Autophagy Genes and Otitis Media Outcomes
Abstract
1. Introduction
1.1. Otitis Media
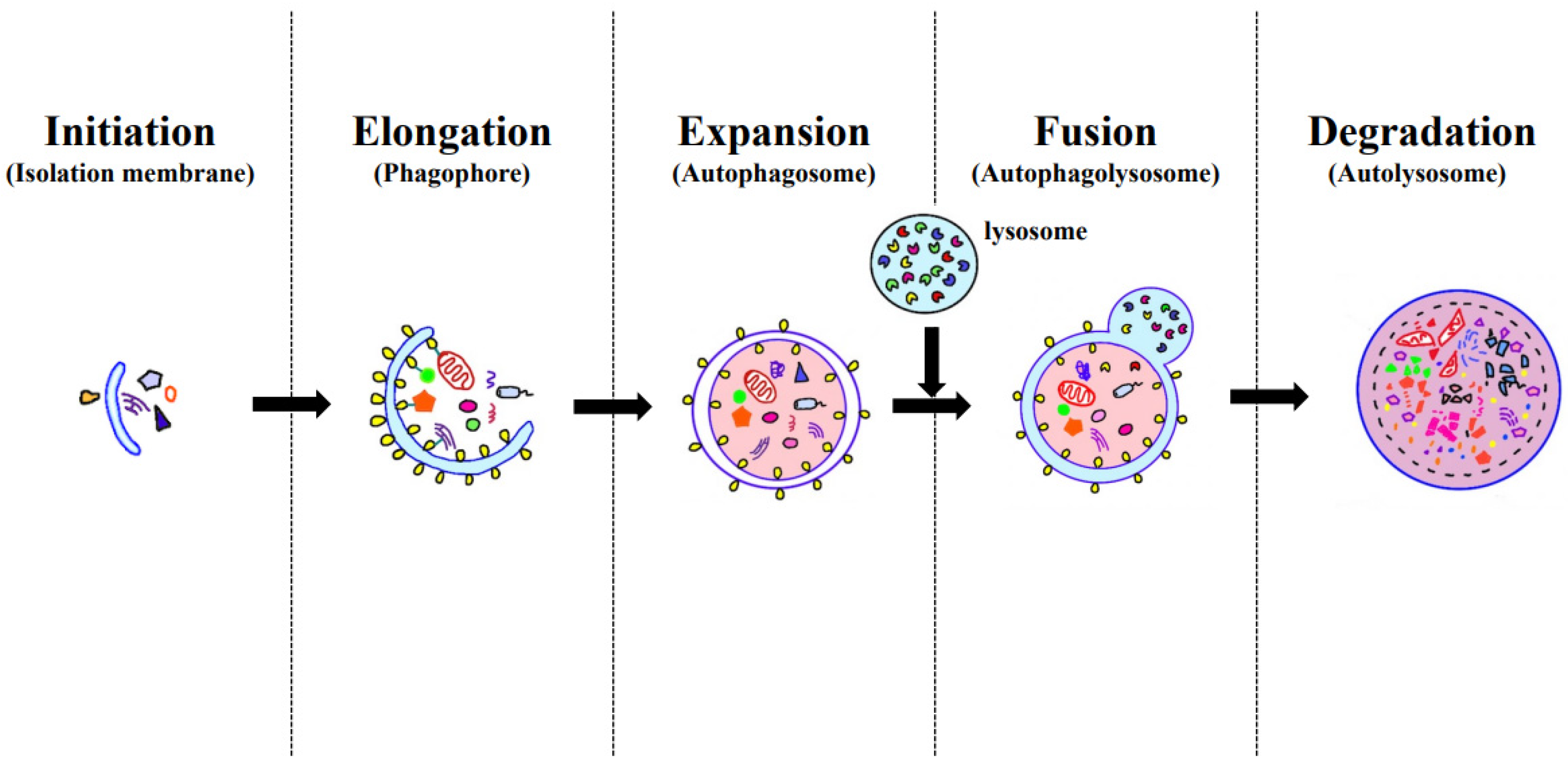
1.2. Autophagy
2. Autophagy in Otitis Media
2.1. Acute Otitis Media (AOM)
2.2. Otitis Media with Effusion (OME)
2.3. Chronic Otitis Media without Cholesteatoma (COM)
2.4. Chronic Otitis Media with Cholesteatoma (CholeOM)
2.5. Chronic Otitis Media without Cholesteatoma (COM) versus Chronic Otitis Media with Cholesteatoma (CholeOM)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bluestone, C.D.; Klein, J.O. Otitis media and eustachian tube dysfunction. In Pediatric Otolaryngology, 4th ed.; Bluestone, C.D., Stool, S.E., Alper, C.M., Eds.; Philadelphia-Saunders: Philadelphia, PA, USA, 2003; pp. 474–685. [Google Scholar]
- Margaretha, L.C.; Ellen, M.M. Acute otitis media and otitis media with effusion. In Cummings Otolaryngology, 6th ed.; Mosby: Philadelphia, PA, USA, 2015; pp. 3019–3037. [Google Scholar]
- Zhu, Z.H.; Shan, Y.J.; Han, Y.; Zhu, L.W.; Ma, Z.X. Pathological study of otitis media with effusion after treatment with intranasal pulmonary surfactant. Laryngoscope 2013, 123, 3148–3155. [Google Scholar] [CrossRef]
- Baek, M.J. Chronic suppurative otitis media. In Otorhinolaryngology—Head and Neck Surgery, 2nd ed.; KoonJa Publishing: Seoul, Republic of Korea, 2018; pp. 399–414. [Google Scholar]
- Bluestone, C.D. Epidemiology and pathogenesis of chronic suppurative otitis media: Implications for prevention and treatment. Int. J. Pediatr. Otorhinolaryngol. 1998, 42, 207–223. [Google Scholar] [CrossRef]
- Verhoeff, M.; Van Der Veen, E.L.; Rovers, M.M.; Sanders, E.A.; Schilder, A.G. Chronic suppurative otitis media: A review. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhou, P.; Zheng, Y.; Zhao, Y.; Li, T.; Zheng, Y. Congenital cholesteatoma clinical and surgical management. Int. J. Pediatr. Otorhinolaryngol. 2023, 164, 111401. [Google Scholar] [CrossRef] [PubMed]
- Ghaheri, B.A.; Kempton, J.B.; Pillers, D.A.; Trune, D.R. Cochlear cytokine gene expression in murine chronic otitis media. Otolaryngol. Head. Neck Surg. 2007, 137, 332–337. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Xia, Y.; Yin, Y.; Zhu, T.; Chen, F.; Hai, C. Autophagy, a double-edged sword for oral tissue regeneration. J. Adv. Res. 2023, S2090-1232(23)00172-8. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Behrends, C.; Sowa, M.E.; Gygi, S.P.; Harper, J.W. Network organization of the human autophagy system. Nature 2010, 466, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H. The Role of Autophagy in the Pathogenesis and Treatment of Metabolic Diseases. Int. J. Biol. Sci. 2020, 16, 2675–2691. [Google Scholar] [CrossRef]
- Arias, E.; Cuervo, M. Chaperone-mediated autophagy in protein quality control. Curr. Opin. Cell Biol. 2011, 23, 184–189. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Long, F.; Kang, R.; Klionsky, D.J.; Yang, M.; Tang, D. The lipid basis of cell death and autophagy. Autophagy 2023, 1–20. [Google Scholar] [CrossRef]
- Chen, F.; Cai, X.; Kang, R.; Liu, J.; Tang, D. Autophagy-Dependent Ferroptosis in Cancer. Antioxid. Redox Signal 2023, 39, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Dong, L.L.; Zhang, Y.J.; Zhao, X.M.; He, H.Y. Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy. Neural Regen. Res. 2021, 16, 813–819. [Google Scholar] [PubMed]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, S.; Liu, J.; Wu, X.; Zhou, S.; Dai, K.; Kou, Y. Mitophagy contributes to the pathogenesis of inflammatory diseases. Inflammation 2018, 41, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Mizushima, N. Autophagy and human diseases. Cell Res. 2014, 24, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhao, T.; Zhang, X.; Kui, L.; Wang, Q.; Wu, Y.; Zheng, T.; Ma, P.; Zhang, Y.; Molteni, H.; et al. Autophagy Contributes to the Rapamycin-Induced Improvement of Otitis Media. Front. Cell Neurosci. 2022, 15, 753369. [Google Scholar] [CrossRef]
- Dong, Y.; Jin, C.; Ding, Z.; Zhu, Y.; He, Q.; Zhang, X.; Ai, R.; Yin, Y.; He, Y. TLR4 regulates ROS and autophagy to control neutrophil extracellular traps formation against Streptococcus pneumoniae in acute otitis media. Pediatr. Res. 2021, 89, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, M.G.; Shim, H.S.; Kim, S.S.; Kim, Y.I.; Yeo, S.G. Lower Beclin-1 mRNA Levels in Pediatric Compared With Adult Patients With Otitis Media With Effusion. J. Int. Adv. Otol. 2018, 14, 48–52. [Google Scholar]
- Jung, J.; Park, D.C.; Kim, Y.I.; Lee, E.H.; Park, H.J.; Kim, S.H.; Yeo, S.G. Decreased expression of autophagy markers in culture-positive patients with chronic otitis media. J. Int. Med. Res. 2020, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Huang, C.J.; Hung, C.C.; Wu, Y.R.; Chiu, C.C.; Chien, C.Y.; Wang, H.M.; Chang, N.C.; Lin, I.L.; Chen, J.Y.F. Autophagy Is Deficient and May be Negatively Regulated by SERPINB3 in Middle Ear Cholesteatoma. Otol. Neurotol. 2020, 41, e881–e888. [Google Scholar] [CrossRef]
- Jung, J.; Jung, S.Y.; Kim, M.G.; Kim, Y.I.; Kim, S.H.; Yeo, S.G. Comparison of Autophagy mRNA expression between Chronic Otitis Media with Cholesteatoma and Chronic Otitis Media without Cholesteatoma. J. Audiol. Otol. 2020, 24, 191–197. [Google Scholar] [CrossRef]
- Li, Q.; Ao, Y.; Yu, Q.; Zhou, S. Role of Autophagy in Acquired Cholesteatoma. Otol. Neurotol. 2019, 40, e993–e998. [Google Scholar] [CrossRef]

| Author /Year [Reference] | Species | Sample | Type of OM | Detection Method | Target Substance(s) Associated with Autophagy | Results Conclusion |
|---|---|---|---|---|---|---|
| Xie D, et al., 2022 [23] | TLR2−/− mice and WT mice | Middle ear | AOM | Histological analysis, immunohistochemistry, immunofluorescence, TUNEL assays | mTOR, LC3-II, beclin-1, ATG7 | Protein expression levels of p-S6, Raptor and mTOR are decreased in TLR2−/− mice after injection of PGPS. Both the autophagosome proteins LC3-II, beclin-1 and ATG7, and autophagy substrate protein p62 accumulated at higher levels in mice with OM than in OM-negative mice. Impaired autophagy is involved in PGPS-induced OM, which is improved by RPM, at least in part, by relieving autophagy impairment. |
| Dong Y, et al., 2017 [24] | C57BL/6 mice | Middle ear | AOM | NETs killing assay, confocal microscopy NETs capture effect assay | LC3-I LC3-II | LC3-II expression is significantly increased 30 min after incubation with S.pn. In addition, the numbers of LC3 puncta per cell significantly increase following S.pn infection. In the course of AOM, TLR4 controls NET formation against S.pn through regulation of autophagy. |
| Kim SH, et al., 2018 [25] | Humans | Effusion | OME | Quantitative PCR, bacterial culture | mTOR, beclin-1, FLIP, Rubicon | Beclin-1 mRNA levels were significantly lower in pediatric than adult patients, regardless of the frequency of surgery or fluid characteristics (p < 0.05). Autophagy-associated mRNAs were expressed in effusion fluids of both pediatric and adult patients with OME. However, the level of beclin-1 mRNA was significantly lower in the effusion fluid of pediatric than adult patients. |
| Jung J, et al., 2020 [26] | Humans | Inflammatory tissues | COM | Quantitative PCR, bacterial culture | mTOR, P13KC3, LC3 II, beclin-1, Rubicon | Autophagy-related mRNAs were detected in all inflammatory tissues of COM patients. LC3-II mRNA expression was highest, followed by expression of beclin-1, P13KC3, Rubicon, and mTOR mRNAs. Beclin-1 mRNA levels were significantly lower in culture-positive than culture-negative patients. Autophagy is involved in the pathogenesis of COM. The finding that the expression of autophagy markers, especially beclin-1, was lower in culture-positive than culture-negative patients suggests that these markers are closely associated with the clinical features of COM. |
| Ho KY, et al., 2020 [27] | Humans | Retroauricular skin and cholesteatoma tissue | CholeOM | Immunoblotting, immunohistochemistry, MTT assays | LC3, Akt, mTOR | Expression of LC3, p-Akt, and p-mTOR in fresh retroauricular skin and cholesteatoma tissue samples was analyzed by immunoblotting and immunohistochemistry. Autophagy is significantly suppressed in cholesteatoma tissues and may not involve the Akt/mTOR signaling pathway. |
| Jung J, et al., 2020 [28] | Humans | Inflammatory tissues | COM CholeOM | Quantitative PCR, bacterial culture | mTOR, PI3KC3, LC3 II, beclin-1, Rubicon | PI3K mRNA expression was lower, whereas Beclin-1 mRNA expression was higher (0.089 ± 0.107 vs. 0.176 ± 0.163; p = 0.034) in the CholeOM group. PI3K mRNA expression was lower in the CholeOM group than in COM subgroups presenting with bacterial infection, otorrhea, and hearing loss > 40 dB. Different autophagy proteins play important roles in chronic OM depending on the presence or absence of cholesteatoma. |
| Li Q, et al., 2019 [29] | Humans | Cholesteatoma epithelium and normal external auditory canal epithelium | COM CholeOM | Immunohistochemistry, Western blotting | LC3, beclin-1, PI3K/AKT pathway | LC3 immunostaining was stronger in cholesteatoma epithelium than in normal EAC epithelium. Western blotting showed significantly higher LC3-II/I ratios and Beclin-1 expression in cholesteatoma epithelium compared with EAC epithelium or COM epithelium, and significantly higher p-PI3K/PI3K and p-AKT/AKT ratios in cholesteatoma epithelium compared with EAC epithelium. Enhanced autophagy might play a role in the pathogenesis of acquired cholesteatoma. PI3Ks might have different regulatory functions in autophagy in the cholesteatoma epithelium. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Rim, H.S.; Kim, J.H.; Kim, S.S.; Yeo, J.H.; Yeo, S.G. Autophagy Genes and Otitis Media Outcomes. Clin. Pract. 2024, 14, 293-304. https://doi.org/10.3390/clinpract14010023
Kim YJ, Rim HS, Kim JH, Kim SS, Yeo JH, Yeo SG. Autophagy Genes and Otitis Media Outcomes. Clinics and Practice. 2024; 14(1):293-304. https://doi.org/10.3390/clinpract14010023
Chicago/Turabian StyleKim, Yong Jun, Hwa Sung Rim, Jeong Hee Kim, Sung Soo Kim, Joon Hyung Yeo, and Seung Geun Yeo. 2024. "Autophagy Genes and Otitis Media Outcomes" Clinics and Practice 14, no. 1: 293-304. https://doi.org/10.3390/clinpract14010023
APA StyleKim, Y. J., Rim, H. S., Kim, J. H., Kim, S. S., Yeo, J. H., & Yeo, S. G. (2024). Autophagy Genes and Otitis Media Outcomes. Clinics and Practice, 14(1), 293-304. https://doi.org/10.3390/clinpract14010023








