Serum Markers of Brain Injury in Pediatric Patients with Congenital Heart Defects Undergoing Cardiac Surgery: Diagnostic and Prognostic Role
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Neurodevelopmental Testing
2.3. Sample Collection and Neuromarkers Analysis
2.4. Perioperative Neurological Monitoring
2.5. Statistical Analysis
3. Results
3.1. Anatomical, Clinical, and Surgical Characteristics
3.2. Serum Neuromarkers (Glial Fibrillary Acidic Protein, Brain-Derived Neurotrophic Factor, Protein s100, and Neuron-Specific Enolase) and Acute Brain Injury
3.2.1. Perioperative Values of Neuromarkers
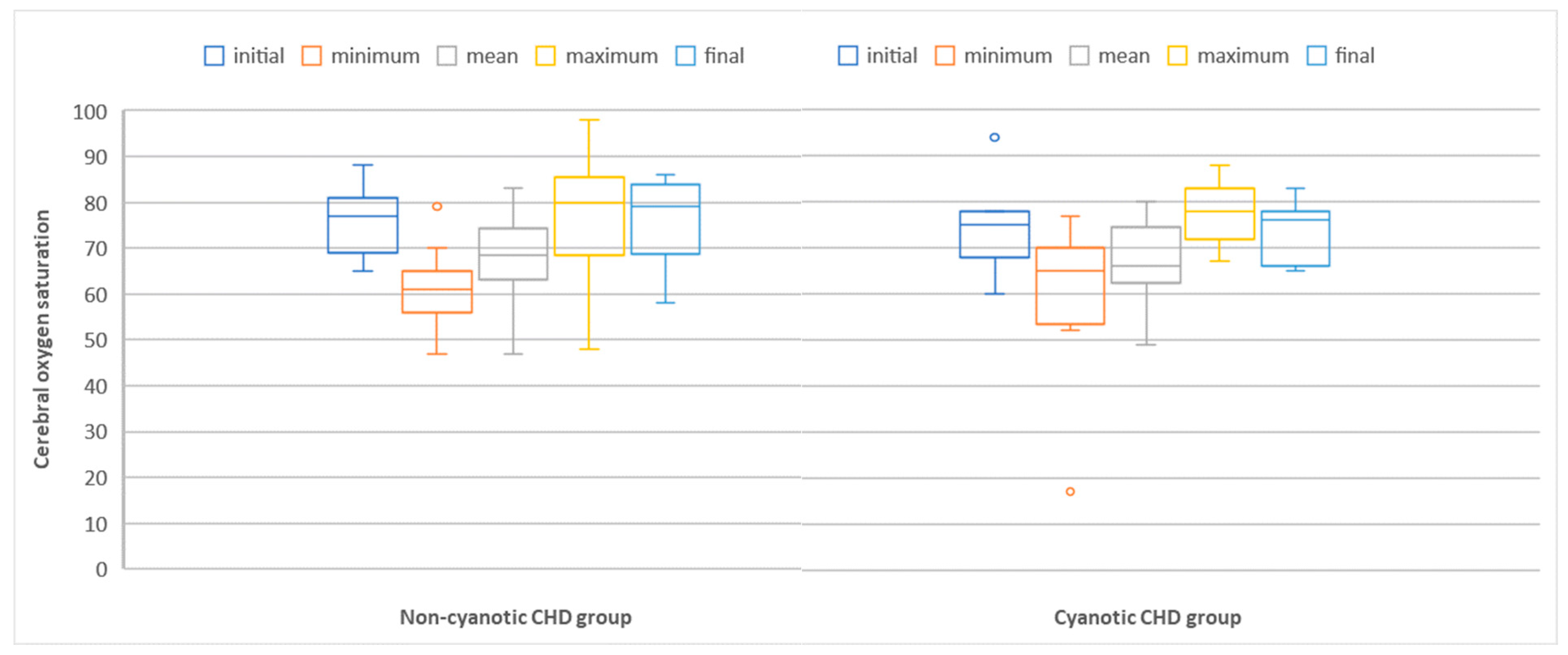
3.2.2. Correlation between Cerebral Oxygen Saturation and Neuromarkers
3.3. Serum Neuromarkers (Glial Fibrillary Acidic Protein, Brain-Derived Neurotrophic Factor, Protein s100, and Neuron-Specific Enolase) and Short-Term Neurodevelopment in Operated CHD Children
3.3.1. Neurodevelopmental Assessment
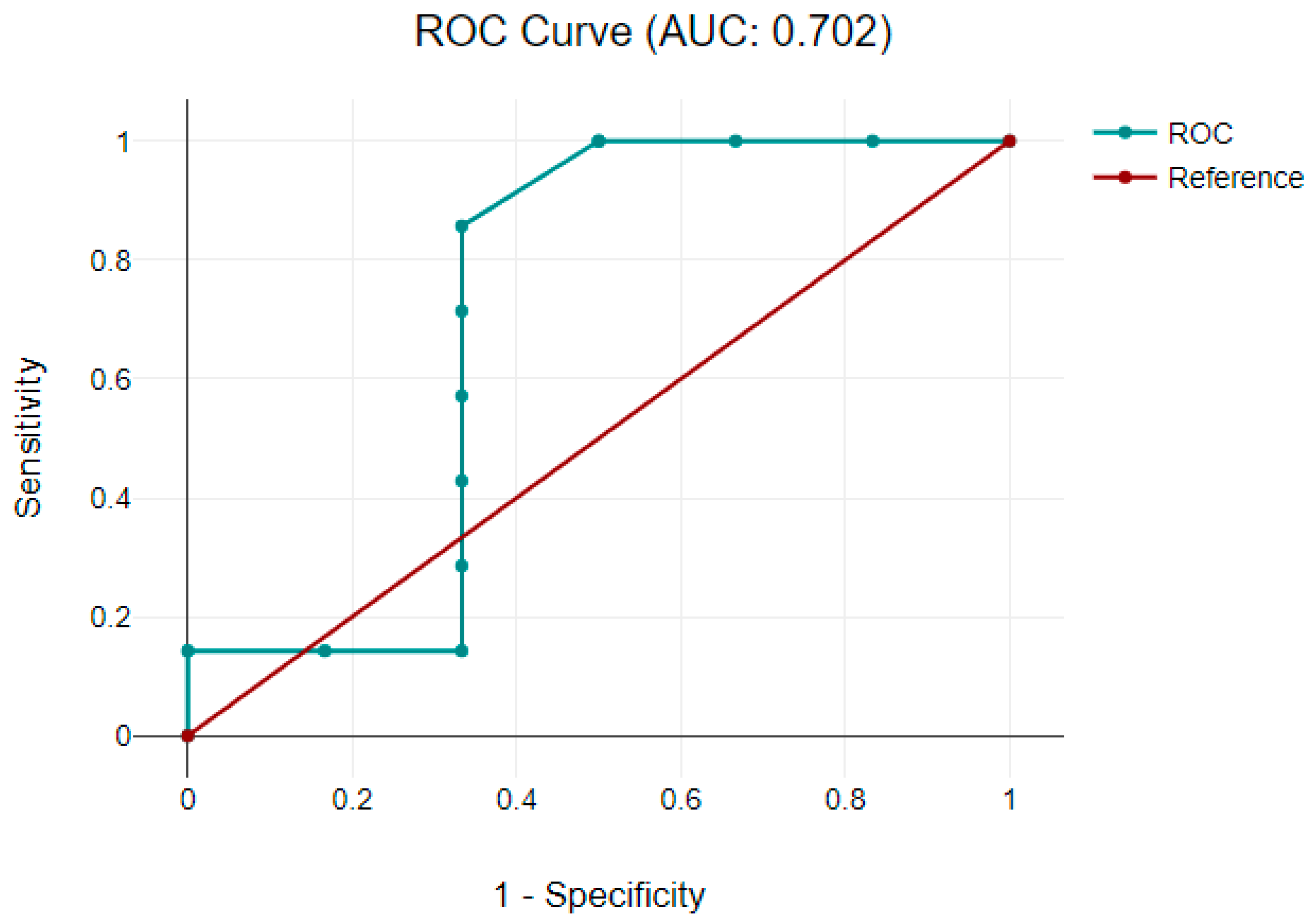
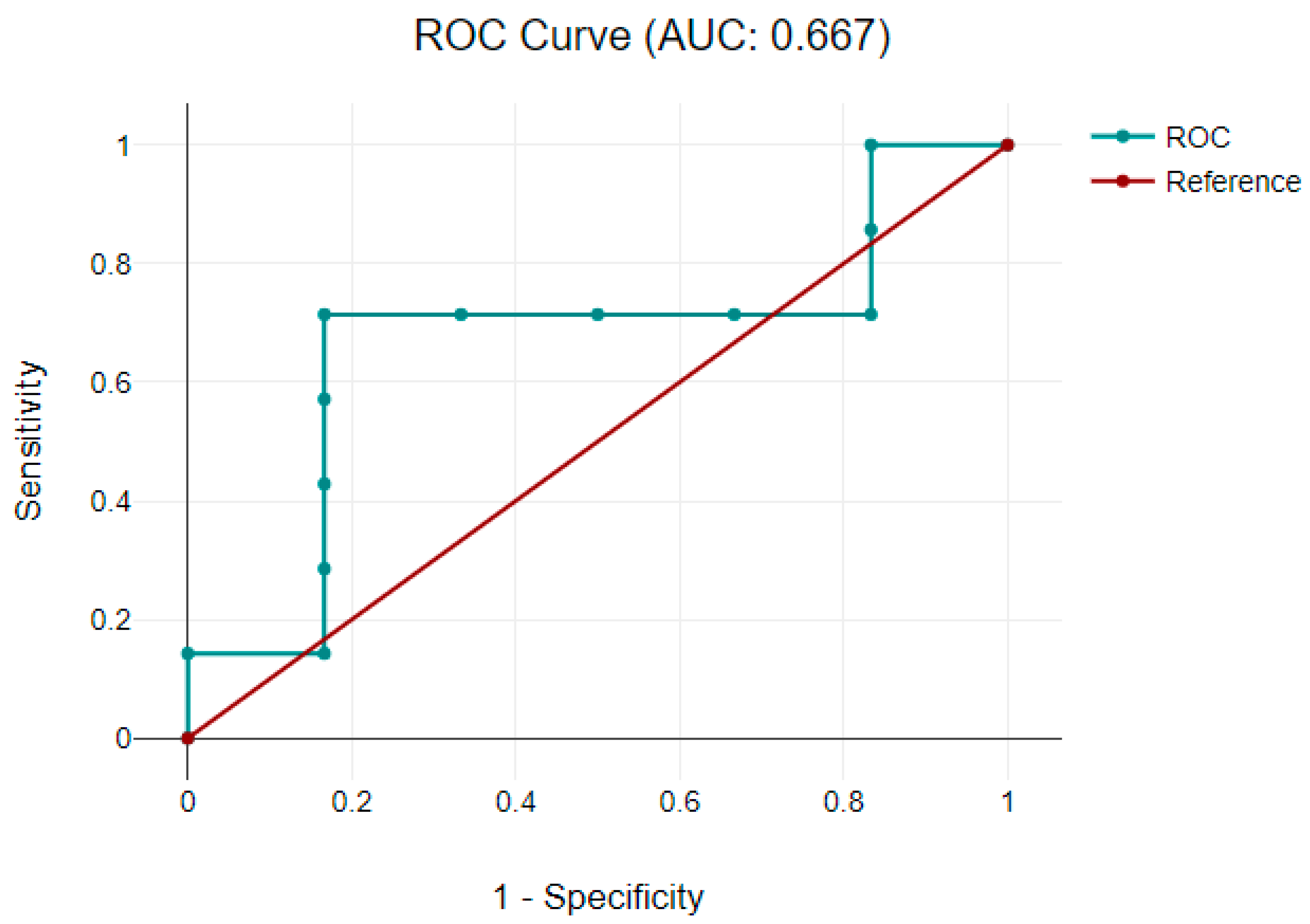
3.3.2. Correlation between Neuromarkers and Neurodevelopmental Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; He, J.; Shao, X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990–2017. Medicine 2020, 99, e20593. [Google Scholar] [CrossRef]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Wernovsky, G. The paradigm shift toward surgical intervention for neonates with hypoplastic left heart syndrome. Arch. Pediatr. Adolesc. Med. 2008, 162, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Corno, A.F.; Utens, E.M.W.J. Editorial: Neuro-Development and Psychological Issues in Congenital Heart Defects. Front. Pediatr. 2018, 5, 297. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Jonas, R.A.; Rappaport, L.A.; Wypij, D.; Wernovsky, G.; Kuban, K.C.; Barnes, P.D.; Holmes, G.L.; Hickey, P.R.; Strand, R.D. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N. Engl. J. Med. 1995, 332, 549–555. [Google Scholar] [CrossRef]
- Massaro, A.N.; Glass, P.; Brown, J.; Chang, T.; Krishnan, A.; Jonas, R.A.; Donofrio, M.T. Neurobehavioral abnormalities in newborns with congenital heart disease requiring open-heart surgery. J. Pediatr. 2011, 158, 678–681.e2. [Google Scholar] [CrossRef]
- Phillips, J.M.; Longoria, J.N. Addressing the neurodevelopmental needs of children and adolescents with congenital heart disease: A review of the existing intervention literature. Child Neuropsychol. 2020, 26, 433–459. [Google Scholar] [CrossRef]
- Sanchez-de-Toledo, J.; Chrysostomou, C.; Munoz, R.; Lichtenstein, S.; Sao-Avilés, C.A.; Wearden, P.D.; Morell, V.O.; Clark, R.S.; Toney, N.; Bell, M.J. Cerebral regional oxygen saturation and serum neuromarkers for the prediction of adverse neurologic outcome in pediatric cardiac surgery. Neurocrit. Care 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Fink, E.L.; Berger, R.P.; Clark, R.S.; Watson, R.S.; Angus, D.C.; Richichi, R.; Panigrahy, A.; Callaway, C.W.; Bell, M.J.; Kochanek, P.M. Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest. Crit. Care Med. 2014, 42, 664–674. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; González-Quevedo, A.; Fernández-Concepción, O.; Peña-Sánchez, M.; Menéndez-Saínz, C.; Hernández-Díaz, Z.; Arteche-Prior, M.; Pando-Cabrera, A.; Fernández-Novales, C. Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin. Biochem. 2012, 45, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Lardner, D.; Davidson, A.; McKenzie, I.; Cochrane, A. Delayed rises in serum S100B levels and adverse neurological outcome in infants and children undergoing cardiopulmonary bypass. Pediatr. Anesth. 2004, 14, 495–500. [Google Scholar] [CrossRef]
- Bechtel, K.; Frasure, S.; Marshall, C.; Dziura, J.; Simpson, C. Relationship of serum S100B levels and intracranial injury in children with closed head trauma. Pediatrics 2009, 124, e697–e704. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.P.; Adelson, P.D.; Richichi, R.; Kochanek, P.M. Serum biomarkers after traumatic and hypoxemic brain injuries: Insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev. Neurosci. 2006, 28, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mediafax.ro/social/in-panedemie-in-romania-s-au-nascut-cei-mai-putini-copii-din-1930-pana-in-prezent-ins-numarul-copiilor-a-scazut-cu-peste-38-000-20112110 (accessed on 31 March 2023).
- Boia, M.; Costescu, O.C.; Cioboata, D.; Popoiu, A.; Lungu, N.; Doandes, F.; Manea, A.M. Congenital heart disease—A public health problem. Rom. J. Pediatr. 2020, 69, 221–225. [Google Scholar] [CrossRef]
- Frankenburg, W.K. The Denver Developmental Screening Test. J. Pediatr. 1967, 71, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Ware, C.J.; Sloss, C.F.; Chugh, C.S.; Budd, K.S. Adaptations of the Denver II Scoring System to Assess the Developmental Status of Children With Medically Complex Conditions. Child. Health Care 2002, 31, 255–272. [Google Scholar] [CrossRef]
- McNeill, S.; Gatenby, J.C.; McElroy, S.; Engelhardt, B. Normal cerebral, renal and abdominal regional oxygen saturations using near-infrared spectroscopy in preterm infants. J. Perinatol. 2011, 31, 51–57. [Google Scholar] [CrossRef]
- Marino, B.S.; Lipkin, P.H.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; Mussatto, K.A.; Uzark, K.; Goldberg, C.S.; Johnson, W.H., Jr.; et al. Neurodevelopmental outcomes in children with congenital heart disease: Evaluation and management: A scientific statement from the American Heart Association. Circulation 2012, 126, 1143–1172. [Google Scholar] [CrossRef]
- Tabbutt, S.; Gaynor, J.W.; Newburger, J.W. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr. Opin. Cardiol. 2012, 27, 82–91. [Google Scholar] [CrossRef]
- Schultz, A.H.; Ittenbach, R.F.; Gerdes, M.; Jarvik, G.P.; Wernovsky, G.; Bernbaum, J.; Solot, C.; Clancy, R.R.; Nicolson, S.C.; Spray, T.L.; et al. Effect of congenital heart disease on 4-year neurodevelopment within multiple-gestation births. J. Thorac. Cardiovasc. Surg. 2017, 154, 273–281.e2. [Google Scholar] [CrossRef]
- White, B.R.; Rogers, L.S.; Kirschen, M.P. Recent advances in our understanding of neurodevelopmental outcomes in congenital heart disease. Curr. Opin. Pediatr. 2019, 31, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Sananes, R.; Manlhiot, C.; Kelly, E.; Hornberger, L.K.; Williams, W.G.; MacGregor, D.; Buncic, R.; McCrindle, B.W. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann. Thorac. Surg. 2012, 93, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Wypij, D.; duPlessis, A.J.; Rappaport, L.A.; Jonas, R.A.; Wernovsky, G.; Newburger, J.W. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1385–1396. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; Rivkin, M.J.; DeMaso, D.R.; Robertson, R.L., Jr.; Dunbar-Masterson, C.; Rappaport, L.A.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: Neuropsychological assessment and structural brain imaging. Circulation 2011, 124, 1361–1369. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Rivkin, M.J.; DeMaso, D.; Robertson, R.L.; Stopp, C.; Dunbar-Masterson, C.; Wypij, D.; Newburger, J.W. Adolescents with tetralogy of Fallot: Neuropsychological assessment and structural brain imaging. Cardiol. Young 2015, 25, 338–347. [Google Scholar] [CrossRef]
- McCusker, C.G.; Doherty, N.N.; Molloy, B.; Casey, F.; Rooney, N.; Mulholland, C.; Sands, A.; Craig, B.; Stewart, M. Determinants of neuropsychological and behavioural outcomes in early childhood survivors of congenital heart disease. Arch. Dis. Child. 2007, 92, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yosef, O.; Greidinger, D.; Iskilova, M.; Hemi, R.; Tirosh, T.; Vardi, A. Neurological deficit is predicted by S100B in children after cardiac surgery. Clin. Chim. Acta 2018, 481, 56–60. [Google Scholar] [CrossRef]
- Trakas, E.; Domnina, Y.; Panigrahy, A.; Baust, T.; Callahan, P.M.; Morell, V.O.; Munoz, R.; Bell, M.J.; Sanchez-de-Toledo, J. Serum Neuronal Biomarkers in Neonates With Congenital Heart Disease Undergoing Cardiac Surgery. Pediatr. Neurol. 2017, 72, 56–61. [Google Scholar] [CrossRef]
- Ennen, C.S.; Huisman, T.A.; Savage, W.J.; Northington, F.J.; Jennings, J.M.; Everett, A.D.; Graham, E.M. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am. J. Obstet. Gynecol. 2011, 205, 251.e1–251.e7. [Google Scholar] [CrossRef]
- Hansen, J.H.; Kissner, L.; Logoteta, J.; Jung, O.; Dütschke, P.; Attmann, T.; Scheewe, J.; Kramer, H.H. S100B and its relation to cerebral oxygenation in neonates and infants undergoing surgery for congenital heart disease. Congenit. Heart Dis. 2019, 14, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Vedovelli, L.; Padalino, M.; Suppiej, A.; Sartori, S.; Falasco, G.; Simonato, M.; Carnielli, V.P.; Stellin, G.; Cogo, P. Cardiopulmonary-Bypass Glial Fibrillary Acidic Protein Correlates With Neurocognitive Skills. Ann. Thorac. Surg. 2018, 106, 792–798. [Google Scholar] [CrossRef]
- Bembea, M.M.; Savage, W.; Strouse, J.J.; Schwartz, J.M.; Graham, E.; Thompson, C.B.; Everett, A. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr. Crit. Care Med. A J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2011, 12, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [PubMed]
- Michetti, F.; Corvino, V.; Geloso, M.C.; Lattanzi, W.; Bernardini, C.; Serpero, L.; Gazzolo, D. The S100B protein in biological fluids: More than a lifelong biomarker of brain distress. J. Neurochem. 2012, 120, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Nagdyman, N.; Kömen, W.; Ko, H.K.; Müller, C.; Obladen, M. Early biochemical indicators of hypoxic-ischemic encephalopathy after birth asphyxia. Pediatr. Res. 2001, 49, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhou, D.; Wang, Y.W. Umbilical artery blood S100beta protein: A tool for the early identification of neonatal hypoxic-ischemic encephalopathy. Eur. J. Pediatr. 2009, 168, 71–77. [Google Scholar] [CrossRef]
- Lindberg, L.; Olsson, A.K.; Anderson, K.; Jögi, P. Serum S-100 protein levels after pediatric cardiac operations: A possible new marker for postperfusion cerebral injury. J. Thorac. Cardiovasc. Surg. 1998, 116, 281–285. [Google Scholar] [CrossRef]
- Abdul-Khaliq, H.; Alexi-Meskhishvili, V.; Lange, P.E. Serum S-100 protein levels after pediatric cardiac surgery: A possible new marker for postperfusion cerebral injury. J. Thorac. Cardiovasc. Surg. 1999, 117, 843–844. [Google Scholar] [CrossRef]
- Gunn, J.K.; Beca, J.; Hunt, R.W.; Goldsworthy, M.; Brizard, C.P.; Finucane, K.; Donath, S.; Shekerdemian, L.S. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch. Dis. Child. 2016, 101, 1010–1016. [Google Scholar] [CrossRef]
- Cheng, F.; Yuan, Q.; Yang, J.; Wang, W.; Liu, H. The prognostic value of serum neuron-specific enolase in traumatic brain injury: Systematic review and meta-analysis. PLoS ONE 2014, 9, e106680. [Google Scholar] [CrossRef]
- Limperopoulos, C.; Tworetzky, W.; McElhinney, D.B.; Newburger, J.W.; Brown, D.W.; Robertson, R.L., Jr.; Guizard, N.; McGrath, E.; Geva, J.; Annese, D.; et al. Brain volume and metabolism in fetuses with congenital heart disease: Evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 2010, 121, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Limperopoulos, C.; Majnemer, A.; Shevell, M.I.; Rosenblatt, B.; Rohlicek, C.; Tchervenkov, C. Neurodevelopmental status of newborns and infants with congenital heart defects before and after open heart surgery. J. Pediatr. 2000, 137, 638–645. [Google Scholar] [CrossRef]
- Licht, D.J.; Wang, J.; Silvestre, D.W.; Nicolson, S.C.; Montenegro, L.M.; Wernovsky, G.; Tabbutt, S.; Durning, S.M.; Shera, D.M.; Gaynor, J.W.; et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J. Thorac. Cardiovasc. Surg. 2004, 128, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Khaliq, H.; Schubert, S.; Stoltenburg-Didinger, G.; Huebler, M.; Troitzsch, D.; Wehsack, A.; Boettcher, W.; Schwaller, B.; Crausaz, M.; Celio, M.; et al. Release patterns of astrocytic and neuronal biochemical markers in serum during and after experimental settings of cardiac surgery. Restor. Neurol. Neurosci. 2003, 21, 141–150. [Google Scholar] [PubMed]
- Wurzelmann, M.; Romeika, J.; Sun, D. Therapeutic potential of brain-derived neurotrophic factor (BDNF) and a small molecular mimics of BDNF for traumatic brain injury. Neural Regen. Res. 2017, 12, 7–12. [Google Scholar] [CrossRef]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional aspects of depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef]
- Monteleone, P.; Serritella, C.; Martiadis, V.; Maj, M. Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord. 2008, 10, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Hashimoto, K.; Shimizu, E.; Niitsu, T.; Iyo, M. Possible involvement of brain-derived neurotrophic factor in eating disorders. IUBMB Life 2012, 64, 355–361. [Google Scholar] [CrossRef]
- Monteleone, P.; Tortorella, A.; Martiadis, V.; Serritella, C.; Fuschino, A.; Maj, M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom. Med. 2004, 66, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Fabrazzo, M.; Martiadis, V.; Serritella, C.; Pannuto, M.; Maj, M. Circulating brain-derived neurotrophic factor is decreased in women with anorexia and bulimia nervosa but not in women with binge-eating disorder: Relationships to co-morbid depression, psychopathology and hormonal variables. Psychol. Med. 2005, 35, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.T.; Andreazza, A.C.; Rej, S.; Rajji, T.K.; Gildengers, A.G.; Lafer, B.; Young, L.T.; Mulsant, B.H. Decreased Brain-Derived Neurotrophic Factor in Older Adults with Bipolar Disorder. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2016, 24, 596–601. [Google Scholar] [CrossRef]
- Korley, F.K.; Diaz-Arrastia, R.; Wu, A.H.; Yue, J.K.; Manley, G.T.; Sair, H.I.; Van Eyk, J.; Everett, A.D.; TRACK-TBI Investigators; Okonkwo, D.O.; et al. Circulating Brain-Derived Neurotrophic Factor Has Diagnostic and Prognostic Value in Traumatic Brain Injury. J. Neurotrauma 2016, 33, 215–225. [Google Scholar] [CrossRef]
- Barbu, M.; Jónsson, K.; Zetterberg, H.; Blennow, K.; Kolsrud, O.; Ricksten, S.E.; Dellgren, G.; Björk, K.; Jeppsson, A. Serum biomarkers of brain injury after uncomplicated cardiac surgery: Secondary analysis from a randomized trial. Acta Anaesthesiol. Scand. 2022, 66, 447–453. [Google Scholar] [CrossRef]
| Cyanotic Defects (n = 13) | Non-Cyanotic Defects (n = 29) |
|---|---|
| Septal defects | |
| Unbalanced atrioventricular septal defect (1) | Ventricular septal defect (20) -Atrial septal defect (1) |
| Conal defects | |
| Double-outlet right ventricle with pulmonary stenosis (5) Tetralogy of Fallot (7) | Tetralogy of Fallot without cyanosis (3) |
| Others | |
| Coarctation of aorta (2) Patent ductus arteriosus (1) Aorto–pulmonary fenestration (1) Cardiac tumor (1) | |
| Characteristics | Non-Cyanotic CHD (n = 29) | Cyanotic CHD (n = 13) | p Value |
|---|---|---|---|
| Neonatal characteristics | |||
| Gestational age (weeks) | 38.6 ± 1.8 | 38.6 ± 2 | 0.66 |
| Apgar score | 9 | 9 | 0.11 |
| Birth weight (g) | 3115 ± 862 | 3442 ± 722 | 0.66 |
| Birth length (cm) | 51.6 ± 4 | 53.4 ± 4.5 | 0.23 |
| Alimentation: breast milk/formula/mixt (n) | 10/14/5 | 7/3/3 | 0.43 |
| Maternal age at birth (years) | 28.2 ± 5.2 | 27.08 ± 4.9 | 0.97 |
| Paternal age at birth (years) | 31.4 ± 4.6 | 30.5 ± 6.03 | 0.7 |
| Clinical characteristics | |||
| Sex M/F (n) | 19/10 | 7/6 | 0.65 |
| Age (months) | 12.6 ± 15 | 9.8 ± 5 | 0.64 |
| Weight (kg) | 7.55 ± 3 | 8.09 ± 1 | 0.27 |
| Height (cm) | 70.8 ± 13 | 70.69 ± 5 | 0.47 |
| Head circumference (cm) | 43.1 ± 4 | 43.9 ± 2 | 0.64 |
| Saturation (%) | 97 ± 1.17 | 82 ± 8 | <0.0001 |
| Hemoglobin (g/dL) | 11.9 ± 1.4 | 13.55 ± 2.4 | 0.046 |
| Albumin (g/dL) | 4.6 ± 0.4 | 4.5 ± 0.2 | 0.28 |
| Surgical characteristics | |||
| Surgery duration (min) | 216 ± 75 | 265.7 ± 56 | 0.006 |
| CBP duration (min) | 97.14 ± 36 | 131 ± 42 | 0.02 |
| Aortic clamp duration (min) | 59 ± 2.89 | 82 ± 35 | 0.002 |
| Mechanical ventilation duration (hours) | 21.44 ± 36 | 80.7 ± 110 | 0.01 |
| ICU admission period (days) | 4.5 ± 1.9 | 7 ± 4.9 | 0.009 |
| Neuromarker | Non-Cyanotic CHD Group | Cyanotic CHD Group | ||||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | p-Value | Preoperative | Postoperative | p-Value | |
| GFAP (pg/mL) | 0.65 ± 0.05 | 0.68 ± 0.02 | 0.08 | 0.64 ± 0.05 | 0.68 ± 0.03 | 0.02 |
| BDNF (pg/mL) | 5723.9 ± 3642 | 3293.5 ± 1788 | 0.0003 | 6334.3 ± 3438 | 2861.5 ± 1424 | 0.0006 |
| pS100 (µg/L) | 21.43 ± 5 | 32.79 ± 10 | 0.002 | 21.51 ± 11 | 39.02 ± 11 | 0.0057 |
| NSE (ng/mL) | 0.21 ± 0.1 | 0.15 ± 0.08 | 0.0009 | 0.19 ± 0.05 | 0.16 ± 0.05 | 0.96 |
| Domain/Number of Cases | Non-Cyanotic CHD n = 29 | Cyanotic CHD n = 13 |
|---|---|---|
| Personal-social | 17 (58.6%) | 6 (46.1%) |
| Fine-motor | 20 (68.9%) | 10 (76.9%) |
| Language | 21 (72.4%) | 4 (30.76%) |
| Gross-motor | 13 (44.8%) | 5 (38.4%) |
| Score | Preoperative | Postoperative | p-Value |
|---|---|---|---|
| Domain-specific developmental quotient Scores | |||
| Personal/social | 0.96 ± 0.2 | 0.92 ± 0.5 | 0.24 |
| Fine motor | 1.06 ± 0.2 | 1.36 ± 0.9 | 0.03 |
| Language | 0.9 ± 0.3 | 1.69 ± 1.9 | 0.8 |
| Gross motor | 0.89 ± 0.2 | 0.81 ± 0.3 | 0.3 |
| Overall developmental quotient score | 1.2 ± 0.8 | 0.95 ± 0.1 | 0.1 |
| Domain-specific developmental gain Scores | |||
| Personal/social | 1 ± 1.7 | ||
| Fine motor | 0.5 ± 1.3 | ||
| Language | 0.006 ± 2.3 | ||
| Gross motor | 1.12 ± 0.7 | ||
| Overall developmental gain score | 0.73 ± 1.2 |
| Score | Preoperative | Postoperative | p-Value |
|---|---|---|---|
| Domain-specific developmental quotient Scores | |||
| Personal/social | 0.86 ± 0.2 | 0.8 ± 0.14 | 0.84 |
| Fine motor | 0.91 ± 0.2 | 1.16 ± 0.1 | 0.02 |
| Language | 0.87 ± 0.2 | 0.79 ± 0.3 | 0.02 |
| Gross motor | 0.81 ± 0.1 | 0.69 ± 0.1 | 0.1 |
| Overall developmental quotient score | 0.86 ± 0.1 | 0.86 ± 0.1 | 0.1 |
| Domain-specific developmental gain Scores | |||
| Personal/social | 1 ± 1.7 | ||
| Fine motor | 0.5 ± 1.3 | ||
| Language | 0.006 ± 2.3 | ||
| Gross motor | 1.12 ± 0.7 | ||
| Overall developmental gain score | 0.73 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiperi, L.E.; Huţanu, A.; Tecar, C.; Muntean, I. Serum Markers of Brain Injury in Pediatric Patients with Congenital Heart Defects Undergoing Cardiac Surgery: Diagnostic and Prognostic Role. Clin. Pract. 2023, 13, 1253-1265. https://doi.org/10.3390/clinpract13050113
Chiperi LE, Huţanu A, Tecar C, Muntean I. Serum Markers of Brain Injury in Pediatric Patients with Congenital Heart Defects Undergoing Cardiac Surgery: Diagnostic and Prognostic Role. Clinics and Practice. 2023; 13(5):1253-1265. https://doi.org/10.3390/clinpract13050113
Chicago/Turabian StyleChiperi, Lacramioara Eliza, Adina Huţanu, Cristina Tecar, and Iolanda Muntean. 2023. "Serum Markers of Brain Injury in Pediatric Patients with Congenital Heart Defects Undergoing Cardiac Surgery: Diagnostic and Prognostic Role" Clinics and Practice 13, no. 5: 1253-1265. https://doi.org/10.3390/clinpract13050113
APA StyleChiperi, L. E., Huţanu, A., Tecar, C., & Muntean, I. (2023). Serum Markers of Brain Injury in Pediatric Patients with Congenital Heart Defects Undergoing Cardiac Surgery: Diagnostic and Prognostic Role. Clinics and Practice, 13(5), 1253-1265. https://doi.org/10.3390/clinpract13050113






