Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Patient and Public Partnership
2.3. Laboratory Analysis
2.4. Surgical Technique
2.5. Clinical Endpoint
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bronicki, R.A.; Hall, M. Cardiopulmonary Bypass-Induced Inflammatory Response: Pathophysiology and Treatment. Pediatr. Crit. Care Med. 2016, 17, S272–S278. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, G.; Tashiro, T. Current Status of Off-Pump Coronary Artery Bypass. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 125–132. [Google Scholar] [CrossRef]
- Chivasso, P.; Guida, G.A.; Fudulu, D.; Bruno, V.D.; Marsico, R.; Sedmakov, H.; Zakkar, M.; Rapetto, F.; Bryan, A.J.; Angelini, G.D. Impact of off-pump coronary artery bypass grafting on survival: Current best available evidence. J. Thorac. Dis. 2016, 8, 808–817. [Google Scholar] [CrossRef] [Green Version]
- Puskas, J.D.; Williams, W.H.; Mahoney, E.M.; Huber, P.R.; Block, P.C.; Duke, P.G.; Staples, J.R.; Glas, K.E.; Marshall, J.J.; Leimbach, M.E.; et al. Off-pump vs conventional coronary artery bypass grafting: Early and 1-year graft patency, cost, and quality-of-life outcomes: A randomized trial. JAMA 2004, 291, 1841–1849. [Google Scholar] [CrossRef]
- Wirtz, P.H.; von Känel, R. Psychological Stress, Inflammation, and Coronary Heart Disease. Curr. Cardiol. Rep. 2017, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.C.; Adhikari, P.; Narra, S.R.; Srivatsa, S.S.; Mills, P.K.; Srivatsa, S.S. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol. J. 2014, 21, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Azab, B.; Zaher, M.; Weiserbs, K.F.; Torbey, E.; Lacossiere, K.; Gaddam, S.; Gobunsuy, R.; Jadonath, S.; Baldari, D.; McCord, D.; et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am. J. Cardiol. 2010, 106, 470–476. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [PubMed]
- Kim, W.H.; Jin, H.S.; Ko, J.S.; Hahm, T.S.; Lee, S.M.; Cho, H.S.; Kim, M.H. The effect of anesthetic techniques on neutrophil-to-lymphocyte ratio after laparoscopy-assisted vaginal hysterectomy. Acta Anaesthesiol. Taiwanica 2011, 49, 83–87. [Google Scholar] [CrossRef]
- Özer, A.; Mardin, B.; Kılıç, Y.; Oktar, L.; İriz, E.; Arslan, M.; Ünal, Y.; Alkan, M. The effect of neutrophil-lymphocyte ratio on the postoperative course of coronary artery bypass graft surgery. Turk. J. Med. Sci. 2018, 48, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, O.; Kumtepe, G.; Ozkan, H.; Karal, I.H.; Velioglu, Y.; Ercan, A.; Yüksel, A.; Ener, S. Predictive Value of Neutrophil-Lymphocyte Ratio for Long-Term Cardiovascular Event Following Coronary Artery Bypass Grafting. Braz. J. Cardiovasc. Surg. 2020, 35, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Larmann, J.; Handke, J.; Scholz, A.S.; Dehne, S.; Arens, C.; Gillmann, H.J.; Uhle, F.; Motsch, J.; Weigand, M.A.; Janssen, H. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non-cardiac surgery. BMC Cardiovasc. Disord. 2020, 20, 230. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.P.; Arekapudi, A.; Metha, J.; Prasad, A.; Venkatraghavan, L. Neutrophil-lymphocyte ratio as predictor of mortality and morbidity in cardiovascular surgery: A systematic review. ANZ J. Surg. 2015, 85, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [Green Version]
- Zernecke, A.; Bot, I.; Djalali-Talab, Y.; Shagdarsuren, E.; Bidzhekov, K.; Meiler, S.; Krohn, R.; Schober, A.; Sperandio, M.; Soehnlein, O.; et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ. Res. 2008, 102, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Chakraborti, T.; Mandal, A.; Mandal, M.; Das, S.; Chakraborti, S. Complement activation in heart diseases: Role of oxidants. Cell Signal. 2000, 12, 607–617. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chung, H.T.; Chang, C.J.; Yeh, K.W.; Chen, L.C.; Huang, J.L. Lymphopenia is a risk factor in the progression of carotid intima-media thickness in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2009, 60, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Challier, B.; Saas, P. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J. Am. Soc. Nephrol. 2003, 14, 767–772. [Google Scholar] [CrossRef] [Green Version]
- Bagger, J.P.; Zindrou, D.; Taylor, K.M. Leukocyte count: A risk factor for coronary artery bypass graft mortality. Am. J. Med. 2003, 115, 660–663. [Google Scholar] [CrossRef]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Intermountain Heart Collaborative Study Group. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; Hu, Q.; Zheng, S.; Tian, B.; Meng, F.; Chen, Z.; Han, J.; Wang, S.; Zhang, H.; et al. Association of early elevated cardiac troponin I concentration and longitudinal change after off-pump coronary artery bypass grafting and adverse events: A prospective cohort study. J. Thorac. Dis. 2020, 12, 6542–6551. [Google Scholar]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atheroscler. Inflamm. Biomol. 2018, 8, 80–91. [Google Scholar]
- Hsi, C.H.-H.; Wang, J.J. Clinical implication of perioperative inflammatory cytokine alteration. Acta Anaesthesiol. Taiwanica 2015, 15, 23–28. [Google Scholar]
- Raja, S.G.; Berg, G.A. Impact of off-pump coronary artery bypass surgery on systemic inflammation: Current best available evidence. J. Card. Surg. 2007, 5, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Al-Ruzzeh, S.; Hoare, G.; Marczin, N.; Asimakopoulos, G.; George, S.; Taylor, K.; Amrani, M. Off-pump coronary artery bypass surgery is associated with reduced neutrophil activation as measured by the expression of CD11b: A prospective randomized study. Heart Surg. Forum. 2003, 6, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Ascione, R.; Lloyd, C.T.; Underwood, M.J.; Lotto, A.A.; Pitsis, A.A.; Angelini, G.D. Inflammatory response after coronary revascularization with or without cardiopulmonary bypass. Ann. Thorac. Surg. 2000, 69, 1198–1204. [Google Scholar] [CrossRef]
- Velissaris, T.; Tang, A.T.; Murray, M.; Mehta, R.L.; Wood, P.J.; Hett, D.A.; Ohri, S.K. A prospective randomized study to evaluate stress response during beating-heart and conventional coronary revascularization. Ann. Thorac. Surg. 2004, 78, 506–512. [Google Scholar] [CrossRef]
- Stoppe, C.; Werker, T.; Rossaint, R.; Dollo, F.; Lue, H.; Wonisch, W.; Menon, A.; Goetzenich, A.; Bruells, C.S.; Coburn, M.; et al. What is the significance of perioperative release of macrophage migration inhibitory factor in cardiac surgery? Antioxid. Redox Signal. 2013, 19, 231–239. [Google Scholar] [CrossRef] [Green Version]
- Min, J.J.; Nam, K.; Kim, T.K.; Kim, H.J.; Seo, J.H.; Hwang, H.Y.; Kim, K.B.; Murkin, J.M.; Hong, D.M.; Jeon, Y. Relationship between early postoperative C-reactive protein elevation and long-term postoperative major adverse cardiovascular and cerebral events in patients undergoing off-pump coronary artery bypass graft surgery: A retrospective study. Br. J. Anaesth. 2014, 113, 391–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awan, N.I.; Jan, A.; Rehman, M.U.; Ayaz, N. The effect of ejection fraction on mortality in Coronary Artery Bypass Grafting (CABG) patients. Pak. J. Med. Sci. 2020, 36, 1454–1459. [Google Scholar] [CrossRef]
- Popovic, B.; Agrinier, N.; Voilliot, D.; Voilliot, D.; Elfarra, M.; Villemot, J.P.; Maureira, P. Ventricular Dysfunction in Patients with Acute Coronary Syndrome Undergoing Coronary Surgical Revascularization: Prognostic Impact on Long-Term Outcomes. PLoS ONE 2016, 11, e0168634. [Google Scholar] [CrossRef]
- Serra, R.; Ielapi, N.; Licastro, N.; Provenzano, M.; Andreucci, M.; Bracale, U.M.; Jiritano, F.; de Franciscis, S.; Mastroroberto, P.; Serraino, G.F. Neutrophil-to-lymphocyte Ratio and Platelet-to-lymphocyte Ratio as Biomarkers for Cardiovascular Surgery Procedures: A Literature Review. Rev. Recent Clin. Trials. 2021, 16, 173–179. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Mukanova, U.; Yessirkepov, M.; Kitas, G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019, 39, 345–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lako, S.; Dedej, T.; Nurka, T.; Ostreni, V.; Demiraj, A.; Xhaxho, R.; Prifti, E. Hematological Changes in Patients Undergoing Coronary Artery Bypass Surgery: A Prospective Study. Med. Arch. 2015, 69, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdoia, M.; Nardin, M.; Gioscia, R.; Negro, F.; Marcolongo, M.; Suryapranata, H.; Kedhi, E.; De Luca, G. Novara Atherosclerosis Study Group (NAS). Higher neutrophil-to-lymphocyte ratio (NLR) increases the risk of suboptimal platelet inhibition and major cardiovascular ischemic events among ACS patients receiving dual antiplatelet therapy with ticagrelor. Vascul. Pharmacol. 2020, 132, 106765. [Google Scholar] [CrossRef] [PubMed]
- Adatia, K.; Farag, M.F.; Gue, Y.X.; Srinivasan, M.; Gorog, D.A. Relationship of Platelet Reactivity and Inflammatory Markers to Recurrent Adverse Events in Patients with ST-Elevation Myocardial Infarction. Thromb. Haemost. 2019, 119, 1785–1794. [Google Scholar] [CrossRef]
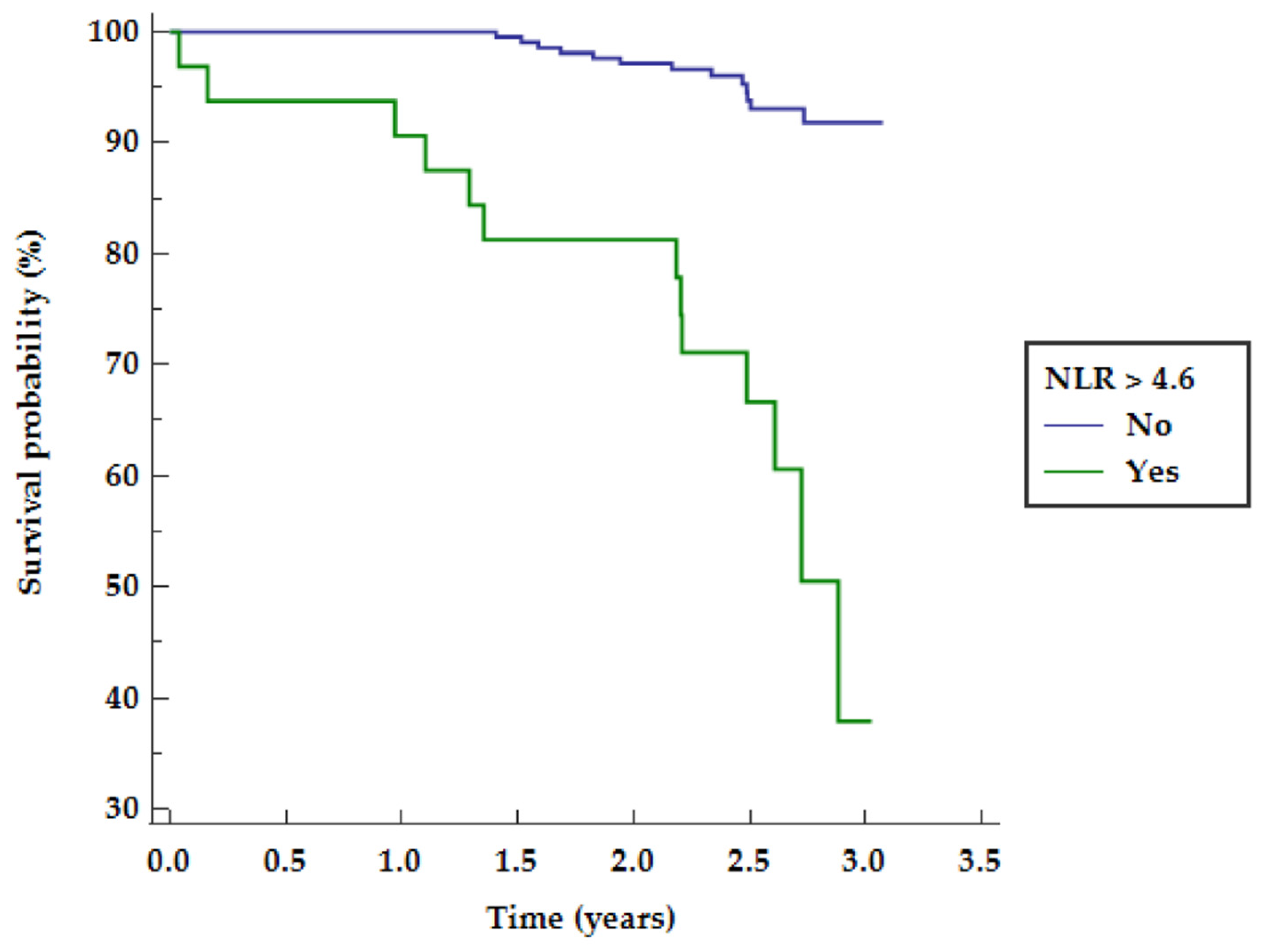
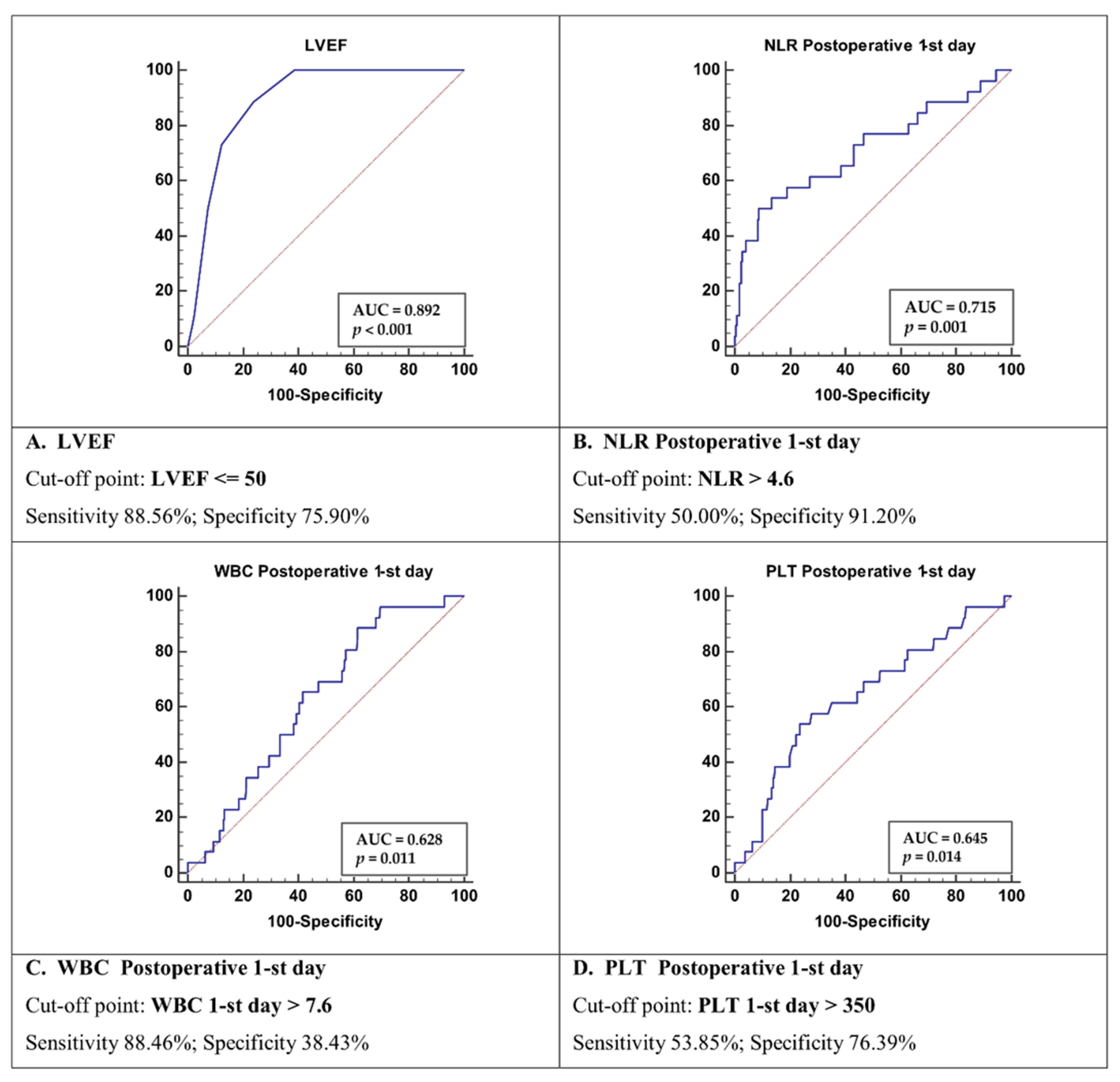
| Parameter | Group 1 Survivors (n = 198) | Group 2 Deceased (n = 26) | p-Value |
|---|---|---|---|
| Gender M/F | 174 (88%)/25 (12%) | 24 (92%)/2 (8%) | p = 0.5483 |
| Age | 65 +/− 9 | 67 +/− 9 | p = 0.2869 |
| Concomitant diseases: | |||
| 1. arterial hypertension | 166 (84%) | 21 (81%) | p = 0.6973 |
| 2. DM | 78 (39%) | 9 (65%) | p = 0.0116 |
| 3. Stroke | 5 (3%) | 8 (31%) | p < 0.0001 |
| 4. Hypercholesterolemia | 134 (68%) | 18 (70%) | p = 0.8368 |
| 5. PAD | 29 (15%) | 11 (42%) | p = 0.0008 |
| Surgical indication: | p = 0.5650 | ||
| 1. LM disease | 102 (52%) | 12 (46%) | p = 0.5629 |
| 2. 3 vessels disease | 88 (44%) | 13 (50%) | p = 1.0000 |
| 3. 2 vessels disease | 8 (4%) | 1 (4%) | |
| Echocardiographic results | |||
| 1. LV diameter (mm) | 47 +/− 6 | 48 +/− 6 | p = 0.4257 |
| 2. LVEF (%) | 54 +/− 8 | 50 +/− 7 | p = 0.0159 |
| Surgery: | |||
| 1. overall time (min) | 141 +/− 42 | 139 +/− 39 | p = 0.8182 |
| 2. Mean anastomosis | 2.3 +/− 0.7 | 2.3 +/− 0.7 | p = 1.0000 |
| Hospitalization time (days) | 8.8 +/− 3 | 13 +/− 10 | p = 0.0433 |
| (excluding in hospital mortality) | (10 +/− 4) |
| Parameters | Group 1 Survivors (n = 198) | Group 2 Deceased (n = 26) | p-Value |
|---|---|---|---|
| Preoperative: | |||
| 1. WBC, ×109/L (mean ± SD) | 8.4 +/− 3.3 | 7.7 +/− 1.9 | p = 0.6780 |
| 2. Neutrophils, ×109/L (mean ± SD) | 5.3 +/− 1.7 | 5.1 +/− 1.5 | p = 0.7849 |
| 3. Lymphocyte, ×109/L (mean ± SD) | 2.2 +/− 2.5 | 1.8 +/− 0.7 | p = 0.4513 |
| 4. Hb, mmol/L (mean ± SD) | 8.7 +/− 0.9 | 8.6 +/− 1.1 | p = 0.9893 |
| 5. Plt, ×109/L (mean ± SD) | 229 +/− 63 | 233 +/− 63 | p = 0.9397 |
| 6. NLR (mean ± SD) | 3.3 +/− 1.8 | 3.2 +/− 1.5 | p = 0.7119 |
| 7. Troponin, ng/mL (mean ± SD) | 0.23 +/− 2.8 | 0.02 +/− 0.4 | p = 0.1442 |
| Postoperative 1st day): | |||
| 1. WBC, ×109/L (mean ± SD) | 9.1 +/− 5 | 12.1 +/− 13 | p = 0.0331 |
| 2. Neutrophils, ×109/L (mean ± SD) | 5.2 +/− 2 | 8.7 +/− 11 | p = 0.0012 |
| 3. Lymphocyte, ×109/L (mean ± SD) | 2.5 +/− 3.4 | 1.8 +/− 0.7 | p = 0.0779 |
| 4. Hb, mmol/L (mean ± SD) | 6.9 +/− 0.6 | 7 +/− 0.5 | p = 0.3570 |
| 5. Plt, ×109/L (mean ± SD) | 304 +/− 92 | 354 +/− 107 | p = 0.0157 |
| 6. NLR (mean ± SD) | 2.8 +/− 1.6 | 5.1 +/− 3.6 | p = 0.0003 |
| 7. Troponin, ng/mL (mean ± SD) | 4 +/− 6.3 | 9.9 +/− 11 | p = 0.1206 |
| Postoperative 7th day): | |||
| 1. WBC, ×109/L (mean ± SD) | 9.1 +/− 4.9 | 9.2 +/− 2.6 | p = 0.4047 |
| 2. Neutrophils, ×109/L (mean ± SD) | 5.1 +/− 1.9 | 5.5 +/− 2.1 | p = 0.3956 |
| 3. Lymphocyte, ×109/L (mean ± SD) | 2.5 +/− 3.4 | 2.4 +/− 1.7 | p = 0.4216 |
| 4. Hb, mmol/L (mean ± SD) | 6.9 +/− 0.6 | 7.1 +/− 0.7 | p = 0.1483 |
| 5. Plt, ×109/L (mean ± SD) | 305.7 +/− 93.4 | 283.5 +/− 70.6 | p = 0.2592 |
| 6. NLR (mean ± SD) | 2.7 +/− 1.4 | 3 +/− 1.9 | p = 0.6694 |
| 7. Troponin, ng/mL (mean ± SD) | 0.2 +/− 1.0 | 0.1 +/− 0.5 | p = 0.7638 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Parameter | HR | 95%CI | p-Value | HR | 95%CI | p-Value |
| Gender M/F Ref. =F | 2.55 | 0.60–10.79 | 0.2034 | |||
| Age | 1.03 | 0.97–1.07 | 0.2823 | |||
| Concomitant diseases: | ||||||
| Arterial hypertension | 1.32 | 0.49–3.51 | 0.5736 | |||
| DM | 0.98 | 0.43–2.23 | 0.9668 | |||
| Stroke | 14.07 | 6.34–31.22 | <0.0001 | |||
| Hypercholesterolemia | 1.35 | 0.59–3.11 | 0.4752 | |||
| PAD | 3.9 | 1.77–8.60 | 0.0007 | |||
| Surgical indication: | ||||||
| LM disease | 0.85 | 0.39–1.85 | 0.6906 | |||
| 3 vessels disease | 1.14 | 0.52–2.50 | 0.7314 | |||
| 2 vessels disease | 1.74 | 0.80–3.81 | 0.1597 | |||
| Echocardiographic results: | ||||||
| LV diameter (mm) | 1.08 | 1.02–1.16 | 0.0191 | |||
| LVEF (%) | 0.88 | 0.85–0.91 | <0.0001 | 0.92 | 0.87–0.95 | <0.0001 |
| Preoperative: | ||||||
| (mean ± SD) | ||||||
| WBC, ×109/L | 0.92 | 0.77–1.09 | 0.3248 | |||
| Neutrophils, ×109/L | 0.94 | 0.75–1.19 | 0.6364 | |||
| Lymphocyte, ×109/L | 0.78 | 0.45–1.35 | 0.3734 | |||
| Hb, mmol/L | 0.98 | 0.62–1.52 | 0.9218 | |||
| Plt, ×109/L | 1 | 0.99 -1.01 | 0.7882 | |||
| NLR | 1.04 | 0.84–1.29 | 0.6986 | |||
| Troponin, ng/mL | 0.91 | 0.34–2.41 | 0.8498 | |||
| Postoperative 1st day: | ||||||
| (mean ± SD) | ||||||
| WBC, ×109/L | 1.05 | 1.01–1.08 | 0.0059 | 1.18 | 1.07–1.30 | 0.0006 |
| Neutrophils, ×109/L | 1.59 | 1.33–1.89 | <0.0001 | 0.36 | 0.22–0.58 | <0.0001 |
| Lymphocyte, ×109/L | 0.64 | 0.37–1.10 | 0.1047 | |||
| Hb, mmol/L | 1.14 | 0.61–2.15 | 0.6848 | |||
| Plt, ×109/L | 1.01 | 1.01–1.01 | 0.0065 | 1.01 | 1.01–1.01 | 0.0038 |
| NLR | 1.47 | 1.30–1.65 | <0.0001 | 1.61 | 1.18–2.18 | 0.0022 |
| Troponin, ng/mL | 0.99 | 0.99–1.01 | 0.8088 | |||
| Postoperative 7th day: | ||||||
| (mean ± SD) | 1.01 | 0.93–1.08 | 0.9178 | |||
| WBC, ×109/L | 1.11 | 0.93–1.32 | 0.2687 | |||
| Neutrophils, ×109/L | 0.99 | 0.87–1.12 | 0.8528 | |||
| Lymphocyte, ×109/L | 1.53 | 0.85–2.74 | 0.1524 | |||
| Hb, mmol/L | 0.98 | 0.99–1.01 | 0.3514 | |||
| Plt, ×109/L | 1.1 | 0.88–1.38 | 0.412 | |||
| NLR | 0.94 | 0.56–1.57 | 0.8105 | |||
| Troponin, ng/mL | ||||||
| Parameter | HR | 95%CI | p-Value |
|---|---|---|---|
| LVEF ≤ 50 | 12.56 | 3.69–42.72 | 0.0001 |
| WBC > 7.6 | 1.04 | 0.32–3.45 | 0.9409 |
| Plt > 350 | 2.70 | 1.19–6.15 | 0.0180 |
| NLR > 4.6 | 9.30 | 3.60–24.02 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowicz, T.; Michalak, M.; Gąsecka, A.; Perek, B.; Rodzki, M.; Bociański, M.; Straburzyńska-Migaj, E.; Jemielity, M. Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures. Clin. Pract. 2021, 11, 587-597. https://doi.org/10.3390/clinpract11030074
Urbanowicz T, Michalak M, Gąsecka A, Perek B, Rodzki M, Bociański M, Straburzyńska-Migaj E, Jemielity M. Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures. Clinics and Practice. 2021; 11(3):587-597. https://doi.org/10.3390/clinpract11030074
Chicago/Turabian StyleUrbanowicz, Tomasz, Michał Michalak, Aleksandra Gąsecka, Bartłomiej Perek, Michał Rodzki, Michał Bociański, Ewa Straburzyńska-Migaj, and Marek Jemielity. 2021. "Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures" Clinics and Practice 11, no. 3: 587-597. https://doi.org/10.3390/clinpract11030074
APA StyleUrbanowicz, T., Michalak, M., Gąsecka, A., Perek, B., Rodzki, M., Bociański, M., Straburzyńska-Migaj, E., & Jemielity, M. (2021). Postoperative Neutrophil to Lymphocyte Ratio as an Overall Mortality Midterm Prognostic Factor following OPCAB Procedures. Clinics and Practice, 11(3), 587-597. https://doi.org/10.3390/clinpract11030074






