Risk Factors and Environmental Preventive Actions for Aspergillosis in Patients with Hematological Malignancies
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Question
- P (population): patients with acute hematologic neoplasms, (HN) acute myeloid leukemia (AML)/recipients of hematopoietic progenitor cell transplantation;
- I (intervention): environmental control measures;
- C (control): does not apply;
- O (outcome): invasive aspergillosis.
2.2. Study Selection
2.3. Inclusion Criteria
- Scientific articles from the years 2009–2023;
- Articles published in Spanish and English;
- Types of studies: Experimental studies, observational studies;
- Articles containing the key descriptors: “Aspergillosis”, “Invasive pulmonary aspergillosis”, “Hematologic neoplasms”, “Hematopoietic Stem Cell Transplantation”, “Leukemia”, “Risk factors” and “Prevention”.
2.4. Exclusion Criteria
- Articles that after applying the STROBE guidelines, for observational studies, respectively, did not reach a minimal punctuation;
- Articles that do not address our research question;
- Articles in pediatric populations.
2.5. Data Extraction
2.6. Methodological Quality Assessment
2.7. Data Synthesis and Analysis
3. Results and Discussion
3.1. Risk Factors for IA
3.2. Environmental Preventive Measures against IA
3.3. Main Recommendations to Fight against IA
3.4. Strengths and Weakness
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pemán, J.; Salavert, M. Epidemiología y Prevención de Las Infecciones Nosocomiales Causadas Por Especies de Hongos Filamentosos y Levaduras. Enferm. Infecc. Microbiol. Clin. 2013, 31, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and Outcome of Invasive Fungal Infection in Adult Hematopoietic Stem Cell Transplant Recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance Registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef]
- Pemán, J.; Salavert, M. Epidemiología General de La Enfermedad Fúngica Invasora. Enferm. Infecc. Microbiol. Clin. 2012, 30, 90–98. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Carratalà, J. Patogenia de La Infección Fúngica Invasora. Enferm. Infecc. Microbiol. Clin. 2012, 30, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Vázquez, L. Invasive Aspergillosis in the Patient with Oncohematologic Disease. Rev. Iberoam. Micol. 2018, 35, 198–205. [Google Scholar] [CrossRef]
- Espinosa, V.; Jhingran, A.; Dutta, O.; Kasahara, S.; Donnelly, R.; Du, P.; Rosenfeld, J.; Leiner, I.; Chen, C.-C.; Ron, Y.; et al. Inflammatory Monocytes Orchestrate Innate Antifungal Immunity in the Lung. PLoS Pathog. 2014, 10, e1003940. [Google Scholar] [CrossRef]
- Crokaert, F.; Walsh, T.J.; Fiere, D.; Selleslag, D.; Maertens, J.; Edwards, J.E.; Bennett, J.E.; Denning, D.W.; Rex, J.H.; Stevens, D.A.; et al. Defining Opportunistic Invasive Fungal Infections in Immunocompromised Patients with Cancer and Hematopoietic Stem Cell Transplants: An International Consensus. Clin. Infect. Dis. 2002, 34, 7–14. [Google Scholar] [CrossRef]
- Sanna, M.; Caocci, G.; Ledda, A.; Orrù, F.; Fozza, C.; Deias, P.; Tidore, G.; Dore, F.; La Nasa, G. Glucose-6-Phosphate Dehydrogenase Deficiency and Risk of Invasive Fungal Disease in Patients with Acute Myeloid Leukemia. Leuk. Lymphoma 2017, 58, 2558–2564. [Google Scholar] [CrossRef]
- Vallejo Llamas, J.C.; Ruiz-Camps, I. Infección Fúngica Invasora En Los Pacientes Hematológicos. Enferm. Infecc. Microbiol. Clin. 2012, 30, 572–579. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Chamilos, G.; Lewis, R.E.; Giralt, S.; Cortes, J.; Raad, I.I.; Manning, J.T.; Han, X. Increased Bone Marrow Iron Stores Is an Independent Risk Factor for Invasive Aspergillosis in Patients with High-Risk Hematologic Malignancies and Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Cancer 2007, 110, 1303–1306. [Google Scholar] [CrossRef]
- Garcia-Vidal, C.; Barba, P.; Arnan, M.; Moreno, A.; Ruiz-Camps, I.; Gudiol, C.; Ayats, J.; Orti, G.; Carratala, J. Invasive Aspergillosis Complicating Pandemic Influenza A (H1N1) Infection in Severely Immunocompromised Patients. Clin. Infect. Dis. 2011, 53, e16–e19. [Google Scholar] [CrossRef]
- Ajmal, S.; Mahmood, M.; Abu Saleh, O.; Larson, J.; Sohail, M.R. Invasive Fungal Infections Associated with Prior Respiratory Viral Infections in Immunocompromised Hosts. Infection 2018, 46, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, B.; Azap, A.; Akan, H.; Yılmaz, G.; Elhan, A. D-Index: A New Scoring System in Febrile Neutropenic Patients for Predicting Invasive Fungal Infections. Turk. J. Hematol. 2016, 33, 102–106. [Google Scholar] [CrossRef]
- Cunha, C.; Giovannini, G.; Pierini, A.; Bell, A.S.; Sorci, G.; Riuzzi, F.; Donato, R.; Rodrigues, F.; Velardi, A.; Aversa, F.; et al. Genetically-Determined Hyperfunction of the S100B/RAGE Axis Is a Risk Factor for Aspergillosis in Stem Cell Transplant Recipients. PLoS ONE 2011, 6, e27962. [Google Scholar] [CrossRef]
- Ruiz Camps, I. Aspergillosis: Beyond the Oncohematological Patient | Aspergilosis: Más Allá Del Paciente Oncohematológico. Enferm. Infecc. Microbiol. Clin. 2020, 38, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Camps, I.; Jarque, I. Enfermedad Fúngica Invasora Por Hongos Filamentosos En Pacientes Hematológicos. Rev. Iberoam. Micol. 2014, 31, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; Ledoux, M.P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-Onset Invasive Aspergillosis and Other Fungal Infections in Patients Treated with Ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef]
- Nedel, W.L.; Kontoyiannis, D.P.; Pasqualotto, A.C. Aspergillosis in Patients Treated with Monoclonal Antibodies. Rev. Iberoam. Micol. 2009, 26, 175–183. [Google Scholar] [CrossRef]
- Meis, J.F.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical Implications of Globally Emerging Azole Resistance in Aspergillus Fumigatus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150460. [Google Scholar] [CrossRef]
- Cornely, O.A.; Koehler, P.; Arenz, D.; Mellinghoff, S.C. EQUAL Aspergillosis Score 2018: An ECMM Score Derived from Current Guidelines to Measure QUALity of the Clinical Management of Invasive Pulmonary Aspergillosis. Mycoses 2018, 61, 833–836. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 Guidelines for the Treatment of Invasive Candidiasis, Aspergillosis and Mucormycosis in Leukemia and Hematopoietic Stem Cell Transplant Patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- De Riesgo, H.; De Trabajo, G.; Ramos Cuadra, A. Recomendaciones Para La Monitorización De La Calidad Microbiologica Del Aire (Bioseguridad Ambiental) En Zonas. Available online: https://www.sociedadandaluzapreventiva.com/wp-content/uploads/Borrador-protocolo-bioseguridad-SAMPSP.pdf (accessed on 1 December 2023).
- Garcia-Vidal, C.; Alastruey-Izquierdo, A.; Aguilar-Guisado, M.; Carratalà, J.; Castro, C.; Fernández-Ruiz, M.; María Aguado, J.; María Fernández, J.; Fortún, J.; Garnacho-Montero, J.; et al. Clinical Practice Guideline for the Management of Invasive Diseases Caused by Aspergillus: 2018 Update by the GEMICOMED-SEIMC/REIPI Documento de Consenso Del GEMICOMED Perteneciente a La Sociedad Española de Enfermedades Infecciosas y Microbiología Clínic. Available online: https://www.seimc.org/contenidos/documentoscientificos/borrador/seimc-GuiaClinica-BR-2018-Management_of_aspergillosis.pdf (accessed on 1 December 2023).
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Camps, I.; Aguado, J.M.; Almirante, B.; Bouza, E.; Ferrer Barbera, C.; Len, O.; López-Cerero, L.; Rodríguez-Tudela, J.L.; Ruiz, M.; Solé, A.; et al. Recomendaciones Sobre La Prevención de La Infección Fúngica Invasora Por Hongos Filamentosos de La Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC). Enferm. Infecc. Microbiol. Clin. 2010, 28, 172.e1–172.e21. [Google Scholar] [CrossRef] [PubMed]
- Peláez, T.; Muñoz, P.; Guinea, J.; Valerio, M.; Giannella, M.; Klaassen, C.H.W.; Bouza, E. Outbreak of Invasive Aspergillosis after Major Heart Surgery Caused by Spores in the Air of the Intensive Care Unit. Clin. Infect. Dis. 2012, 54, e24–e31. [Google Scholar] [CrossRef] [PubMed]
- Vonberg, R.-P.; Gastmeier, P. Nosocomial Aspergillosis in Outbreak Settings. J. Hosp. Infect. 2006, 63, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Risk of Bias Tools—Current Version of RoB 2. Available online: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed on 18 August 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; Von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, 1628–1654. [Google Scholar] [CrossRef] [PubMed]
- Combariza, J.F.; Toro, L.F.; Orozco, J.J. Effectiveness of Environmental Control Measures to Decrease the Risk of Invasive Aspergillosis in Acute Leukaemia Patients during Hospital Building Work. J. Hosp. Infect. 2017, 96, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Kanda, J.; Hishizawa, M.; Kitano, T.; Kondo, T.; Yamashita, K.; Takaori-Kondo, A. Effect of Laminar Air Flow and Building Construction on Aspergillosis in Acute Leukemia Patients: A Retrospective Cohort Study. BMC Infect. Dis. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Loschi, M.; Thill, C.; Gray, C.; David, M.; Bagatha, M.-F.; Chamseddine, A.; Contentin, N.; Jardin, F.; Lanic, H.; Lemasle, E.; et al. Invasive Aspergillosis in Neutropenic Patients During Hospital Renovation: Effectiveness of Mechanical Preventive Measures in a Prospective Cohort of 438 Patients. Mycopathologia 2015, 179, 337–345. [Google Scholar] [CrossRef]
- Park, J.H.; Ryu, S.H.; Lee, J.Y.; Kim, H.J.; Kwak, S.H.; Jung, J.; Lee, J.; Sung, H.; Kim, S.H. Airborne Fungal Spores and Invasive Aspergillosis in Hematologic Units in a Tertiary Hospital during Construction: A Prospective Cohort Study. Antimicrob. Resist. Infect. Control 2019, 8, 88. [Google Scholar] [CrossRef] [PubMed]
- Friese, C.; Breuckmann, K.; Hüttmann, A.; Eisele, L.; Dührsen, U. Neutropenia-Related Aspergillosis in Non-Transplant Haematological Patients Hospitalised under Ambient Air versus Purified Air Conditions. Mycoses 2023, 66, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gerlinger, M.-P.; Jannot, A.-S.; Rigaudeau, S.; Lambert, J.; Eloy, O.; Mignon, F.; Farhat, H.; Castaigne, S.; Merrer, J.; Rousselot, P. The Plasmair Decontamination System Is Protective Against Invasive Aspergillosis in Neutropenic Patients. Infect. Control Hosp. Epidemiol. 2016, 37, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, S.; Reboux, G.; Larosa, F.; Scherer, E.; Daguindeau, E.; Berceanu, A.; Deconinck, E.; Millon, L.; Bellanger, A.-P. Evaluation of Invasive Aspergillosis Risk of Immunocompromised Patients Alternatively Hospitalized in Hematology Intensive Care Unit and at Home. Indoor Air 2014, 24, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Busca, A.; Candoni, A.; Cattaneo, C.; Cesaro, S.; Fanci, R.; Nadali, G.; Potenza, L.; Russo, D.; Tumbarello, M.; et al. Risk Stratification for Invasive Fungal Infections in Patients with Hematological Malignancies: SEIFEM Recommendations. Blood Rev. 2017, 31, 17–29. [Google Scholar] [CrossRef]
- Pagano, L.; Akova, M.; Dimopoulos, G.; Herbrecht, R.; Drgona, L.; Blijlevens, N. Risk Assessment and Prognostic Factors for Mould-Related Diseases in Immunocompromised Patients. J. Antimicrob. Chemother. 2011, 66, i5–i14. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Martino, B.; Specchia, G.; Pastore, D.; Stanzani, M.; Cattaneo, C.; Fanci, R.; et al. Invasive Aspergillosis in Patients with Acute Myeloid Leukemia: A SEIFEM-2008 Registry Study. Haematologica 2010, 95, 644–650. [Google Scholar] [CrossRef]
- Lien, M.Y.; Chou, C.H.; Lin, C.C.; Bai, L.Y.; Chiu, C.F.; Yeh, S.P.; Ho, M.W. Epidemiology and Risk Factors for Invasive Fungal Infections during Induction Chemotherapy for Newly Diagnosed Acute Myeloid Leukemia: A Retrospective Cohort Study. PLoS ONE 2018, 13, e0197851. [Google Scholar] [CrossRef]
- Warris, A.; Onken, A.; Gaustad, P.; Janssen, W.; van der Lee, H.; Verweij, P.E.; Abrahamsen, T.G. Point-of-Use Filtration Method for the Prevention of Fungal Contamination of Hospital Water. J. Hosp. Infect. 2010, 76, 56–59. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The Epidemiology of Fungal Infections in Patients with Hematologic Malignancies: The SEIFEM-2004 Study. Haematologica 2006, 91, 1068–1075. [Google Scholar]
- Alberti, C.; Bouakline, A.; Ribaud, P.; Lacroix, C.; Rousselot, P.; Leblanc, T.; Derouin, F. Relationship between Environmental Fungal Contamination and the Incidence of Invasive Aspergillosis in Haematology Patients. J. Hosp. Infect. 2001, 48, 198–206. [Google Scholar] [CrossRef]
- Hruszkewycz, V.; Ruben, B.; Hypes, C.M.; Bostic, G.D.; Staszkiewicz, J.; Band, J.D. A Cluster of Pseudofungemia Associated with Hospital Renovation Adjacent to the Microbiology Laboratory. Infect. Control Hosp. Epidemiol. 1992, 13, 147–150. [Google Scholar] [CrossRef]
- Aisner, J.; Schimpff, S.C.; Bennett, J.E.; Young, V.M.; Wiernik, P.H. Aspergillus Infections in Cancer Patients: Association with Fireproofing Materials in a New Hospital. JAMA J. Am. Med. Assoc. 1976, 235, 411–412. [Google Scholar] [CrossRef]
- Lai, K.K. A Cluster of Invasive Aspergillosis in a Bone Marrow Transplant Unit Related to Construction and the Utility of Air Sampling. Am. J. Infect. Control 2001, 29, 333–337. [Google Scholar] [CrossRef]
- Flynn, P.M.; Williams, B.G.; Hetherington, S.V.; Williams, B.F.; Giannini, M.A.; Pearson, T.A. Aspergillus Terreus During Hospital Renovation. Infect. Control Hosp. Epidemiol. 1993, 14, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Weems, J.J.; Davis, B.J.; Tablan, O.C.; Kaufman, L.; Martone, W.J. Construction Activity: An Independent Risk Factor for Invasive Aspergillosis and Zygomycosis in Patients with Hematologic Malignancy. Infect. Control 1987, 8, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Abdul Salam, Z.-H.; Karlin, R.B.; Ling, M.L.; Yang, K.S. The Impact of Portable High-Efficiency Particulate Air Filters on the Incidence of Invasive Aspergillosis in a Large Acute Tertiary-Care Hospital. Am. J. Infect. Control 2010, 38, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Herbrecht, R.; Bories, P.; Moulin, J.C.; Ledoux, M.P.; Letscher-Bru, V. Risk Stratification for Invasive Aspergillosis in Immunocompromised Patients. Ann. N. Y. Acad. Sci. 2012, 1272, 23–30. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Oren, I.; Haddad, N.; Finkelstein, R.; Rowe, J.M. Invasive Pulmonary Aspergillosis in Neutropenic Patients during Hospital Construction: Before and after Chemoprophylaxis and Institution of HEPA Filters. Am. J. Hematol. 2001, 66, 257–262. [Google Scholar] [CrossRef]
- Diaz-Arevalo, D.; Kalkum, M. CD4+ T Cells Mediate Aspergillosis Vaccine Protection; Humana Press: New York, NY, USA, 2017; pp. 281–293. [Google Scholar]
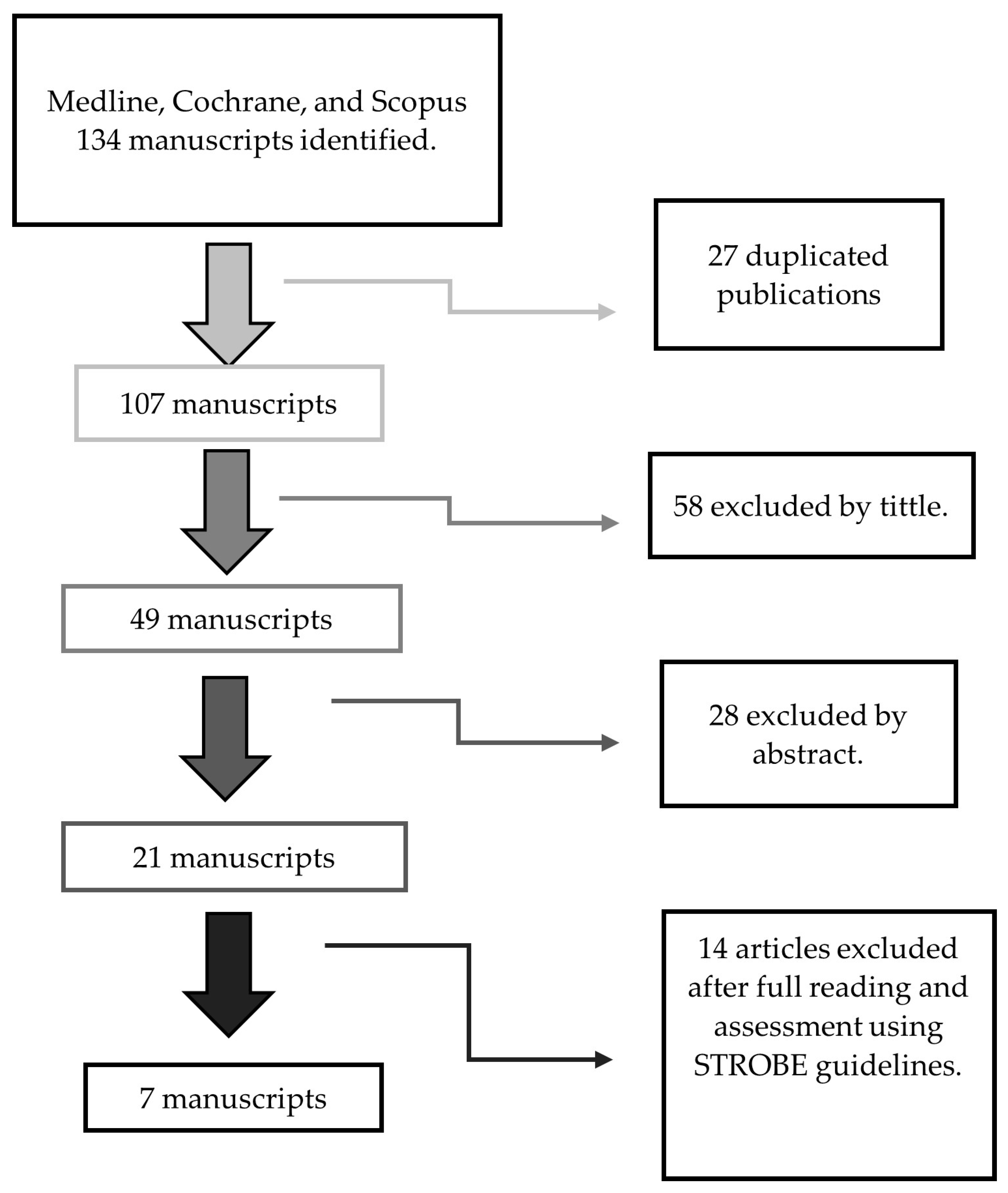
| Source of Data | Search Strategy | Number of Manuscripts |
|---|---|---|
| PubMed | MeSH: (“Aspergillosis”[Mesh] OR “Invasive Pulmonary Aspergillosis”[Mesh]) AND (“Hematologic Neoplasms”[Mesh] OR “Hematopoietic Stem Cell Transplantation”[Mesh] OR “Leukemia” [Mesh]) AND (“Risk Factors”[Mesh] OR “Primary Prevention”[Mesh]) | 79 |
| SCOPUS | (Aspergillosis OR “invasive pulmonary aspergillosis”) AND (“hematologic stem cell transplantation” OR “hematologic neoplasms” OR leukemia) AND (“Risk factors” OR “Primary prevention”) AND NOT index (MEDLINE). | 46 |
| Cochrane Library | “aspergillosis” | 9 |
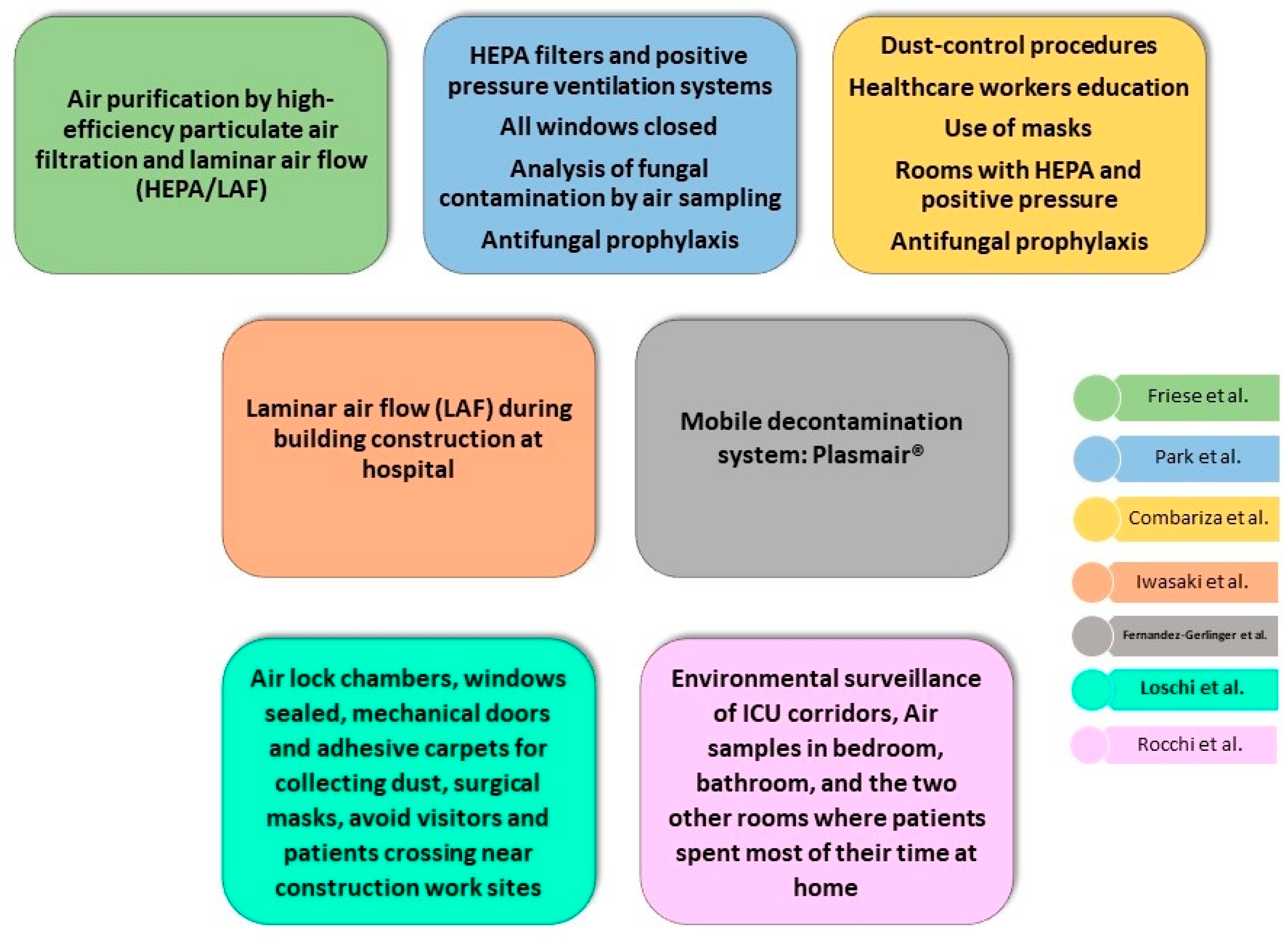
| Authors/Year/Country 1 | SD | SDu | Location | Patients | Sample | IAI | Assessment |
|---|---|---|---|---|---|---|---|
| Friese et al. [34]/2023/Germany | RCS | 7 | Hospitalization during construction of a new building with HEPA/LAF | AML or ALL | 204 No HEPA/LAF 126 with HEPA/LAF | 51 No HEPA/LAF 26 With HEPA/LAF | Efficiency of HEPA/LAF against IA |
| Park et al. [33]/2019/South Korea | PCS | 0.5 | Hospitalization during construction of new building with HEPA | Patients with HM | 29 | 15 First period 14 s period | Environmental spore surveillance Preventive effect of HEPA system against IA |
| Iwasaki et al. [31]/2019/Japan | RCS | 20 | Hospitalization during construction of a new building with LAF | AML or ALL | 124 | 14 | Preventive effect of long-term LAF isolation against IA |
| Combariza et al. [30]/2017/Colombia | RCS | 2.5 | Hospitalization during construction of a new building with and without ECM | AML or ALL | 175 62 No ECM 113 W ECM | 29 16 No ECM 13 W ECM | Impact of ECM for the prevention of IA |
| Fernandez-Gerlinger et al. [35]/2016/France | RCS | 2 | HICU with and without Plasmair® | Patients with HM and CIN | 156 | 11 | Impact of Plasmair® on IAI |
| Loschi et al. [32]/2015/France | PCS | 5 | HICU during hospital renovation | with neutropenia for more than 7 days | 438 | 84 | Effectiveness of mechanical preventive measures against IA |
| Rocchi et al. [36]/2014/France | PCS | 2 | HICU and PH | Hospitalized in the HICU | 53 | 14 | ES of HICU and PH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raposo Puglia, D.; Raposo Puglia, J.Á.; García-Cabrera, E.; Morales, F.; Camacho-Vega, J.C.; Vilches-Arenas, Á. Risk Factors and Environmental Preventive Actions for Aspergillosis in Patients with Hematological Malignancies. Clin. Pract. 2024, 14, 280-292. https://doi.org/10.3390/clinpract14010022
Raposo Puglia D, Raposo Puglia JÁ, García-Cabrera E, Morales F, Camacho-Vega JC, Vilches-Arenas Á. Risk Factors and Environmental Preventive Actions for Aspergillosis in Patients with Hematological Malignancies. Clinics and Practice. 2024; 14(1):280-292. https://doi.org/10.3390/clinpract14010022
Chicago/Turabian StyleRaposo Puglia, Daniel, José Ángel Raposo Puglia, Emilio García-Cabrera, Fátima Morales, Juan Carlos Camacho-Vega, and Ángel Vilches-Arenas. 2024. "Risk Factors and Environmental Preventive Actions for Aspergillosis in Patients with Hematological Malignancies" Clinics and Practice 14, no. 1: 280-292. https://doi.org/10.3390/clinpract14010022
APA StyleRaposo Puglia, D., Raposo Puglia, J. Á., García-Cabrera, E., Morales, F., Camacho-Vega, J. C., & Vilches-Arenas, Á. (2024). Risk Factors and Environmental Preventive Actions for Aspergillosis in Patients with Hematological Malignancies. Clinics and Practice, 14(1), 280-292. https://doi.org/10.3390/clinpract14010022






