Clinical Characteristics, Treatment, and Short-Term Outcome in Patients with Heart Failure and Cancer
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
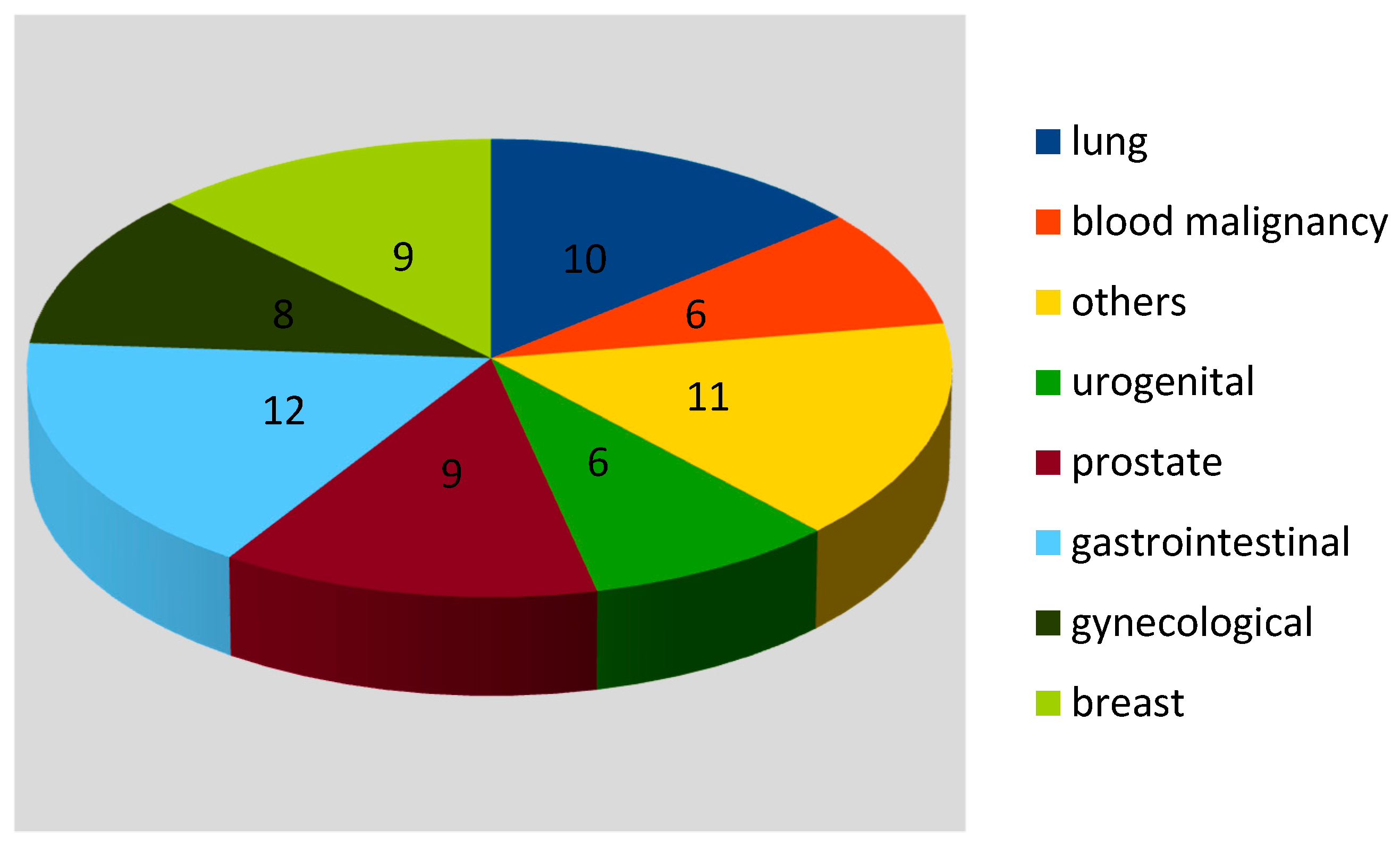
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coebergh, J.; Janssen-Heijnen, M.; Post, P.; Razenberg, P. Serious Co-morbidity among Unselected Cancer Patients Newly Diagnosed in the Southeastern Part of The Netherlands in 1993–1996. J. Clin. Epidemiol. 1999, 52, 1131–1136. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Oliveira, G.H. Prevalence of Preexisting Cardiovascular Disease in Patients with Different Types of Cancer. Mayo Clin. Proc. 2016, 91, 81–83. [Google Scholar] [CrossRef]
- Hasin, T.; Gerber, Y.; McNallan, S.M.; Weston, S.A.; Kushwaha, S.S.; Nelson, T.J.; Cerhan, J.R.; Roger, V.L. Patients with Heart Failure Have an Increased Risk of Incident Cancer. J. Am. Coll. Cardiol. 2013, 62, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Cuomo, A.; Pirozzi, F.; Attanasio, U.; Franco, R.; Elia, F.; De Rosa, E.; Russo, M.; Ghigo, A.; Ameri, P.; Tocchetti, C.G.; et al. Cancer Risk in the Heart Failure Population: Epidemiology, Mechanisms, and Clinical Implications. Curr. Oncol. Rep. 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Loosen, S.H.; Jahn, J.K.; Gänsbacher, J.; Luedde, T.; Kostev, K.; Luedde, M. Heart failure is associated with an increased incidence of cancer diagnoses. ESC Hear. Fail. 2021, 8, 3628–3633. [Google Scholar] [CrossRef]
- Cuomo, A.; Paudice, F.; D’Angelo, G.; Perrotta, G.; Carannante, A.; Attanasio, U.; Iengo, M.; Fiore, F.; Tocchetti, C.G.; Mercurio, V.; et al. New-Onset Cancer in the HF Population: Epidemiology, Pathophysiology, and Clinical Management. Curr. Hear. Fail. Rep. 2021, 18, 191–199. [Google Scholar] [CrossRef]
- Banke, A.B.S.; Schou, M.; Videbaek, L.; Møller, J.E.; Torp-Pedersen, C.; Gustafsson, F.; Dahl, J.S.; Køber, L.; Hildebrandt, P.R.; Gislason, G.H. Incidence of cancer in patients with chronic heart failure: A long-term follow-up study. Eur. J. Heart Fail. 2016, 18, 260–266. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. Task Force Members; ESC Committee for Practice Guidelines (CPG): 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 21, 2768–2801. [Google Scholar]
- Piotrowski, G.; Gawor, R.; Gawor, Z.; Szmit, S.; Kasprzak, J.D.; Miśkiewicz, Z.; Opolski, G.; Torbicki, A.; Krzakowski, M.; Filipiak, K.J.; et al. Polskie Kliniczne Forum Obrazowania Serca i Naczyń. Role of echocardiography in monitoring of cardiac toxicity of cancer pharmacotherapy. Expert consensus statement of the Polish Clinical Forum for Cardiovascular Imaging. Kardiol. Pol. 2014, 72, 558–575. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Canepa, M.; Anker, M.S.; Belenkov, Y.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; López-Fernández, T.; Lainscak, M.; Pudil, R.; et al. Cancer diagnosis in patients with heart failure: Epidemiology, clinical implications and gaps in knowledge. Eur. J. Heart Fail. 2018, 20, 879–887. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 8, 891–975. [Google Scholar]
- Driver, J.A.; Djoussé, L.; Logroscino, G.; Gaziano, J.M.; Kurth, T. Incidence of cardiovascular disease and cancer in advanced age: Prospective cohort study. BMJ 2008, 337, 2467–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.; Deswal, A. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population with Heart Failure and Preserved Versus Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef] [Green Version]
- Tuzovic, M.; Yang, E.H.; Packard, R.R.S.; Ganz, P.A.; Fonarow, G.C.; Ziaeian, B. National Outcomes in Hospitalized Patients with Cancer and Comorbid Heart Failure. J. Card. Fail. 2019, 25, 516–521. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Qadir, H.; Tai, F.; Croxford, R.; Austin, P.C.; Amir, E.; Calvillo-Argüelles, O.; Ross, H.; Lee, D.S.; Thavendiranathan, P. Characteristics and Outcomes of Women Developing Heart Failure after Early Stage Breast Cancer Chemotherapy: A Population-Based Matched Cohort Study. Circ. Hear. Fail. 2021, 14, e008110. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, T.; Cha, W.C.; Yoon, H.; Hwang, S.Y.; Shin, T.G.; Sim, M.S.; Jo, I.; Lee, S.; Park, H.; et al. Cardiac troponin I predicts clinical outcome of patients with cancer at emergency department. Clin. Cardiol. 2020, 43, 1585–1591. [Google Scholar] [CrossRef]
- Song, M.; Kim, T.; Kang, E.-J.; Park, J.E.; Park, S.H.; Cha, W.C.; Yoon, H.; Hwang, S.Y.; Shin, T.G.; Sim, M.S.; et al. Prognostic implication of elevated cardiac troponin I in patients visiting emergency department without diagnosis of coronary artery disease. Clin. Chem. Lab. Med. 2021, 59, 1107–1113. [Google Scholar] [CrossRef]
- Horwich, T.B.; Patel, J.; MacLellan, W.R.; Fonarow, G.C. Cardiac Troponin I Is Associated with Impaired Hemodynamics, Progressive Left Ventricular Dysfunction, and Increased Mortality Rates in Advanced Heart Failure. Circulation 2003, 108, 833–838. [Google Scholar] [CrossRef] [Green Version]
- Salata, C.; Ferreira-Machado, S.C.; De Andrade, C.B.; Mencalha, A.L.; De-Lacerda, C.A.M.; de Almeida, C.E. Apoptosis induction of cardiomyocytes and subsequent fibrosis after irradiation and neoadjuvant chemotherapy. Int. J. Radiat. Biol. 2014, 90, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Moslehi, J.; Zhang, Q.; Moore, K. Crosstalk between the Heart and Cancer. Circulation 2020, 142, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Peacock, W.F.; De Marco, T.; Fonarow, G.; Diercks, D.B.; Wynne, J.; Apple, F.S.; Wu, A.H. Cardiac Troponin and Outcome in Acute Heart Failure. N. Engl. J. Med. 2008, 358, 2117–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, W.T.; Fonarow, G.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B. Predictors of In-Hospital Mortality in Patients Hospitalized for Heart Failure: Insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J. Am. Coll. Cardiol. 2008, 52, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, S.W.; Daraban, N.; Abbasi, N.; Lei, X.; Durand, J.B.; Daher, I.N. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin. Cardiol. 2012, 35, 443–450. [Google Scholar] [CrossRef]
- Tini, G.; Bertero, E.; Signori, A.; Sormani, M.P.; Maack, C.; De Boer, R.A.; Canepa, M.; Ameri, P. Cancer Mortality in Trials of Heart Failure with Reduced Ejection Fraction: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.A.; Ashraf, A.; Singh, R.; Rahim, A.; Rostom, W.; Hussain, M.; Renner, I.; Collins, N.J. Incidence, time of occurrence and response to heart failure therapy in patients with anthracycline cardiotoxicity. Intern. Med. J. 2017, 47, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameter | Group A (n = 71 (%)) | Group B (n = 70 (%)) | p-Value |
|---|---|---|---|
| Age | 72.03 ± 12.82 | 71.37 ± 13.71 | 0.39 |
| Gender (male) | 33 (54.93%) | 39 (47.14%) | 0.36 |
| NYHA | 3.06 ± 0.91 | 3.47 ± 0.5 | <0.001 |
| Smoking | 16 (22.54%) | 12 (17.14%) | 0.42 |
| Comorbidities | |||
| Hypertension | 54 (76.06%) | 63 (90.00%) | 0.03 |
| Diabetes mellitus | 27 (38.03%) | 26 (37.14%) | 0.91 |
| Dyslipidaemia | 33 (46.48%) | 33 (47.14%) | 0.94 |
| Ischemic heart diseases/myocardial infarction in the past | 33 (46.48%) | 37 (52.86%) | 0.45 |
| Atrial fibrillation | 28 (39.44%) | 38 (54.29%) | 0.08 |
| Chronic kidney disease | 23 (32.39%) | 30 (42.86%) | 0.20 |
| HFpEF | 31 (43.66%) | 16 (22.86%) | 0.01 |
| In-hospital bleedings | 2 (2.82%) | 2 (2.86%) | 0.62 |
| In-hospital mortality | 6 (8.45%) | 2 (2.86%) | 0.28 |
| Duration of hospitalization (days) | 6.17 ± 4.16 | 5.92 ± 3.33 | 0.35 |
| Laboratory Parameters | |||
| Creatinine (mg/dL) | 1.25 ± 0.55 | 1.37 ± 0.71 | 0.13 |
| GFR (mL/min/m2) | 50.88 ± 12.47 | 49.38 ± 13.68 | 0.25 |
| K+ (mEq/L) | 4.42 ± 0.64 | 4.52 ± 0.70 | 0.19 |
| Na+ (mEq/L) | 139.24 ± 4.98 | 136.6 ± 15.34 | 0.08 |
| HgB (g/dL) | 12.51 ± 2.33 | 13.03 ± 2.05 | 0.08 |
| WBC (tys/L) | 11.08 ± 4.57 | 9.38 ± 3.69 | 0.01 |
| PLT (tys/L) | 250.79 ± 125.85 | 217 ± 68.23 | 0.03 |
| ALT (U/L) | 71.27 ± 270.24 | 81.39 ± 293.31 | 0.43 |
| AST (U/L) | 78.58 ± 235.74 | 125.74 ± 584.00 | 0.31 |
| INR | 1.81 ± 2.55 | 1.77 ± 1.40 | 0.46 |
| TnT (ng/mL) | 0.12 ± 0.23 | 0.05 ± 0.05 | 0.007 |
| Echocardiography | |||
| LVEF (%) | 43.08 ± 15.79 | 37.60 ± 15.33 | 0.02 |
| LAD (mm) | 45.70 ± 7.86 | 49.12 ± 7.04 | 0.006 |
| LAV index (mL/m2) | 58.86 ± 19.69 | 67.86 ± 24.49 | 0.04 |
| E/A | 1.32 ± 0.81 | 1.47 ± 0.93 | 0.25 |
| E/e’ | 15.95 ± 8.15 | 22.32 ± 31.77 | 0.07 |
| NT proBNP (ng/mL) | 8725.17 ± 7777.91 | 7244.95 ± 9570.28 | 0.31 |
| Medication | Group A (n = 71 (%)) | Group B (n = 70 (%)) | p-Value |
|---|---|---|---|
| ACE-I/ARA | 48 (67.61%) | 55(78.57%) | 0.35 |
| Beta-blockers | 53 (74.65%) | 62 (88.57%) | 0.03 |
| MRA | 35 (49.30%) | 50 (71.43%) | 0.01 |
| Diuretics (on discharge) | 57 (80.28%) | 63 (90.0%) | 0.11 |
| Ivabradine | 3 (4.23%) | 3 (4.29%) | 0.69 |
| Statin | 48 (67.61%) | 55 (78.57%) | 0.14 |
| Aetiogy of Heart Failure | Group A (n = 71 (%)) | Group B (n = 70 (%)) | p-Value |
|---|---|---|---|
| Coronary artery disease | 30 (42.25%) | 38 (54.29%) | 0.12 |
| Hypertension | 9 (12.68%) | 4 (5.71%) | 0.15 |
| Valvular heart disease | 10 (14.09%) | 8 (11.43%) | 0.16 |
| Cardiomyopathy | 11 (15.49%) | 12 (17.14%) | 0.41 |
| Tachyarrhythmias/bradyarrhythmias | 7 (9.86%) | 12 (17.14%) | 0.81 |
| Others | 4 (5.63%) | 3 (4.29%) | 0.21 |
| Triggering Factor | |||
| Uncontrolled blood pressure | 9 (12.68%) | 10 (14.298%) | 0.67 |
| Tachyarrhythmia/bradyarrhytmia | 27 (38.03%) | 20 (28.57%) | 0.15 |
| Infection | 20 (28.17%) | 20 (28.57%) | 0.96 |
| Kidney disease exacerbation | 2 (2.82%) | 3 (4.29%) | 0.99 |
| Nonadherence with medications | 9 (12.68%) | 7 (10%) | 0.25 |
| Others | 2 (2.82%) | 3 (4.29%) | 0.28 |
| Unknown | 4 (5.63%) | 9 (12%) | 0.09 |
| Parameter | Subgroup A1 (n = 6) | Subgroup A2 (n = 65) | p-Value |
|---|---|---|---|
| NYHA | 3.4 ± 0.2 | 3.0 ± 0.9 | 0.008 |
| HgB (g/dL) | 10.5 ± 2.5 | 12.7 ± 2.2 | 0.045 |
| GFR (mL/min/m2) | 34 ± 18 | 53 ± 11 | 0.008 |
| K+ (mEq/L) | 5.6 ± 0.9 | 4.3 ± 0.5 | <0.001 |
| AST (U/L) | 109 ± 124 | 74 ± 248 | 0.021 |
| Diabetes Mellitus type 2 | 5.0 (83%) | 26 (40%) | 0.014 |
| HFpEF | 5.0 (83%) | 26 (40%) | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, J.; Timler, M.; Kozłowski, R.; Stasiak, A.; Stasiak, J.; Bissinger, A.; Timler, D.; Timler, W.; Marczak, M.; Załuska, R.; et al. Clinical Characteristics, Treatment, and Short-Term Outcome in Patients with Heart Failure and Cancer. Clin. Pract. 2021, 11, 933-941. https://doi.org/10.3390/clinpract11040107
Piotrowski J, Timler M, Kozłowski R, Stasiak A, Stasiak J, Bissinger A, Timler D, Timler W, Marczak M, Załuska R, et al. Clinical Characteristics, Treatment, and Short-Term Outcome in Patients with Heart Failure and Cancer. Clinics and Practice. 2021; 11(4):933-941. https://doi.org/10.3390/clinpract11040107
Chicago/Turabian StylePiotrowski, Jędrzej, Małgorzata Timler, Remigiusz Kozłowski, Arkadiusz Stasiak, Joanna Stasiak, Andrzej Bissinger, Dariusz Timler, Wojciech Timler, Michał Marczak, Roman Załuska, and et al. 2021. "Clinical Characteristics, Treatment, and Short-Term Outcome in Patients with Heart Failure and Cancer" Clinics and Practice 11, no. 4: 933-941. https://doi.org/10.3390/clinpract11040107
APA StylePiotrowski, J., Timler, M., Kozłowski, R., Stasiak, A., Stasiak, J., Bissinger, A., Timler, D., Timler, W., Marczak, M., Załuska, R., & Piotrowski, G. (2021). Clinical Characteristics, Treatment, and Short-Term Outcome in Patients with Heart Failure and Cancer. Clinics and Practice, 11(4), 933-941. https://doi.org/10.3390/clinpract11040107







