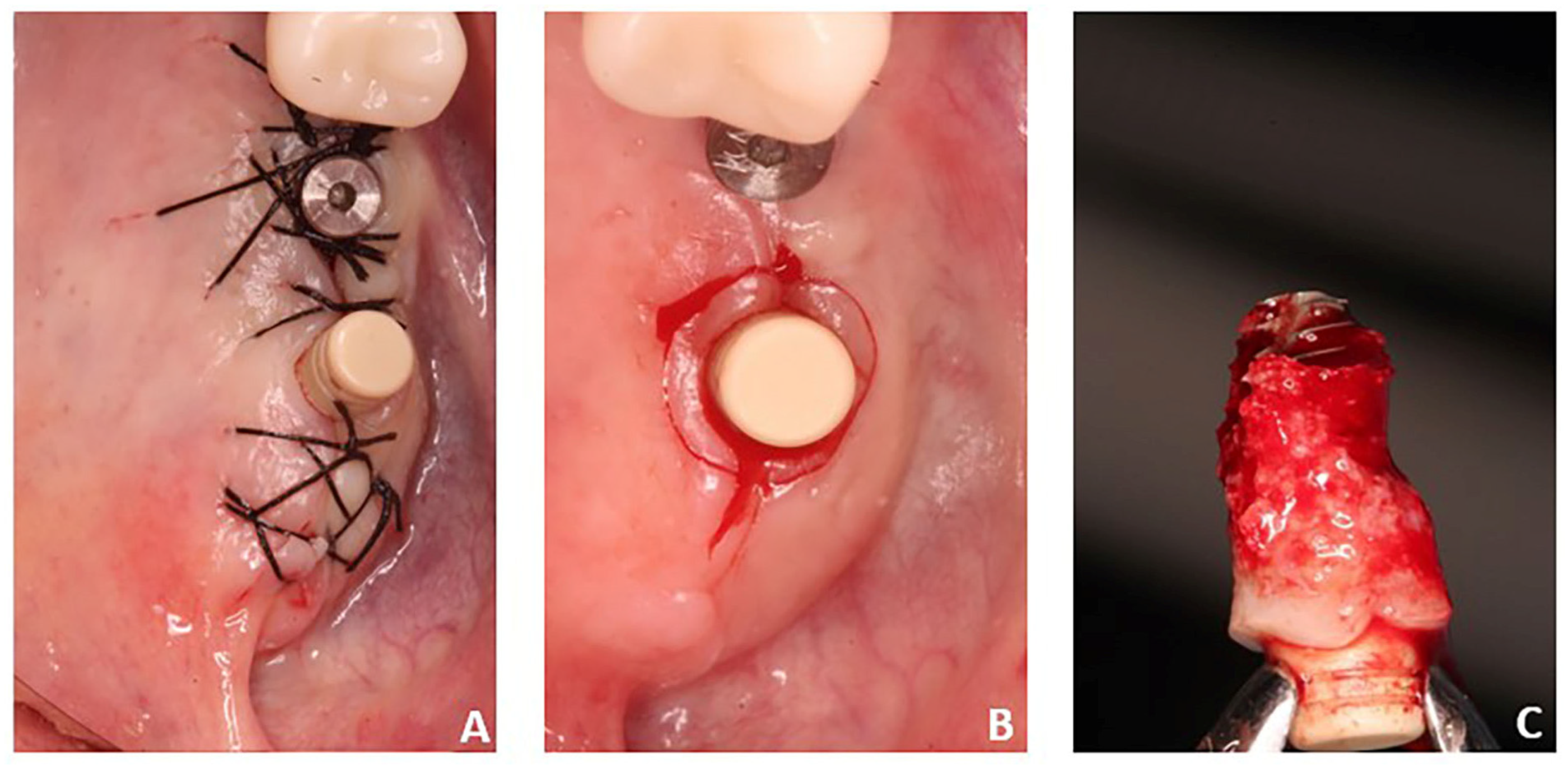
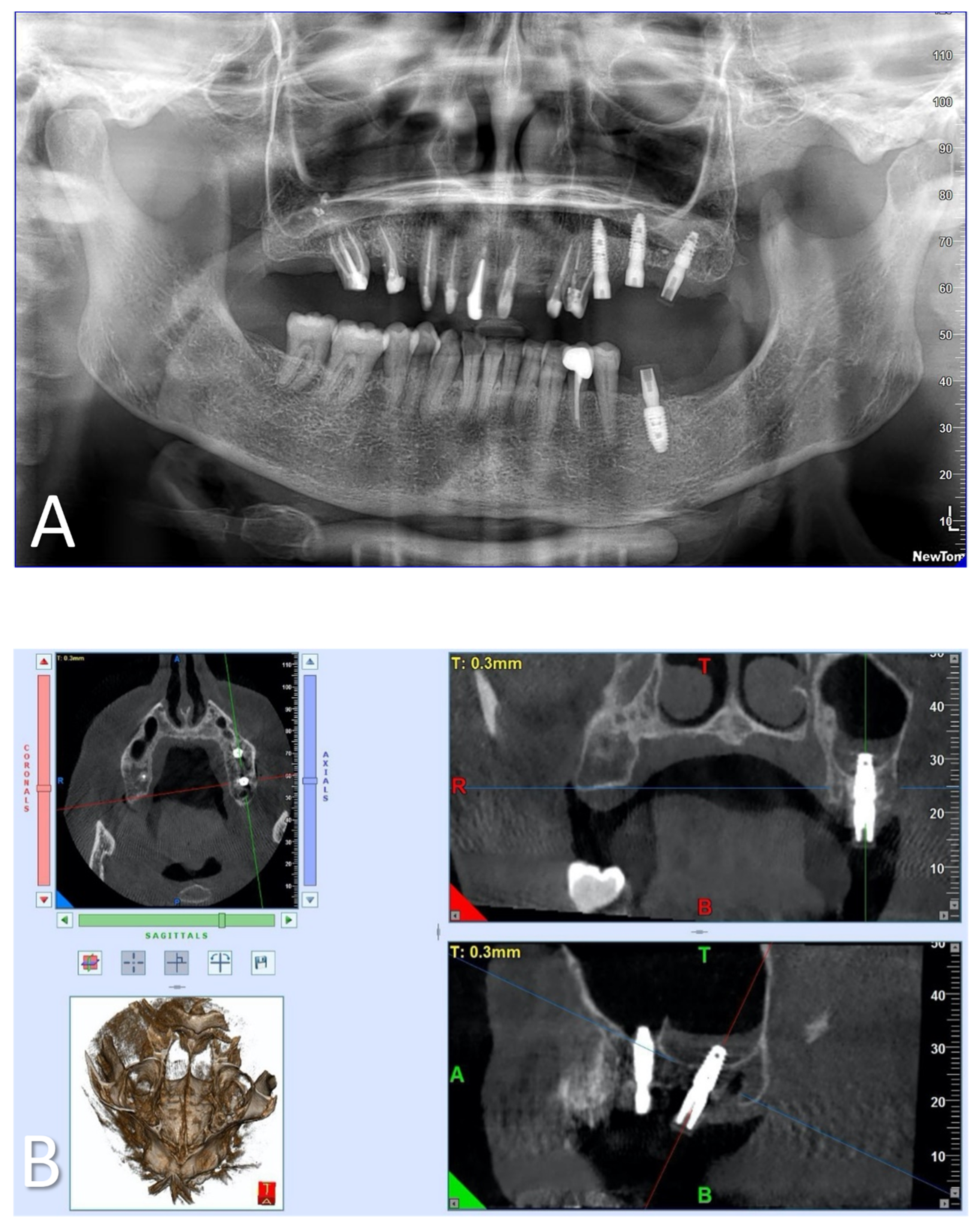
2.1. Samples
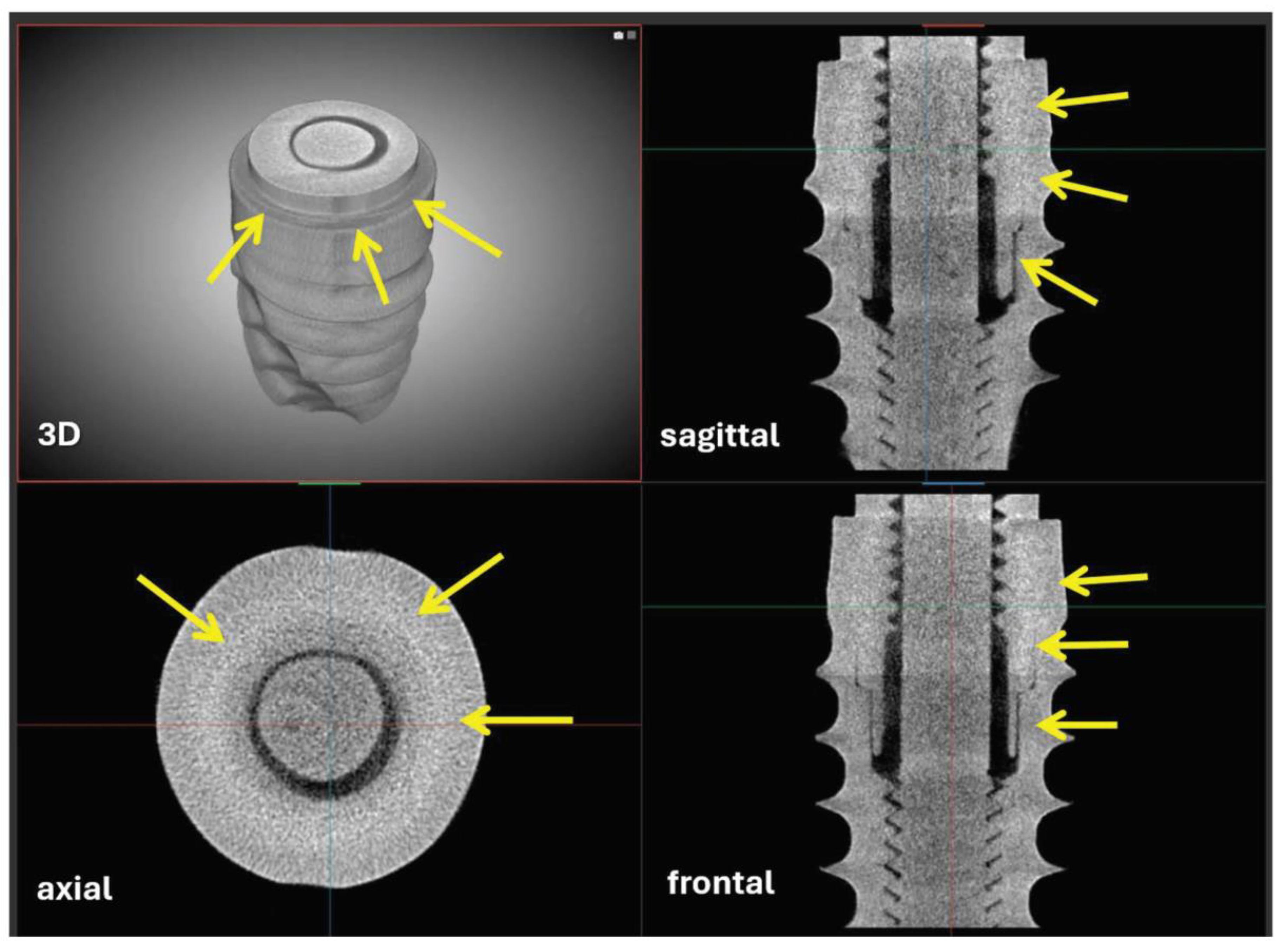
2.2. Laboratory-Based Micro-CT Investigation of the Implant
2.3. Synchrotron Light-Based Phase-Contrast Micro-CT of Peri-Implant Connective Tissue
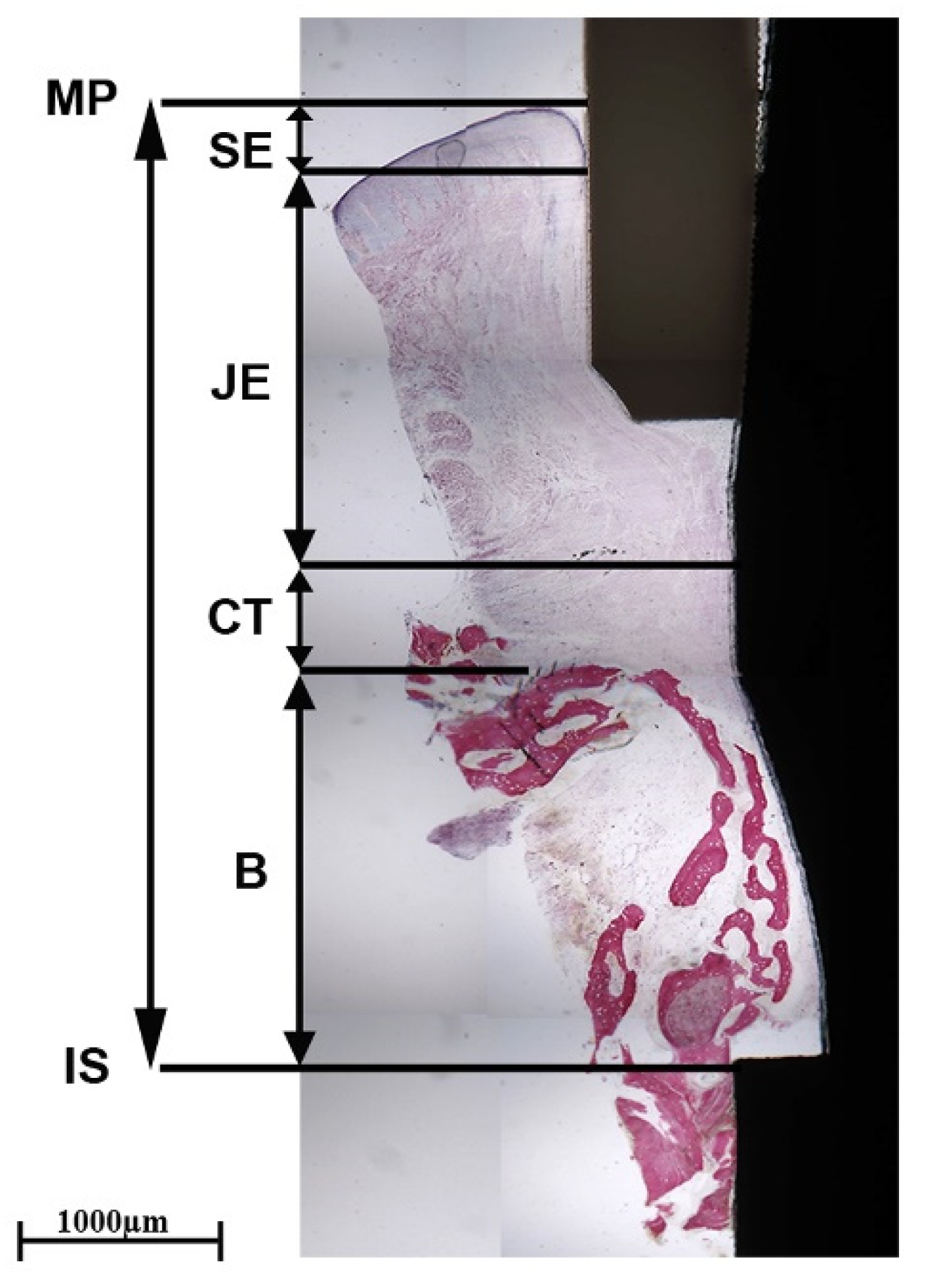
2.4. Histological Investigation
2.5. Polarized Light Microscopy
3. Results
3.1. Laboratory-Based Micro-CT
3.2. Synchrotron Micro-CT
3.3. Histology and Polarized Light Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannobile, W.V.; Jung, R.E.; Schwarz, F. Evidence-Based Knowledge on the Aesthetics and Maintenance of Peri-Implant Soft Tissues: Osteology Foundation Consensus Report Part 1-Effects of Soft Tissue Augmentation Procedures on the Maintenance of Peri-Implant Soft Tissue Health. Clin. Oral. Implant. Res. 2018, 29 (Suppl. S15), 7–10. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S313–S318. [Google Scholar] [CrossRef]
- Thoma, D.S.; Gil, A.; Hämmerle, C.H.F.; Jung, R.E. Management and Prevention of Soft Tissue Complications in Implant Dentistry. Periodontology 2000 2022, 88, 116–129. [Google Scholar] [CrossRef]
- Schwarz, F.; Giannobile, W.V.; Jung, R.E. Evidence-Based Knowledge on the Aesthetics and Maintenance of Peri-Implant Soft Tissues: Osteology Foundation Consensus Report Part 2-Effects of Hard Tissue Augmentation Procedures on the Maintenance of Peri-Implant Tissues. Clin. Oral. Implant. Res. 2018, 29 (Suppl. S15), 11–13. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Silva, E.; Félix, S.; Rodriguez-Archilla, A.; Oliveira, P.; Martins dos Santos, J. Revisiting peri-implant soft tissue—Histopathological study of the peri-implant soft tissue. Int. J. Clin. Exp. Pathol. 2014, 7, 611–618. [Google Scholar]
- Schierano, G.; Ramieri, G.; Cortese, M.G.; Aimetti, M.; Preti, G. Organization of the Connective Tissue Barrier around Long-Term Loaded Implant Abutments in Man. Clin. Oral. Implant. Res. 2002, 13, 460–464. [Google Scholar] [CrossRef]
- Cinquini, C.; Marchio, V.; Di Donna, E.; Cinquini, C.; Marchio, V.; Di Donna, E.; Alfonsi, F.; Derchi, G.; Nisi, M.; Barone, A. Histologic Evaluation of Soft Tissues around Dental Implant Abutments: A Narrative Review. Materials 2022, 15, 3811, Published 2022 May 27. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S230–S236. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef]
- Dellavia, C.; Canullo, L.; Allievi, C.; Lang, N.P.; Pellegrini, G. Soft Tissue Surrounding Switched Platform Implants: An Immunohistochemical Evaluation. Clin. Oral. Implant. Res. 2013, 24, 63–70. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The Mucosal Attachment to Titanium Implants with Different Surface Characteristics: An Experimental Study in Dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef]
- Iezzi, G.; Di Lillo, F.; Furlani, M.; Degidi, M.; Piattelli, A.; Giuliani, A. The Symmetric 3D Organization of Connective Tissue around Implant Abutment: A Key-Issue to Prevent Bone Resorption. Symmetry 2021, 13, 1126. [Google Scholar] [CrossRef]
- Canullo, L.; Giuliani, A.; Furlani, M.; Menini, M.; Piattelli, A.; Iezzi, G. Influence of Abutment Macro- and Micro-geometry on Morphologic and Morphometric Features of Peri-implant Connective Tissue. Clin. Oral. Implant. Res. 2023, 34, 920–933. [Google Scholar] [CrossRef]
- Riberti, N.; Furlani, M.; D’Amico, E.; Comuzzi, L.; Piattelli, A.; Iezzi, G.; Giuliani, A. Deep Learning for Microstructural Characterization of Synchrotron Radiation-Based Collagen Bundle Imaging in Peri-Implant Soft Tissues. Appl. Sci. 2023, 13, 4423. [Google Scholar] [CrossRef]
- Sailer, I.; Mühlemann, S.; Zwahlen, M.; Hämmerle, C.H.; Schneider, D. Cemented and screw-retained implant reconstructions: A systematic review of the survival and complication rates. Clin. Oral. Implant. Res. 2012, 23, 163–201. [Google Scholar] [CrossRef]
- Wittneben, J.-G.; Millen, C.; Brägger, U. Clinical Performance of Screw- versus Cement-Retained Fixed Implant-Supported Reconstructions--a Systematic Review. Int. J. Oral. Maxillofac. Implant. 2014, 29, 84–98. [Google Scholar] [CrossRef]
- Ma, S.; Fenton, A. Screw- versus Cement-Retained Implant Prostheses: A Systematic Review of Prosthodontic Maintenance and Complications. Int. J. Prosthodont. 2015, 28, 127–145. [Google Scholar] [CrossRef]
- Block, M.S. Evidence-Based Criteria for an Ideal Abutment Implant Connection-A Narrative Review. J. Oral. Maxillofac. Surg. 2022, 80, 1670–1675. [Google Scholar] [CrossRef]
- Saravia-Rojas, M.A.; Geng-Vivanco, R. Clinical Protocol for Intraoral Repair of a Chipped All-Ceramic Crown: A Case Report. Gen. Dent. 2023, 71, 54–57. [Google Scholar]
- Mesquita, A.M.M.; Al-Haj Husain, N.; Molinero-Mourelle, P.; Özcan, M. An Intraoral Repair Method for Chipping Fracture of a Multi-Unit Fixed Zirconia Reconstruction: A Direct Dental Technique. Eur. J. Dent. 2021, 15, 174–178. [Google Scholar] [CrossRef]
- Bressan, E.; Lops, D. Conometric Retention for Complete Fixed Prosthesis Supported by Four Implants: 2-Years Prospective Study. Clin. Oral. Implant. Res. 2014, 25, 546–552. [Google Scholar] [CrossRef]
- Bressan, E.; Lops, D.; Tomasi, C.; Ricci, S.; Stocchero, M.; Carniel, E.L. Experimental and Computational Investigation of Morse Taper Conometric System Reliability for the Definition of Fixed Connections between Dental Implants and Prostheses. Proc Inst Mech Eng H. 2014, 228, 674–681. [Google Scholar] [CrossRef]
- Degidi, M.; Nardi, D.; Sighinolfi, G.; Degidi, D. The Conometric Concept for the Definitive Rehabilitation of a Single Posterior Implant by Using a Conical Indexed Abutment: A Technique. J. Prosthet. Dent. 2020, 123, 576–579. [Google Scholar] [CrossRef]
- Albiero, A.M.; Benato, R.; Momic, S.; Degidi, M. Guided-Welded Approach Planning Using a Computer-Aided Designed Prosthetic Shell for Immediately Loaded Complete-Arch Rehabilitations Supported by Conometric Abutments. J. Prosthet. Dent. 2019, 122, 510–515. [Google Scholar] [CrossRef]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Bambace, M.; Lops, D.; Tomasi, C. Five-Year Prospective Study on Conometric Retention for Complete Fixed Prostheses. Int. J. Oral. Implantol. 2019, 12, 105–113. [Google Scholar]
- Albiero, A.; Benato, R.; Momic, S.; Degidi, M. Computer-Aided Crown Design Using Digital Scanning Technology for Immediate Postextraction Single-Implant Restorations Supported by Conical Indexed Abutments. Int. J. Periodontics Restor. Dent. 2021, 41, 135–140. [Google Scholar] [CrossRef]
- Bressan, E.; Venezze, A.; Magaz, V.; Lops, D.; Ghensi, P. Fixed Conometric Retention with CAD/CAM Conic Coupling Abutments and Prefabricated Syncone Caps: A Case Series. Int. J. Periodontics Restor. Dent. 2018, 38, 277–280. [Google Scholar] [CrossRef]
- Lupi, S.M.; Todaro, C.; De Martis, D.; Blasi, P.; Rodriguez y Baena, R.; Storelli, S. The Conometric Connection for the Implant-Supported Fixed Prosthesis: A Narrative Review. Prosthesis 2022, 4, 458–467. [Google Scholar] [CrossRef]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous Phase and Amplitude Extraction from a Single Defocused Image of a Homogeneous Object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- Makovetsky, R.; Piche, N.; Marsh, M. Dragonfly as a Platform for Easy Image-Based Deep Learning Applications. Microsc. Microanal. 2018, 24, 532–533. [Google Scholar] [CrossRef]
- Domander, R.; Felder, A.A.; Doube, M. BoneJ2—Refactoring Established Research Software. Wellcome Open Res. 2021, 6, 37. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv 2015, arXiv:1505.04597. [Google Scholar]
- Sasada, Y.; Cochran, D.L. Implant-Abutment Connections: A Review of Biologic Consequences and Peri-implantitis Implications. Int. J. Oral Maxillofac. Implant. 2017, 32, 1296–1307. [Google Scholar] [CrossRef]
- D’Ercole, S.; Dotta, T.C.; Farani, M.R.; Etemadi, N.; Iezzi, G.; Comuzzi, L.; Piattelli, A.; Petrini, M. Bacterial Microleakage at the Implant-Abutment Interface: An In Vitro Study. Bioengineering 2022, 9, 277. [Google Scholar] [CrossRef]
- Bittencourt, A.B.B.C.; Neto, C.L.M.M.; Penitente, P.A.; Pellizzer, E.P.; Dos Santos, D.M.; Goiato, M.C. Comparison of the Morse Cone Connection with the Internal Hexagon and External Hexagon Connections Based on Microleakage—Review. Prague Med. Rep. 2021, 122, 181–190. [Google Scholar] [CrossRef]
- Mao, Z.; Beuer, F.; Wu, D.; Zhu, Q.; Yassine, J.; Schwitalla, A.; Schmidt, F. Microleakage along the implant-abutment interface: A systematic review and meta-analysis of in vitro studies. Int. J. Implant Dent. 2023, 9, 34. [Google Scholar] [CrossRef]
- De Castro, D.S.M.; De Araujo, M.A.R.; Benfatti, C.A.M.; De Araujo, C.D.R.P.; Piattelli, A.; Perrotti, V.; Iezzi, G. Comparative Histological and Histomorphometrical Evaluation of Marginal Bone Resorption around External Hexagon and Morse Cone Implants: An Experimental Study in Dogs. Implant. Dent. 2014, 23, 270–276. [Google Scholar] [CrossRef]
- Yao, K.T.; Kao, H.C.; Cheng, C.K.; Fang, H.W.; Huang, C.H.; Hsu, M.L. Mechanical performance of conical implant-abutment connections under different cyclic loading conditions. J. Mech. Behav. Biomed. Mater. 2019, 90, 426–432. [Google Scholar] [CrossRef]
- Coray, R.; Zeltner, M.; Özcan, M. Fracture strength of implant abutments after fatigue testing: A systematic review and a meta-analysis. J. Mech. Behav. Biomed. Mater. 2016, 62, 333–346. [Google Scholar] [CrossRef]
- Rack, A.; Rack, T.; Stiller, M.; Riesemeier, H.; Zabler, S.; Nelson, K. In vitro synchrotron-based radiography of micro-gap formation at the implant-abutment interface of two-piece dental implants. J. Synchrotron Radiat. 2010, 17, 289–294. [Google Scholar] [CrossRef]
- Rack, T.; Zabler, S.; Rack, A.; Riesemeier, H.; Nelson, K. An in vitro pilot study of abutment stability during loading in new and fatigue-loaded conical dental implants using synchrotron-based radiography. Int. J. Oral Maxillofac. Implant. 2013, 28, 44–50. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riberti, N.; D’Amico, E.; Pierfelice, T.V.; Furlani, M.; Giuliani, A.; Piattelli, A.; Iezzi, G.; Comuzzi, L. Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance. Clin. Pract. 2024, 14, 556-569. https://doi.org/10.3390/clinpract14020043
Riberti N, D’Amico E, Pierfelice TV, Furlani M, Giuliani A, Piattelli A, Iezzi G, Comuzzi L. Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance. Clinics and Practice. 2024; 14(2):556-569. https://doi.org/10.3390/clinpract14020043
Chicago/Turabian StyleRiberti, Nicole, Emira D’Amico, Tania Vanessa Pierfelice, Michele Furlani, Alessandra Giuliani, Adriano Piattelli, Giovanna Iezzi, and Luca Comuzzi. 2024. "Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance" Clinics and Practice 14, no. 2: 556-569. https://doi.org/10.3390/clinpract14020043
APA StyleRiberti, N., D’Amico, E., Pierfelice, T. V., Furlani, M., Giuliani, A., Piattelli, A., Iezzi, G., & Comuzzi, L. (2024). Case Report of a Dental Implant with Conometric Abutment–Prosthetic Cap Connection: Advanced High-Resolution Imaging and Peri-Implant Connective Tissue Performance. Clinics and Practice, 14(2), 556-569. https://doi.org/10.3390/clinpract14020043











