Diagnostic and Therapeutic Challenges of Cerebral Venous Thrombosis in SARS-CoV-2 Infection: A Case Report and Review of Literature
Abstract
:1. Introduction
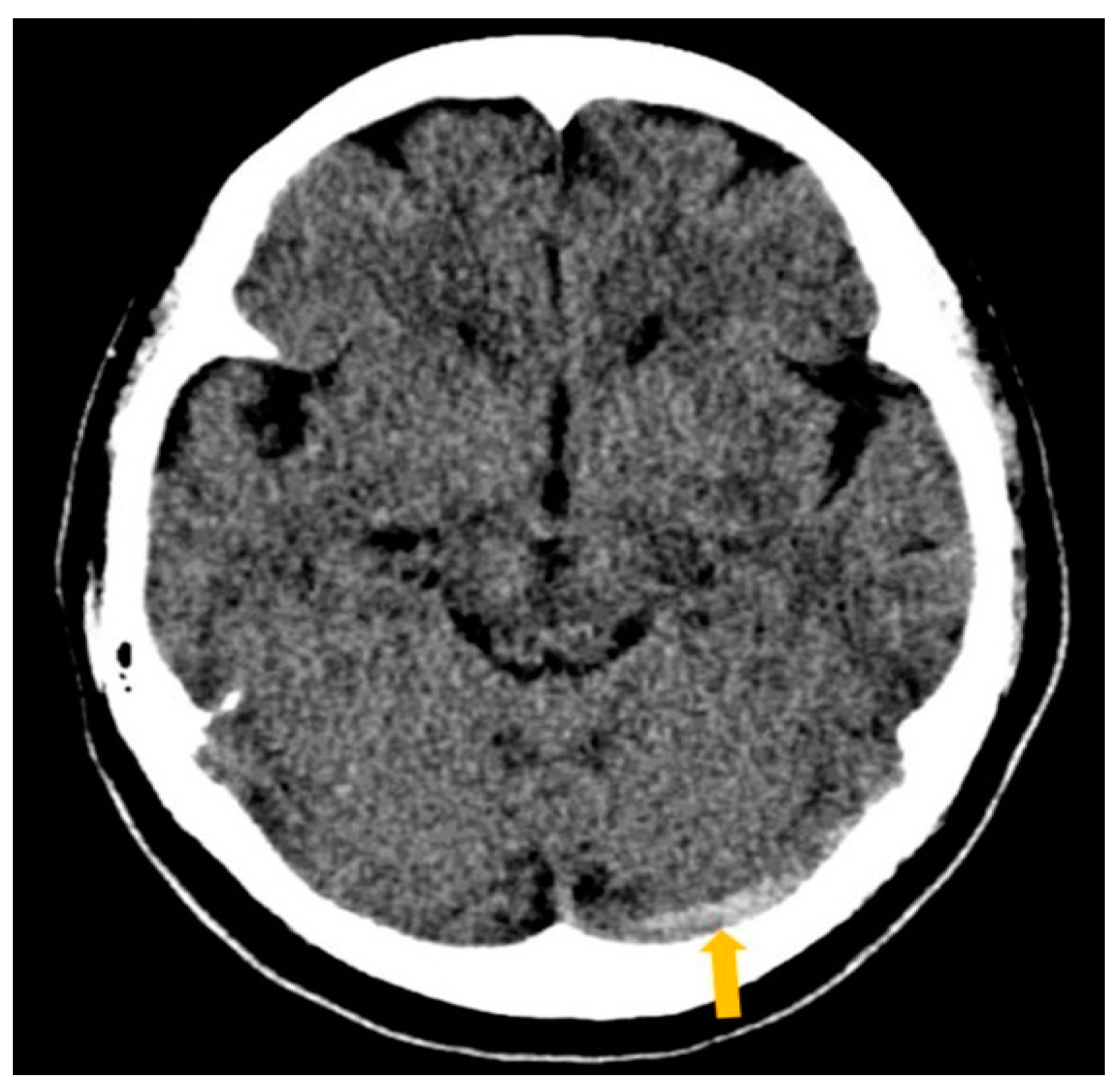
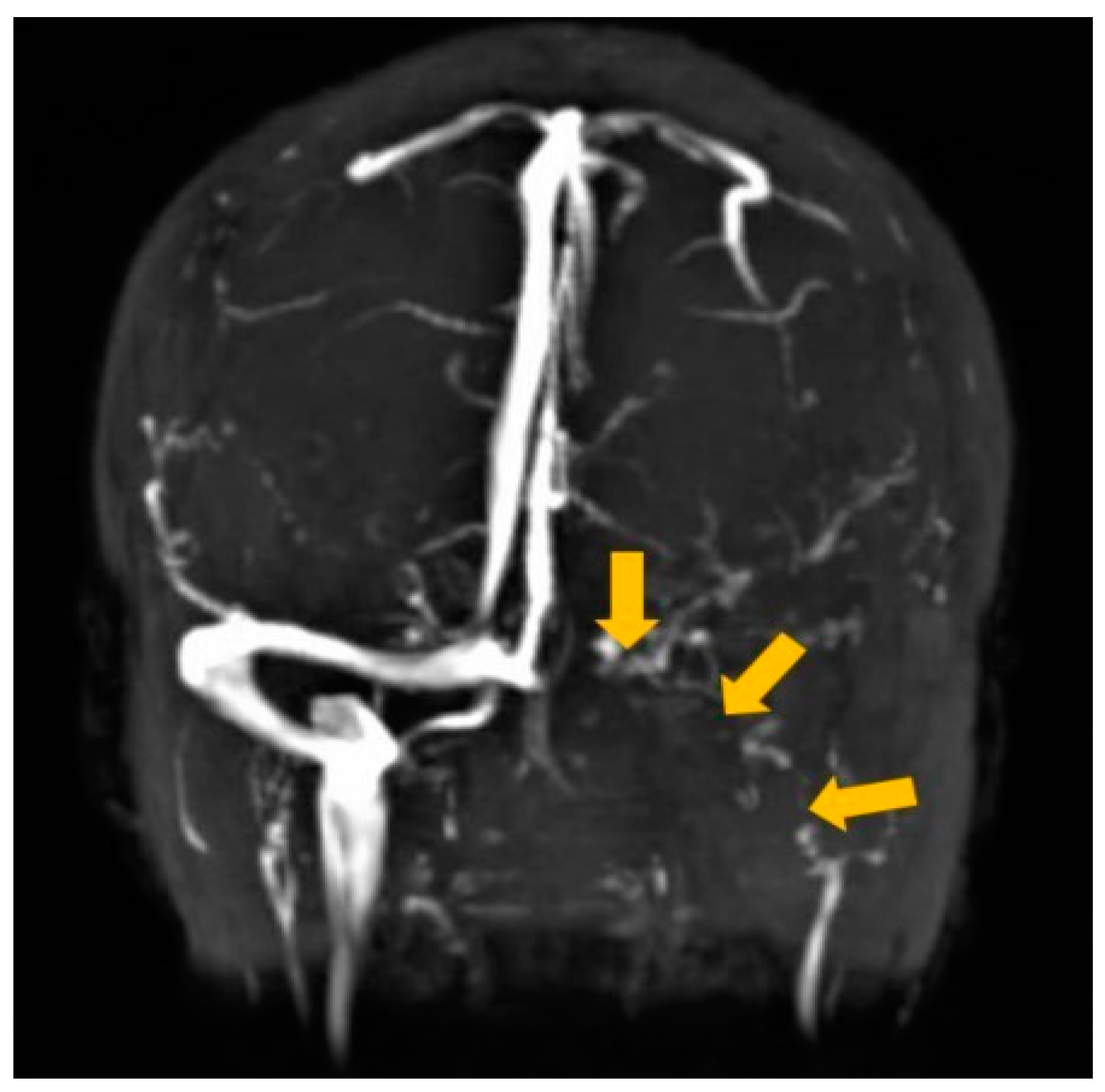
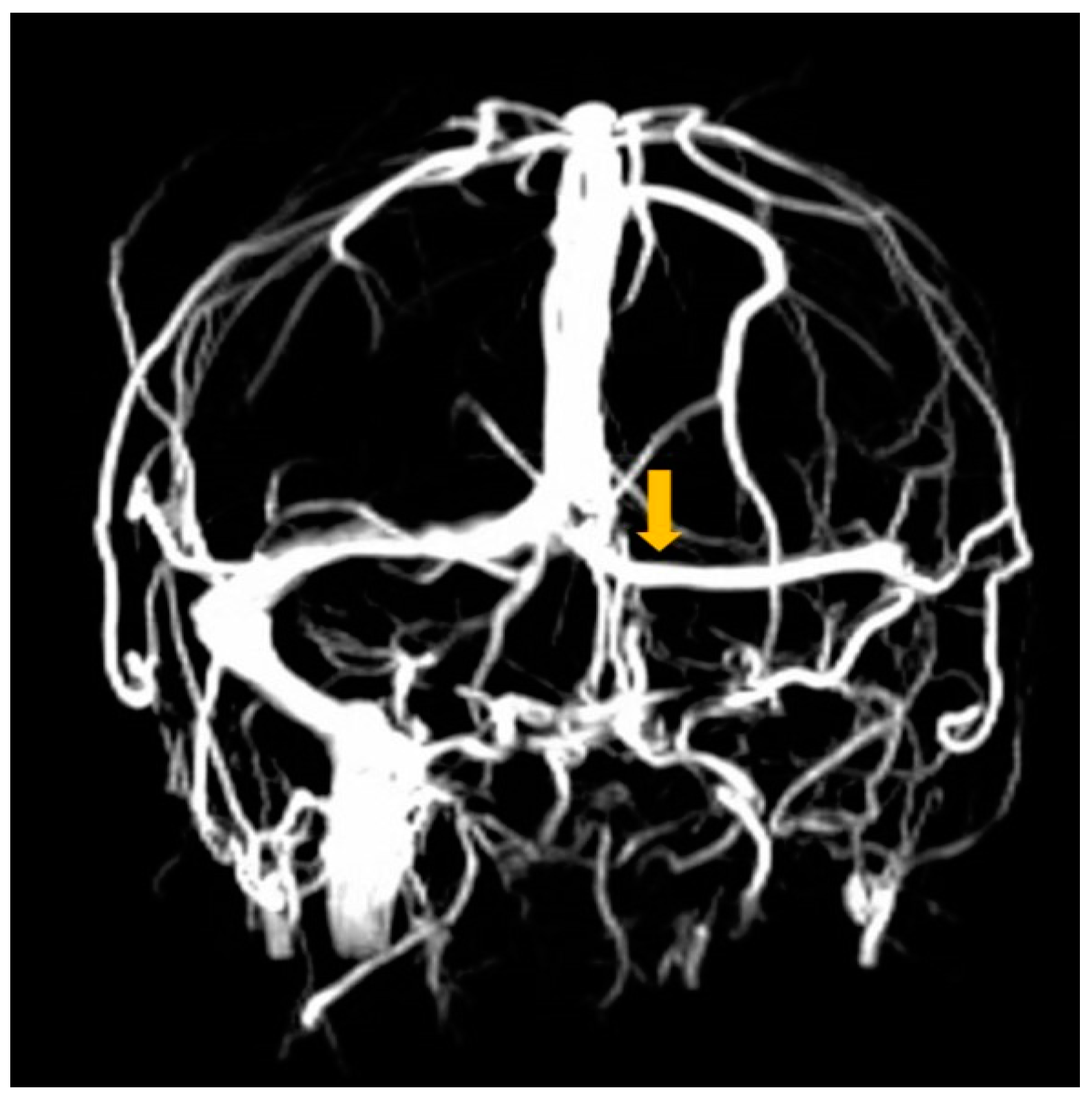
2. Case Report
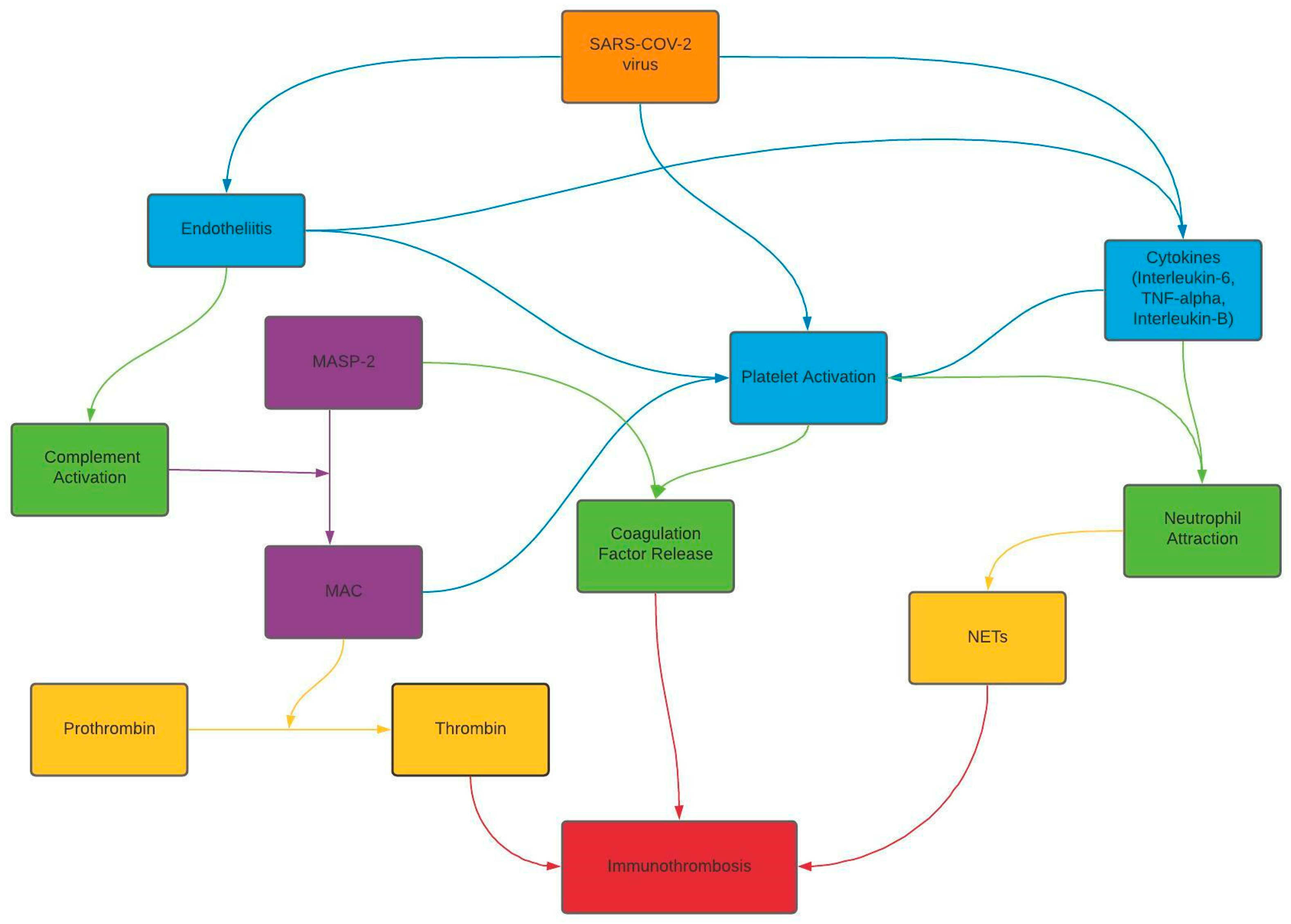
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdalkader, M.; Shaikh, S.P.; Siegler, J.E.; Cervantes-Arslanian, A.M.; Tiu, C.; Radu, R.A.; Tiu, V.E.; Jillella, D.V.; Mansour, O.Y.; Vera, V.; et al. Cerebral Venous Sinus Thrombosis in COVID-19 Patients: A Multicenter Study and Review of Literature. J. Stroke Cerebrovasc. Dis. 2021, 30, 105733. [Google Scholar] [CrossRef]
- Alons, I.M.; Jellema, K.; Wermer, M.J.; Algra, A. D-dimer for the exclusion of cerebral venous thrombosis: A meta-analysis of low risk patients with isolated headache. BMC Neurol. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Seyam, M.; Sharma, N.; Din, M.U.; Bansal, V. New Horizons for Diagnostic Pitfalls of Cerebral Venous Thrombosis: Clinical Utility of a Newly Developed Cerebral Venous Thrombosis Diagnostic Score: A Case Report and Literature Review. Am. J. Case Rep. 2021, 22, e932123-1. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Bousser, M.; Canhão, P.; Coutinho, J.M.; Crassard, I.; Dentali, F.; di Minno, M.; Maino, A.; Martinelli, I.; Masuhr, F.; et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—Endorsed by the European Academy of Neurology. Eur. J. Neurol. 2017, 24, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Stecker, E.; Warden, B.A. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J. Am. Heart Assoc. 2020, 9, e017559. [Google Scholar] [CrossRef] [PubMed]
- Ferro, J.M.; Coutinho, J.M.; Dentali, F.; Kobayashi, A.; Alasheev, A.; Canhão, P.; Karpov, D.; Nagel, S.; Posthuma, L.; Roriz, J.M.; et al. Safety and Efficacy of Dabigatran Etexilate vs. Dose-Adjusted Warfarin in Patients With Cerebral Venous Thrombosis. JAMA Neurol. 2019, 76, 1457–1465. [Google Scholar] [CrossRef] [Green Version]
- Hsu, A.; Mistry, H.; Lala, N.; Reagan, J.L. Preliminary findings regarding the use of direct oral anticoagulants in cerebral venous thrombosis. Clin. Neurol. Neurosurg. 2020, 198, 106204. [Google Scholar] [CrossRef]
- Hughes, C.; Nichols, T.; Pike, M.; Subbe, C.; Elghenzai, S. Cerebral Venous Sinus Thrombosis as a Presentation of COVID-19. Eur. J. Case Rep. Intern. Med. 2020, 7, 001691. [Google Scholar]
- Sugiyama, Y.; Tsuchiya, T.; Tanaka, R.; Ouchi, A.; Motoyama, A.; Takamoto, T.; Hara, N.; Yanagawa, Y. Cerebral venous thrombosis in COVID-19-associated coagulopathy: A case report. J. Clin. Neurosci. 2020, 79, 30–32. [Google Scholar] [CrossRef]
- Thompson, A.; Morgan, C.; Smith, P. Cerebral venous sinus thrombosis associated with COVID-19. Am. J. Neuroradiol. 2020, 41, 1370–1376. [Google Scholar] [CrossRef]
- Tu, T.M.; Goh, C.; Tan, Y.K.; Leow, A.S.; Pang, Y.Z.; Chien, J.; Shafi, H.; Chan, B.P.; Hui, A.; Koh, J.; et al. Cerebral Venous Thrombosis in Patients with COVID-19 Infection: A Case Series and Systematic Review. J. Stroke Cerebrovasc. Dis. 2020, 29, 105379. [Google Scholar] [CrossRef] [PubMed]
- Bolaji, P.; Kukoyi, B.; Ahmad, N.; Wharton, C. Extensive cerebral venous sinus thrombosis: A potential complication in a patient with COVID-19 disease. BMJ Case Rep. 2020, 13, e236820. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.Z.; Shafi, H.; Lee, Z.C.; Ting, S.K.S.; De Silva, D.A. Cerebral venous thrombosis in a patient with mild COVID-19 infection. Ann. Acad. Med. Singap. 2021, 50, 188–190. [Google Scholar] [CrossRef]
- Hameed, S.; Wasay, M.; Soomro, B.A.; Mansour, O.; Abd-Allah, F.; Tu, T.; Farhat, R.; Shahbaz, N.; Hashim, H.; Alamgir, W.; et al. Cerebral Venous Thrombosis Associated with COVID-19 Infection: An Observational, Multicenter Study. Cerebrovasc. Dis. Extra 2021, 11, 55–60. [Google Scholar] [CrossRef]
- Caronna, E.; Ballvé, A.; Llauradó, A.; Gallardo, V.J.; Ariton, D.M.; Lallana, S.; Maza, S.L.; Gadea, M.O.; Quibus, L.; Restrepo, J.L.; et al. Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia 2020, 40, 1410–1421. [Google Scholar] [CrossRef]
- Chaumont, H.; Etienne, P.; Roze, E.; Couratier, C.; Roger, P.-M.; Lannuzel, A. Acute meningoencephalitis in a patient with COVID-19. Rev. Neurol. 2020, 176, 519–521. [Google Scholar] [CrossRef]
- McCracken, I.R.; Saginc, G.; He, L.; Huseynov, A.; Daniels, A.; Fletcher, S.; Peghaire, C.; Kalna, V.; Andaloussi-Mäe, M.; Muhl, L.; et al. Lack of Evidence of Angiotensin-Converting Enzyme 2 Expression and Replicative Infection by SARS-CoV-2 in Human Endothelial Cells. Circulation 2021, 143, 865–868. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Lou, M.; Yuan, D.; Liao, S.; Tong, L.; Li, J. Potential mechanisms of cerebrovascular diseases in COVID-19 patients. J. NeuroVirol. 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Alvis, H.; Castellar-Leones, S.M.; Alcala-Cerra, G.; Moscote-Salazar, L.R. Cerebral sinus venous thrombosis. J. Neurosci. Rural Pract. 2013, 4, 427–438. [Google Scholar] [CrossRef]
- Tayyebi, S.; Akhavan, R.; Shams, M.; Salehi, M.; Farrokh, D.; Yousefi, F.; Abbasi, B. Diagnostic value of non-contrast brain computed tomography in the evaluation of acute cerebral venous thrombosis. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dmytriw, A.A.; Song, J.S.A.; Yu, E.; Poon, C.S. Cerebral venous thrombosis: State of the art diagnosis and management. Neuroradiology 2018, 60, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Mokin, M.; Hetts, S.W.; Fifi, J.T.; Bousser, M.-G.; Fraser, J.F. Current endovascular strategies for cerebral venous thrombosis: Report of the SNIS Standards and Guidelines Committee. J. Neurointerventional Surg. 2018, 10, 803–810. [Google Scholar] [CrossRef]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Baldini, T.; Asioli, G.M.; Romoli, M.; Dias, M.C.; Schulte, E.C.; Hauer, L.; De Sousa, D.A.; Sellner, J.; Zini, A. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: A systematic review and meta-analysis. Eur. J. Neurol. 2021. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Skalij, P.; Tokajuk, P.; Politynska, B.; Wojtukiewicz, A.M.; Tucker, S.C.; Honn, K.V. Direct Oral Anticoagulants in Cancer Patients. Time for a Change in Paradigm. Cancers 2020, 12, 1144. [Google Scholar] [CrossRef]
- Wadsworth, D.; Sullivan, E.; Jacky, T.; Sprague, T.; Feinman, H.; Kim, J. A review of indications and comorbidities in which warfarin may be the preferred oral anticoagulant. J. Clin. Pharm. Ther. 2021, 46, 560–570. [Google Scholar] [CrossRef]
- Sales, C.; Wijeratne, T.; Lucero, A. Is JAK2-Mutation Associated with Extensive Clot Burden in Cerebral Venous Thrombosis? Ann. Clin. Case Rep. 2021, 6, 1911. [Google Scholar]
- Ostovan, V.R.; Foroughi, R.; Rostami, M.; Almasi-Dooghaee, M.; Esmaili, M.; Bidaki, A.A.; Behzadi, Z.; Farzadfard, F.; Marbooti, H.; Rahimi-Jaberi, A.; et al. Cerebral venous sinus thrombosis associated with COVID-19: A case series and literature review. J. Neurol. 2021, 1–12. [Google Scholar] [CrossRef]
| Article | Study | Clinical Presentation | Location | Diagnostic Imaging | COVID-19 Diagnosis | Therapeutics | Outcome |
|---|---|---|---|---|---|---|---|
| Hughes 2020 [8] | Case Report | Right-sided frontotemporal progressive headache, ipsilateral numbness, slurred speech, and expressive dysphasia | Right sigmoid and transverse sinus | Head CT a: hyperdensity of superior sagittal sinus, right transverse sinus, sigmoid sinus, and right upper jugular vein. CTV b: filling defect in right sigmoid and transverse sinus involving the torcula | Nasopharyngeal COVID-19 c swab | 1. Low molecular weight Heparin for 24 h (dosage unknown) 2. Apixaban 10 mg d BID e for 7 days | Patient is at home recovering as of publication. |
| Sugiyama 2020 [9] | Case Report | Fever and malaise | Confluence of sinus to left transverse sinus | Non-contrast CT a Head: hyperdensity of left transverse sinus (cord sign) T2-weighted MRI f demonstrated isointensity, T2-FLAIR g MRI f hyperintensity in the left transverse sinus T2-weighted MRI f hypointensity left transverse sinus. | First SARS-CoV-2 h RT PCR i test was negative. Repeat SARS-CoV-2 h RT PCR i was positive. | 1. subcutaneous unfractionated Heparin 10,000 units (duration unknown); switched to IV m unfractionated Heparin, dose adjusted (specific dose unknown) for 18 days 2. Edoxaban 60 mg d QD j duration of at least 29 days | Significant improvement of sinus thrombus and discharged on day 33; continued Edoxaban and remained free of symptoms for 14 days after discharge. |
| Thompson 2020 [10] | Case Report | Delirium, executive dysfunction and dyspraxia | Superior sagittal sinus, left transverse sinus and left sigmoid sinus down to the level of the jugular foramen | Non-contrast CT a of the head was normal 1 week later: Repeat Head CT a and CTV b: thrombosis of superior sagittal sinus, left transverse sinus, left sigmoid sinus, jugular foramen, and the vein of Labbé 7 × 8 mm l parenchymal haemorrhage in left temporal lobe 2 weeks from admission: radiological improvement with recanalisation of the vein of Labbe, partial recanalisation of the left transverse sinus and superior sagittal sinus. Acute haemorrhage within the slender hygroma. | Negative PCR k nasopharyngeal swabs for SARS-CoV-2 h (however probable COVID-19 c diagnosis was made, based on European Centre for Disease Control case definition) | 1. subcutaneous Enoxaparin 40 mg d QD j for 7 days, switched to IV m Heparin 1.5 mg/kg QD j (duration unknown) 2. Apixaban 5 mg d BID e for minimum 3–6 months (personal communication) | Discharged from hospital and positive response to treatment to date of publication. Continuing anticoagulation for a minimum of 3-6 months. |
| Tu 2020 [11] | Case series Systematic Review | 1. Chest pain, fever, and chills | 1. Left transverse, sigmoid sinus | Not Applicable | Nasopharyngeal swab SARS-CoV-2 h RT PCRi was positive. | 1.Dabigatran (dosage/duration unknown) | 1. Resolution of Cerebral Venous Thrombosis after 4 weeks |
| Bolaji 2020 [12] | Case report | Left sided weakness, left facial twitch, inability to stand, and left-sided extensor plantar response | Right transverse sinus | CT a, CTV b revealed venous sinus thrombosis, bilateral venous cortical infarcts, acute cortical hemorrhage | Nasopharyngeal swab SARS-CoV-2 h positive | 1. Therapeutic doses of IV m low molecular weight Heparin (specific dose unknown, started on admission, full duration unknown) 2. Edoxaban (dosage/duration unknown) | Discharged to long-term care facility for physical therapy and monitoring, then discharged home |
| Pang 2021 [13] | Case report | 3 day history of fever, cough, and headache | Left transverse and sigmoid sinuses | MRI f Brain, MRV n (T2 flow void) | RT-PCR i Nasopharyngeal swab SARS-CoV-2 h positive | 1. Heparin use unknown 2. Dabigatran 150 mg d BID e for 3 months | CTV b 1 month later revealed resolution, then patient was lost to follow up |
| Hameed 2021 [14] | Multicenter Study and Review of Literature | Headache, seizures, altered mental status, hemiparesis, and heminumbness | Superior sagittal, transverse, sigmoid, cavernous, straight sinuses and internal jugular vein | Neuroimaging revealed infarction in affected sinuses | RT-PCR i Nasopharyngeal swab SARS-CoV-2 h positive | 1.Low molecular weight Heparin (dosage/duration unknown) 10 of 18 patients 2.Unfractionated Heparin (dosage/duration unknown) 4 of 18 patients 3. Direct oral anticoagulants (dosage/duration unknown) 6 of 18 patients; Rivaroxaban (dosage/duration unknown) 5 of 6 patients; Dabigatran (dosage/duration unknown) 1 of 6 patients | mRS o score 0–2 o |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Sharma, N.; Ud Din, M.; Chetram, R. Diagnostic and Therapeutic Challenges of Cerebral Venous Thrombosis in SARS-CoV-2 Infection: A Case Report and Review of Literature. Clin. Pract. 2021, 11, 598-606. https://doi.org/10.3390/clinpract11030075
Khan F, Sharma N, Ud Din M, Chetram R. Diagnostic and Therapeutic Challenges of Cerebral Venous Thrombosis in SARS-CoV-2 Infection: A Case Report and Review of Literature. Clinics and Practice. 2021; 11(3):598-606. https://doi.org/10.3390/clinpract11030075
Chicago/Turabian StyleKhan, Faisal, Neha Sharma, Moin Ud Din, and Ryan Chetram. 2021. "Diagnostic and Therapeutic Challenges of Cerebral Venous Thrombosis in SARS-CoV-2 Infection: A Case Report and Review of Literature" Clinics and Practice 11, no. 3: 598-606. https://doi.org/10.3390/clinpract11030075
APA StyleKhan, F., Sharma, N., Ud Din, M., & Chetram, R. (2021). Diagnostic and Therapeutic Challenges of Cerebral Venous Thrombosis in SARS-CoV-2 Infection: A Case Report and Review of Literature. Clinics and Practice, 11(3), 598-606. https://doi.org/10.3390/clinpract11030075






