Bridging the Gap between Ophthalmology and Emergency Medicine in Community-Based Emergency Departments (EDs): A Neuro-Ophthalmology Guide for ED Practitioners
Abstract
1. Introduction
2. Case 1
Could the Visual Loss Have Been Prevented? What Would Have Been an Optimal Work Up in the Patient?
3. Case 2
What Would Have Been an Optimal Work Up in the Patient? Could the Visual Loss Have Been Prevented?
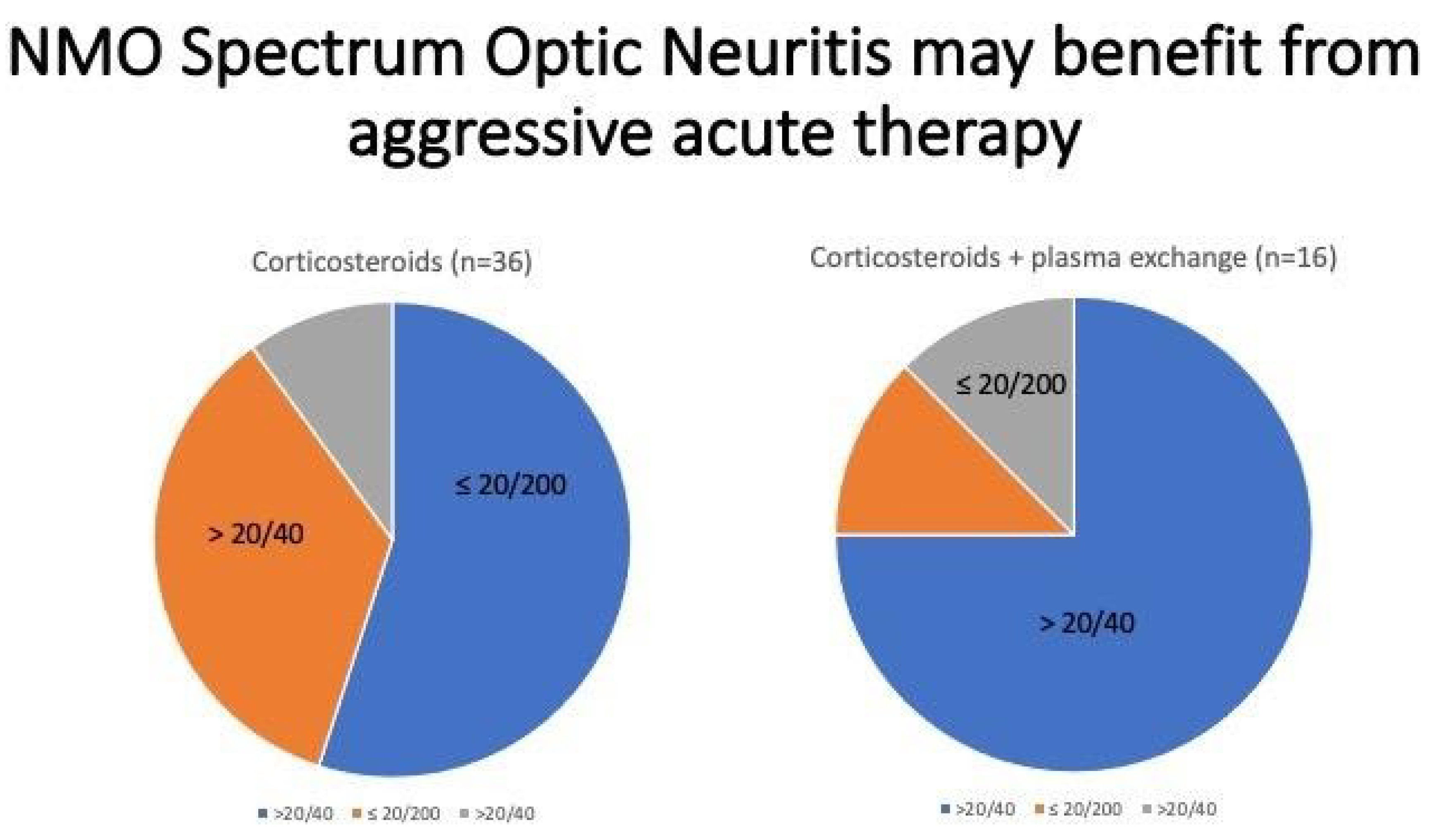
4. Case 3
What Would Have Been an Optimal Work Up in the Patient? Could the Visual Loss Have Been Prevented?
5. Case 4
What Would Have Been an Optimal Work Up in the Patient? Could This Have Been Prevented?
6. Case 5
What Would Have Been an Optimal Work Up in the Patient? Could Visual Loss Have Been Prevented?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Albert, D.M.; Bartley, G.B. A Proposal to Improve Ophthalmic Education in Medical Schools. Ophthalmology 2014, 121, 1157–1159. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Knoch, D.; Waxman, E. The State of Ophthalmology Medical Student Education in the United States and Canada, 2012 through 2013. Ophthalmology 2014, 121, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, L.R.; Tsao, J.W.; Levine, S.R.; Swain-Eng, R.J.; Adams, R.J.; Demaerschalk, B.M.; Hess, D.C.; Moro, E.; Schwamm, L.H.; Steffensen, S.; et al. Teleneurology applications: Report of the Telemedicine Work Group of the American Academy of Neurology. Neurology 2013, 80, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Mehta, S. Acute Vision Loss. Prim. Care Clin. Off. Pract. 2015, 42, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Borooah, S.; Dhillon, A.; Dhillon, B. Gradual loss of vision in adults. BMJ 2015, 350, h2093. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Smit, E.; O’Sullivan, E.; Mackey, D.A.; Hewitt, A.W. Giant cell arteritis: Ophthalmic manifestations of a systemic disease. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 2291–2306. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, K.; Jacobsson, L.; Mohammad, A.J.; Nilsson, J.-Å.; Warrington, K.; Matteson, E.L.; Turesson, C. The effect of clinical features and glucocorticoids on biopsy findings in giant cell arteritis. BMC Musculoskelet. Disord. 2016, 17, 363. [Google Scholar] [CrossRef] [PubMed]
- Sammel, A.M.; Fraser, C. Update on giant cell arteritis. Curr. Opin. Ophthalmol. 2018, 29, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Ninan, J.; Hissaria, P. Diagnosis and management of giant cell arteritis: Major review. Clin. Exp. Ophthalmol. 2021, 49, 169–185. [Google Scholar] [CrossRef]
- Oliveira, F.; Butendieck, R.R.; Ginsburg, W.W.; Parikh, K.; Abril, A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin. Exp. Rheumatol. 2014, 32 (Suppl. S82), S76–S78. [Google Scholar] [PubMed]
- Parikh, M.; Miller, N.R.; Lee, A.G.; Savino, P.J.; Vacarezza, M.N.; Cornblath, W.; Eggenberger, E.; Antonio-Santos, A.; Golnik, K.; Kardon, R.; et al. Prevalence of a Normal C-Reactive Protein with an Elevated Erythrocyte Sedimentation Rate in Biopsy-Proven Giant Cell Arteritis. Ophthalmology 2006, 113, 1842–1845. [Google Scholar] [CrossRef]
- Weis, E.; Waite, C.; Roelofs, K.A. A Predictive Model for Temporal Artery Biopsy in the Setting of Suspected Giant Cell Arteritis: A Validation Study. Ophthalmic Plast. Reconstr. Surg. 2021, 37, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Maz, M.; Chung, S.A.; Abril, A.; Langford, C.A.; Gorelik, M.; Guyatt, G.; Mustafa, R.A. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of giant cell arteritis and Takayasu arteritis. Arthritis Care Res. 2021, 73, 1071–1087. [Google Scholar] [CrossRef]
- Avisar, I.; Gaton, D.D.; Dania, H.; Stiebel-Kalish, H. The Prevalence of Polycystic Ovary Syndrome in Women with Idiopathic Intracranial Hypertension. Science 2012, 2012, 708042. [Google Scholar] [CrossRef]
- Biousse, V. Idiopathic intracranial hypertension: Diagnosis, monitoring and treatment. Rev. Neurol. 2012, 168, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Fridley, J.; Foroozan, R.; Sherman, V.; Brandt, M.L.; Yoshor, D. Bariatric surgery for the treatment of idiopathic intracranial hypertension. J. Neurosurg. 2011, 114, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Incidence of Idiopathic Intracranial Hypertension Parallels Rising Rate of Obesity. Available online: www.mayoclinic.org/medical-professionals/ophthalmology/news/incidence-of-idiopathic-intracranial-hypertension-parallels-rising-rate-of-obesity/MAC-20430150 (accessed on 22 July 2021).
- Jensen, R.H.; Radojicic, A.; Yri, H. The diagnosis and management of idiopathic intracranial hypertension and the associated headache. Ther. Adv. Neurol. Disord. 2016, 9, 317–326. [Google Scholar] [CrossRef]
- Lee, A.G.; Wall, M. Idiopathic Intracranial Hypertension (Pseduotumor Cerebri): Epidemiology and Pathogenesis. Available online: www.uptodate.com/contents/idiopathic-intracranial-hypertension-pseudotumor-cerebri-epidemiology-and-pathogenesis (accessed on 13 August 2021).
- Bruce, B.B.; Kedar, S.; Van Stavern, G.P.; Monaghan, D.; Acierno, M.D.; Braswell, R.A.; Preechawat, P.; Corbett, J.J.; Newman, N.J.; Biousse, V. Idiopathic intracranial hypertension in men. Neurology 2009, 72, 304–309. [Google Scholar] [CrossRef]
- Panikkath, R.; Karukote, A.; Ruthirago, D.; Panikkath, D.; Julayanont, P. Idiopathic intracranial hypertension: Ongoing clinical challenges and future prospects. J. Pain Res. 2016, 9, 87–99. [Google Scholar] [CrossRef]
- Marmura, M.J.; Silberstein, S.D.; Schwedt, T.J. The Acute Treatment of Migraine in Adults: The American Headache Society Evidence Assessment of Migraine Pharmacotherapies. Headache J. Head Face Pain 2015, 55, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Wall, M. The Importance of Visual Field Testing in Idiopathic Intracranial Hypertension. Contin. Lifelong Learn. Neurol. 2014, 20, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Kanagalingam, S.; Subramanian, P.S. Cerebral venous sinus stenting for pseudotumor cerebri: A review. Saudi J. Ophthalmol. 2015, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Sexton, D. Lumbar Puncture Technique, Indications, Contraindications and Complications in Adults. Available online: www.uptodate.com/contents/lumbar-puncture-technique-indications-contraindications-and-complications-in-adults (accessed on 12 September 2021).
- Palay, D.A.; Krachmer, J.H. Ophthalmology for the Primary Care Physician; Elsevier Mosby: St. Louis, MO, USA, 1997. [Google Scholar]
- Mukherjee, N.; El-Dairi, M.A.; Bhatti, M.T. Optic Nerve Sheath Fenestration—Indications and Techniques. US Ophthalmic Rev. 2013, 6, 125. [Google Scholar] [CrossRef]
- Adesina, O.-O.O.; McNally, J.S.; Salzman, K.L.; Katz, B.J.; Warner, J.E.A.; McFadden, M.; Digre, K.B. Diffusion-Weighted Imaging and Post-contrast Enhancement in Differentiating Optic Neuritis and Non-arteritic Anterior Optic Neuropathy. Neuro Ophthalmol. 2017, 42, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Pau, D.; Al-Zubidi, N.; Yalamanchili, S.; Plant, G.T.; Lee, A.G. Optic neuritis. Eye 2011, 25, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.D.; Lapidus, S.; Stefani-Hunyady, D.; Levy, M. Patient attitudes towards NMOSD diagnosis and treatment: Final survey results. In Proceedings of the American Academy of Neurology, Virtual, 17–22 April 2021. [Google Scholar]
- Cree, B.; Bennett, J.; Weinshenker, B.; Wingerchuk, D.; Paul, F.; Kim, H.J.; Katz, E. Long term efficacy outcomes with inebilizumab treatment in NMOSD: The N-MOmentum trial. In Proceedings of the American Academy of Neurology, Virtual, 17–22 April 2021. [Google Scholar]
- Kim, H.J.; Smith, M.; Katz, E.; Rees, W.; Cree, B. Inebilizumab treatment reduces the occurrence of pain in NMOSD patients. In Proceedings of the American Academy of Neurology, Virtual, 17–22 April 2021. [Google Scholar]
- Tullman, M.; Ratchford, J.; She, D.; Katz, E.; Cree, B. Evaluation of infusion reactions and infusion times in the N-MOmentum study of inebilizumab for NMOSD. In Proceedings of the American Academy of Neurology, Virtual, 17–22 April 2021. [Google Scholar]
- Li, Y.; Wang, B.; She, D.; Mitchel, B.; Criste, R.; Cimbora, D.; Rees, W. Pharmacodynamic modeling and exposure-response assessment of inebilizumab in subjects with neuromyelitis optica spectrum disorder. In Proceedings of the American Academy of Neurology, Virtual, 17–22 April 2021. [Google Scholar]
- Iliescu, D.A.; Timaru, C.M.; Alexe, N.; Gosav, E.; De Simone, A.; Batras, M.; Stefan, C. Management of diplopia. Rom. J. Ophthalmol. 2017, 61, 166–170. [Google Scholar] [CrossRef]
- Rucker, J.C.; Tomsak, R.L. Binocular diplopia. A practical approach. Neurologist 2005, 11, 98–110. [Google Scholar] [CrossRef] [PubMed]
- O’Colmain, U.; Gilmour, C.; MacEwen, C.J. Acute-onset diplopia. Acta Ophthalmol. 2013, 92, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Dinkin, M. Diagnostic Approach to Diplopia. Contin. Lifelong Learn. Neurol. 2014, 20, 942–965. [Google Scholar] [CrossRef]
- Morillon, P.; Bremner, F. Trochlear nerve palsy. Br. J. Hosp. Med. 2017, 78, C38–C40. [Google Scholar] [CrossRef]
- Elder, C.; Hainline, C.; Galetta, S.L.; Balcer, L.J.; Rucker, J.C. Isolated Abducens Nerve Palsy: Update on Evaluation and Diagnosis. Curr. Neurol. Neurosci. Rep. 2016, 16, 69. [Google Scholar] [CrossRef]
- Patel, A.; Yang, H.; Douglas, R.S. A New Era in the Treatment of Thyroid Eye Disease. Am. J. Ophthalmol. 2019, 208, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Siakallis, L.C.; Uddin, J.M.; Miszkiel, K.A. Imaging Investigation of Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2018, 34, S41–S51. [Google Scholar] [CrossRef]
- Strianese, D. Efficacy and Safety of Immunosuppressive Agents for Thyroid Eye Disease. Ophthalmic Plast. Reconstr. Surg. 2018, 34, S56–S59. [Google Scholar] [CrossRef]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235. [Google Scholar] [CrossRef]
- Farrugia, M.E.; Goodfellow, J.A. A Practical Approach to Managing Patients With Myasthenia Gravis—Opinions and a Review of the Literature. Front. Neurol. 2020, 11, 604. [Google Scholar] [CrossRef]
- Narayanaswami, P.; Sanders, D.B.; Wolfe, G.; Benatar, M.; Cea, G.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.L.; Massey, J.; et al. International Consensus Guidance for Management of Myasthenia Gravis. Neurology 2021, 96, 114–122. [Google Scholar] [CrossRef]
- Patil-Chhablani, P.; Venkatramani, D.V.; Gandhi, R.A.; Nair, A.G. Ocular myasthenia gravis: A review. Indian J. Ophthalmol. 2014, 62, 985–991. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Park, Y.; Chung, K.J. Considerations for the Management of Medial Orbital Wall Blowout Fracture. Arch. Plast. Surg. 2016, 43, 229–236. [Google Scholar] [CrossRef]
- Mansour, T.N.; Rudolph, M.; Brown, D.; Mansour, N.; Taheri, M.R. Orbital blowout fractures: A novel CT measurement that can predict the likelihood of surgical management. Am. J. Emerg. Med. 2017, 35, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-H.; Yu, J.-H.; Wang, Y.-H.; Wang, A.-A.; Liao, H.-F. Analysis of the effect of repair materials for orbital blowout fracture on complications. Int. J. Ophthalmol. 2019, 12, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- Baruah, M.P.; Ranabir, S. Pituitary apoplexy. Indian J. Endocrinol. Metab. 2011, 15, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Bujawansa, S.; Thondam, S.K.; Steele, C.; Cuthbertson, D.; Gilkes, C.E.; Noonan, C.; Bleaney, C.W.; Macfarlane, I.A.; Javadpour, M.; Daousi, C. Presentation, management and outcomes in acute pituitary apoplexy: A large single-centre experience from the United Kingdom. Clin. Endocrinol. 2013, 80, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Capatina, C.; Inder, W.; Karavitaki, N.; Wass, J.A.H. Management Of Endocrine Disease: Pituitary tumour apoplexy. Eur. J. Endocrinol. 2015, 172, R179–R190. [Google Scholar] [CrossRef]
- Chanson, P.; Lepeintre, J.F.; Ducreux, D. Management of pituitary apoplexy. Expert Opin. Pharmacother. 2004, 5, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Doglietto, F.; Costi, E.; Villaret, A.B.; Mardighian, D.; Fontanella, M.; Giustina, A. New oral anticoagulants and pituitary apoplexy. Pituitary 2014, 19, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, P.P.; Crawford, J.R.; Khanna, P.; Malicki, D.M.; Ciacci, J.D.; Levy, M.L. Pituitary Tumor Apoplexy in Adolescents. World Neurosurg. 2015, 83, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Chang, C.N.; Pai, P.C.; Wei, K.C.; Jung, S.M.; Chen, N.Y.; Chuang, C.C. Clinical features and surgical outcome of clinical and subclinical pituitary apoplexy. J. Clin. Neurosci. 2010, 17, 694–699. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Vanderpump, M.; Baldeweg, S.; Drake, W.; Reddy, N.; Lanyon, M.; Markey, A.; Plant, G.; Powell, M.; Sinha, S.; et al. UK guidelines for the management of pituitary apoplexy. Clin. Endocrinol. 2011, 74, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.B.A.; França, M.M.; Hirosawa, R.M.; Marivo, M.; Zanini, M.A.; Nunes, V.S. Conservative management of pituitary tumor apoplexy. Arq. Bras. Endocrinol. Metabol. 2011, 55, 345–348. [Google Scholar] [CrossRef][Green Version]
- Shimon, I. Clinical Features of Pituitary Apoplexy. Pituit. Apoplexy 2014, 2013, 49–54. [Google Scholar] [CrossRef]
- Singh, T.D.; Valizadeh, N.; Meyer, F.B.; Atkinson, J.L.D.; Erickson, D.; Rabinstein, A.A. Management and outcomes of pituitary apoplexy. J. Neurosurg. 2015, 122, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Badakere, A.; Chhablani, P.P. Orbital Apex Syndrome: A Review. Eye Brain 2019, 11, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Lee, S.; Gupta, N.; Kumar, Y.; Mangla, M.; Hooda, K.; Li, S.; Mangla, R. Orbital apex disorders: Imaging findings and management. Neuroradiol. J. 2018, 31, 104–125. [Google Scholar] [CrossRef]
- Pfeiffer, M.L.; Merritt, H.A.; Bailey, L.A.; Richani, K.; Phillips, M.E. Orbital apex syndrome from bacterial sinusitis without orbital cellulitis. Am. J. Ophthalmol. Case Rep. 2018, 10, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Rattan, V. Traumatic superior orbital fissure syndrome: Review of literature and report of three cases. Natl. J. Maxillofac. Surg. 2012, 3, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Saini, L.; Chakrabarty, B.; Kumar, A. Orbital apex syndrome: A clinico-anatomical diagnosis. J. Pediatr. Neurosci. 2020, 15, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tong, Y.; Jiang, F.; Wu, W. Successful delayed treatment of the traumatic orbital apex syndrome by nasal endoscopic decompression surgery. Indian J. Ophthalmol. 2015, 63, 728–730. [Google Scholar] [CrossRef]
- Warburton, R.; Brookes, C.; Golden, B.; Turvey, T. Orbital apex disorders: A case series. Int. J. Oral Maxillofac. Surg. 2016, 45, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Moy, W.L. Orbital Apex Syndrome Resulting from Mixed Bacterial Sphenoid Sinusitis. Eur. J. Case Rep. Intern. Med. 2018, 5, 000905. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Oh, S.Y. The clinical features and outcomes of Tolosa-Hunt syndrome. BMC Ophthalmol. 2021, 21, 237. [Google Scholar] [CrossRef]
- Tolosa, E. Periarteritic Lesions of the Carotid Siphon with the Clinical Features of a Carotid Infraclinoidal Aneurysm. J. Neurol. Neurosurg. Psychiatry 1954, 17, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.E.; Meagher, J.N.; LeFever, H.E.; Zeman, W. Painful ophthalmoplegia: Its relation to indolent inflammation of the cavernous sinus. Neurology 1961, 11, 56. [Google Scholar] [CrossRef]
- Colnaghi, S.; Versino, M.; Marchioni, E.; Pichiecchio, A.; Bastianello, S.; Cosi, V.; Nappi, G. ICHD-II Diagnostic Criteria for Tolosa—Hunt Syndrome in Idiopathic Inflammatory Syndromes of the Orbit and/or the Cavernous Sinus. Cephalalgia 2008, 28, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Kline, L.B. Nosological Entities?: The Tolosa-Hunt syndrome. J. Neurol. Neurosurg. Psychiatry 2001, 71, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Chen, M.-C.; Ho, Y.-H.; Chong, P.-N. A rare case of septic cavernous sinus thrombosis as a complication of sphenoid sinusitis. Tzu Chi Med. J. 2019, 31, 63–65. [Google Scholar] [CrossRef]
- Dolapsakis, C.; Kranidioti, E.; Katsila, S.; Samarkos, M. Cavernous sinus thrombosis due to ipsilateral sphenoid sinusitis. BMJ Case Rep. 2019, 12, e227302. [Google Scholar] [CrossRef]
- Eltayeb, A.S.; Karrar, M.A.; Elbeshir, E.I. Orbital Subperiosteal Abscess Associated with Mandibular Wisdom Tooth Infection: A Case Report. J. Maxillofac. Oral Surg. 2019, 18, 30–33. [Google Scholar] [CrossRef]
- Kasha, S.; Bandari, G. Bilateral posterior fracture-dislocation of shoulder following seizures secondary to cavernous sinus venous thrombosis—A rare association. J. Orthop. Case Rep. 2018, 8, 49–52. [Google Scholar]
- Matthew, T.J.H.; Hussein, A. Atypical Cavernous Sinus Thrombosis: A Diagnosis Challenge and Dilemma. Cureus 2018, 10, e3685. [Google Scholar] [CrossRef]
- Asai, K.; Hasuo, K.; Hara, T.; Miyagishima, T.; Terano, N. Traumatic Persistent Trigeminal Artery - Cavernous Sinus Fistula Treated by Transcatheter Arterial Embolization. Interv. Neuroradiol. 2010, 16, 93–96. [Google Scholar] [CrossRef]
- Goto, K.; Hieshima, G.B.; Higashida, R.T.; Halbach, V.V.; Bentson, J.R.; Mehringer, C.M.; Pribram, H.F. Treatment of direct carotid cavernous sinus fistulae. Various therapeutic approaches and results in 148 cases. Acta Radiol. Suppl. 1986, 369, 576–579. [Google Scholar]
- Lau, L.-I.; Wu, H.-M.; Wang, A.-G.; Yen, M.-Y.; Hsu, W.-M. Paradoxical worsening with superior ophthalmic vein thrombosis after gamma knife radiosurgery for dural arteriovenous fistula of cavernous sinus: A case report suggesting the mechanism of the phenomenon. Eye 2006, 20, 1426–1428. [Google Scholar] [CrossRef][Green Version]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- USC Roski Eye Institute. Ask the Expert: How COVID-19 Affects the Eyes. Available online: https://eye.keckmedicine.org/how-COVID-19-affects-the-eyes/ (accessed on 14 August 2021).
- Eriksen, A.Z.; Møller, R.; Makovoz, B.; Uhl, S.A.; Tenoever, B.R.; Blenkinsop, T.A. SARS-CoV-2 infects human adult donor eyes and hESC-derived ocular epithelium. Cell Stem Cell 2021, 28, 1205–1220. [Google Scholar] [CrossRef]
- Makhija, S.C.; Walinjkar, J.A.; Sharma, H.R.; Morekar, S.R.; Natarajan, S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J. Ophthalmol. 2020, 68, 2572–2574. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, O.M.; Volod, O.; Bose, S.; Wang, Y.; Biousse, V.; Lyden, P.D. Acute ophthalmic artery occlusion in a COVID-19 patient on apixaban. J. Stroke Cerebrovasc. Dis. 2020, 29, 104982. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sacchetti, D.; Finn, A.; Dapaah-Afriyie, K. Acute Vision Loss in a Patient with COVID-19. Rhode Isl. Med. J. 2020, 103, 37–38. [Google Scholar]
- Cyr, D.G.; Vicidomini, C.M.; Siu, N.Y.; Elmann, S.E. Severe Bilateral Vision Loss in 2 Patients With Coronavirus Disease 2019. J. Neuro Ophthalmol. 2020, 40, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, C.M.; Mull, M.; Schwarz, M.; Koch, B.; Biniek, R.; Schläfer, J.; Milkereit, E.; Willmes, K.; Schiefer, J. Do Normal D-dimer Levels Reliably Exclude Cerebral Sinus Thrombosis? Stroke 2004, 35, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Fogarty, H.; Dyer, A.; Martin-Loeches, I.; Bannan, C.; Nadarajan, P.; Bergin, C.; Farrelly, C.O.; Conlon, N.; Bourke, N.M.; et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J. Thromb. Haemost. 2021, 19, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Biousse, V.; Nahab, F.; Newman, N.J. Management of Acute Retinal Ischemia. Ophthalmology 2018, 125, 1597–1607. [Google Scholar] [CrossRef]
| Symptom | Treatment Dosage |
|---|---|
| Eye pain | Prednisone 60 mg PO each am Eye exam within 24 h * |
| Transient visual obscurations (TVOs) | Prednisone 60 mg PO each am Eye exam within 24 h * |
| Blurred vision or mild visual acuity (VA) change | Prednisone 60 mg PO each am Eye exam within 24 h * |
| Devastating visual loss (anterior ischemic optic neuropathy, posterior ischemic optic neuropathy, cilioretinal artery occlusion, central retinal artery occlusion) resulting in VA < 20/400, no light perception not uncommon. Occipital lobe infarctions have also been described. | Admit for IV corticosteroids—start first dose in the ED. Ophthalmology consult as inpatient. |
| Double vision due to cranial nerve (CN) III, IV, VI involvement | Discharge on prednisone 60 mg PO each am Ophthalmology exam within 24 h * |
| Lab Value | Sensitivity |
|---|---|
| ESR | 76–86% (Parikh et al., 2006) [11] |
| CRP | 97.5% (Parikh et al., 2006) [11] |
| ESR and CRP | 99% (Parikh et al., 2006) [11] |
| Platelets | 71.2% (Franzco et al., 2021) [12] |
| Acute serum amyloid A (A-SAA) | 97% (Franzco et al., 2021) [12] |
| Normocytic normochromic anemia | 20–50% (Franzco et al., 2021) [12] |
| PLT > 634 k, ESR > 90, CRP > 115 | All had positive TA BX (Weis et al., 2021) [13] |
| PLT < 224, CRP < 2, ESR < 9 | All had negative TA BX (Weis et al., 2021) [13] |
| Symptoms | Diagnostic Work-Up |
|---|---|
| Headache often worse in the am or wakes patient from sleep | Eye Vitals: vision, intraocular pressure (IOP), red desaturation, amsler grid, confrontation visual field, motility, and fundus photo (optic nerve and central retina) |
| Transient graying out or blacking out of vision | MRI/MRV |
| Double vision (CN VI dysfunction) | Followed by lumbar puncture (LP) in lateral decubitus position for opening pressure, cell, protein, and glucose |
| Visual field defects | If central visual acuity is affected, especially if papilledema looks ischemic (cotton wool spots), admit |
| Decreased vision | - |
| Retina Symptoms and Signs | Optic Nerve Symptoms and Signs |
|---|---|
| Retina Classic Symptoms: Flashing lights Floaters Shadow that progresses from the periphery | Optic Nerve Classic Symptoms: Graying out or blacking out Eye pain or ache Eye pain worse on eye movement Woke up with visual loss |
| Amsler grid: wavy lines Red color is normal Ultrasound: vitreous opacification can be blood or infection. Retinal detachment may be visible. (need to have gain turned all of the way up) | Amsler grid: Absent lines or regions Red desaturation Ultrasound: can detect severe disc edema |
| Fundus photo of Retina can show: Vitreous hemorrhage Diabetic retinopathy Macular degeneration with hemorrhage Macular fluid (easier to see on OCT) Central retinal artery occlusion Branch retinal artery occlusion Central retinal vein occlusion | Fundus photo of optic nerve can be:
|
| Age | Unilateral or Bilateral Visual Loss | Unilateral Visual Loss | Bilateral Visual Loss |
|---|---|---|---|
| AGE > 60 | - | Non-arteritic ischemic optic neuropathy Giance cell arteritisCompressive (orbital mass) | Compressive (parasellar mass) Toxic (Alcohol, ethambutol) Infectious (TB, syphilis, Lyme) |
| AGE < 60 | Optic Neuritis Neruomyelitis Optica Spectrum Disorder (NMOSD) Lupus Saracoidosis | - | Compressive (parasellar mass) Toxic (alchol, ethambutol) Infectious (TB, syphilis, lyme) |
| Unilateral Visual Loss Testing in ED: | Bilateral Visual Loss Testing in ED | ||
| Eye vitals, fundus photo CBC, ESR, CRP, RPR, Quantiferon MRI of brain and orbit with/without GAD | Eye vitals including fundus photo ANA, ACE, RPR, Quantiferon Anti-aquaporin-4 antibody (AQP4) MRI of brain and orbit with/without GAD Lumbar puncture | ||
| Presenting Symptoms | Signs |
|---|---|
| MS-Related Optic Neuritis | NMO-Related Optic Neuritis |
| Unilateral visual loss better than 20/100 improves in 6–8 weeks | Unilateral or bilateral visual loss worse than 20/100 and often permanent |
| No biomarker | anti-AQP4 biomarker |
| Can be retrobulbar or with disc edema Eye pain worse on eye movement More common in women than men | Can be retrobulbar or with disc edema Eye pain worse on eye movement More common in women than men |
| Short regions of enhancement on MRI orbits | Long regions of optic nerve enhancement that extend to the chiasm and may be bilateral. Longitudinally extensive transverse myelitis (LETM) that spans 3 or more vertebral segments |
| Periventricular plaques on MRI brain | Subcortical and deep white matter lesions on T2-weighted or fluid-attenuated inversion recovery sequences. Diencephalic lesions around the third ventricle, thalamus, hypothalamus, and midbrain. Dorsal brainstem adjacent to the fourth ventricle also reported that it causes intractable hiccups, nausea, and vomiting. Nystagmus, dysarthria, dysphagia, ataxia and ophthalmoplegia (multiple cranial nerves causing dysmotility) can also occur. Can also present with antidiuretic hormone secretion, narcolepsy, hypothermia, hypotension, hypersomnia, obesity, hypothyroidism, hyperprolactinemia, amenorrhea, galactorrhea, and behavioral changes. |
| LP: leukocytosis | LP: oligoclonal bands |
| Cranial Nerve Palsy | Diagnostic Work-Up |
|---|---|
| Complete CN III with pupil involvement [35,36,37,38] Eyelid drooping (ptosis), eye position is down and out. Pupil is larger than contralateral pupil | Aneurysm until proven otherwise. If Computed tomography angiography (CTA) is normal, the standard of care is to transfer to hospital with interventional radiology to perform angiogram |
| Incomplete CN III [35,36,37,38] Limitation of up gaze with ptosis and/or limitation of downgaze and contralateral gaze (adduction). | CTA and close follow-up for progression HBA1c CBC, ESR, CRP if patient > 60 years old |
| CN IV [35,36,37,38,39,40] Vertical deviation worse with tilt of head on side of higher eye | MRI with and without gadolinium HBA1c CBC, ESR, CRP if patient > 60 years old |
| CN VI [35,38,40] Limitation of lateral gaze | MRI with and without Gad HBA1c LP for opening pressure, cells, protein, glucose |
| Thyroid eye disease [41,42,43] Vertical deviation in primary gaze Limitation of up gaze Limitation of lateral gaze Esotropia in primary gaze Proptosis (bulging of eyes) Eyelid retraction (appearance of stare) | CT scan of orbits (include corneal views) TSH, free T3 and T4, Thyroid stimulating immunoglobulin |
| Myasthenia Gravis [43,44,45,46,47] Double vision worse at end of day or with reading Any deviation pattern possible Associated with drooping of eyelid (ptosis) | CT scan of chest looking for thymoma Acetylcholine binding, blocking and modulating antibodies and anti-musk |
| Orbital Fracture [48,49,50] Recent trauma to orbit with limitation of up gaze (or less commonly lateral and downgaze) | CT scan of orbit to look for blow out fracture |
| Pituitary apoplexy [51,52,53,54,55,56,57,58,59,60,61] Any or all cranial nerves can be involved | Non-contrast CT will typically identify the parasellar hemorrhage and necrosis. IV corticosteroids should be initiated and urgent transfer to a hospital with Neurosurgery. |
| Orbital Apex Syndrome [62,63,64,65,66,67,68,69] Any or all cranial nerves can be involved Orbital pain and visual field/visual loss also typically present | MRI of orbits/brain with and without GAD Differential includes mucor, aspergillosis, non-specific inflammatory process, sarcoidosis, and lymphoma. ENT and ophthalmology consultation. Admit for IV corticosteroids and anti-fungal medications. |
| Tolosa Hunt [70,71,72,73,74] Any or all cranial nerves can be involved Orbital pain characteristic | MRI of brain with and without Gad To detect enhancement of cavernous sinus Differential includes mucor, aspergillosis, non-specific inflammatory process, sarcoidosis, lymphoma, and cavernous sinus thrombosis. Admit for IV corticosteroids. |
| Cavernous sinus fistula/Sinus Thrombosis [75,76,77,78,79,80,81,82] Any or all cranial nerves can be involved Conjunctival injection and chemosis typical Proptosis (eye bulging may be present) Tinnitus (whooshing in the ears) | CT/CTA will show superior ophthalmic vein enlargement, extraocular muscle swelling and convexity to the normally concave wall of the cavernous sinus. Initiate transfer to hospital with interventional |
| Ocular Manifestations of COVID-19 |
|---|
| Conjunctivitis (hemorrhagic) |
| Scleritis |
| Retinal infarcts |
| Orbital infiltration |
| Central retinal vein occlusion; branch retinal vein occlusion |
| Central retinal artery occlusion; branch retinal artery occlusion |
| Anterior visual pathway strokes resulting in visual loss |
| Posterior visual pathway strokes resulting in visual loss |
| Cerebral venous thrombosis with papilledema |
| Cavernous sinus thrombosis with diplopia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, K.; Ocran, C.; Monterastelli, A.; Sadun, A.A.; Cockerham, K.P. Bridging the Gap between Ophthalmology and Emergency Medicine in Community-Based Emergency Departments (EDs): A Neuro-Ophthalmology Guide for ED Practitioners. Clin. Pract. 2021, 11, 919-932. https://doi.org/10.3390/clinpract11040106
Thomas K, Ocran C, Monterastelli A, Sadun AA, Cockerham KP. Bridging the Gap between Ophthalmology and Emergency Medicine in Community-Based Emergency Departments (EDs): A Neuro-Ophthalmology Guide for ED Practitioners. Clinics and Practice. 2021; 11(4):919-932. https://doi.org/10.3390/clinpract11040106
Chicago/Turabian StyleThomas, Kristina, Cindy Ocran, Anna Monterastelli, Alfredo A. Sadun, and Kimberly P. Cockerham. 2021. "Bridging the Gap between Ophthalmology and Emergency Medicine in Community-Based Emergency Departments (EDs): A Neuro-Ophthalmology Guide for ED Practitioners" Clinics and Practice 11, no. 4: 919-932. https://doi.org/10.3390/clinpract11040106
APA StyleThomas, K., Ocran, C., Monterastelli, A., Sadun, A. A., & Cockerham, K. P. (2021). Bridging the Gap between Ophthalmology and Emergency Medicine in Community-Based Emergency Departments (EDs): A Neuro-Ophthalmology Guide for ED Practitioners. Clinics and Practice, 11(4), 919-932. https://doi.org/10.3390/clinpract11040106





