Effect of Temperature on Immunocompetence of the Blue Mussel (Mytilus edulis)
Abstract
Introduction
Materials and Methods
Animals
Experimental design
Histological sex identification
Index analysis
Viability, cellularity and phagocytosis
Lysozyme activity
Inflammation level
Statistical analysis
Results
Gender difference and sexual maturity
Indices
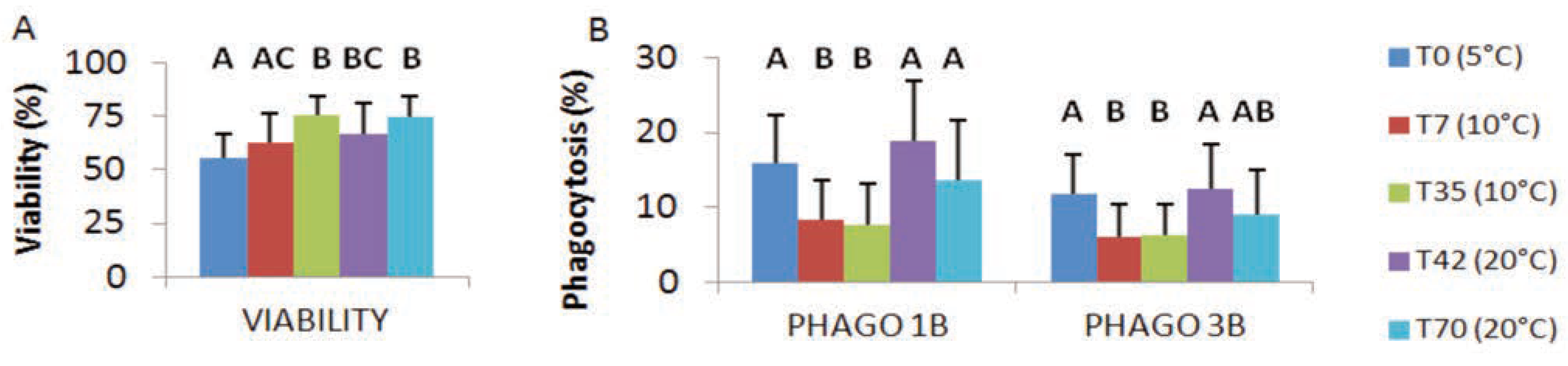
Viability and phagocytosis
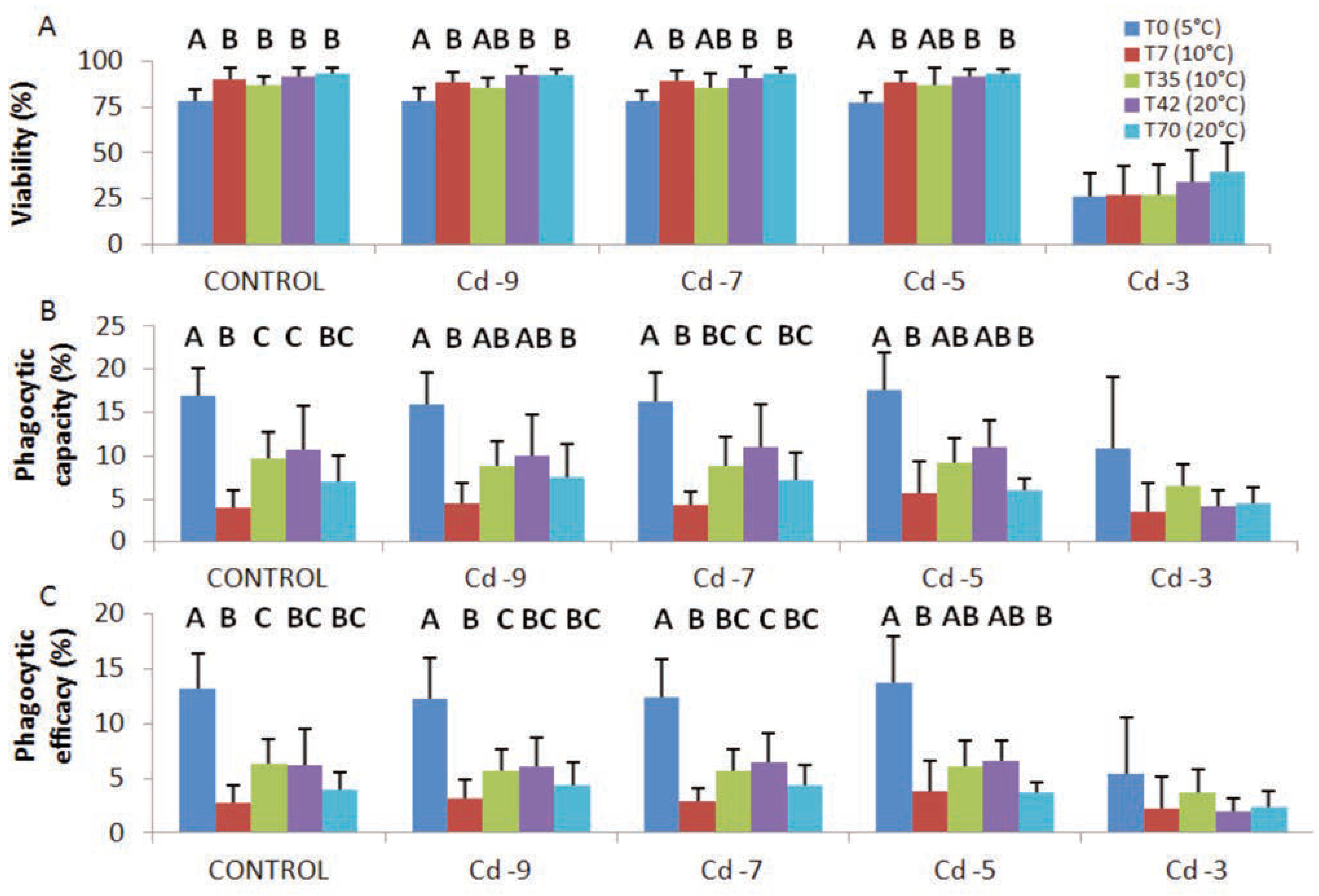
Cadmium exposure: viability and phagocytosis
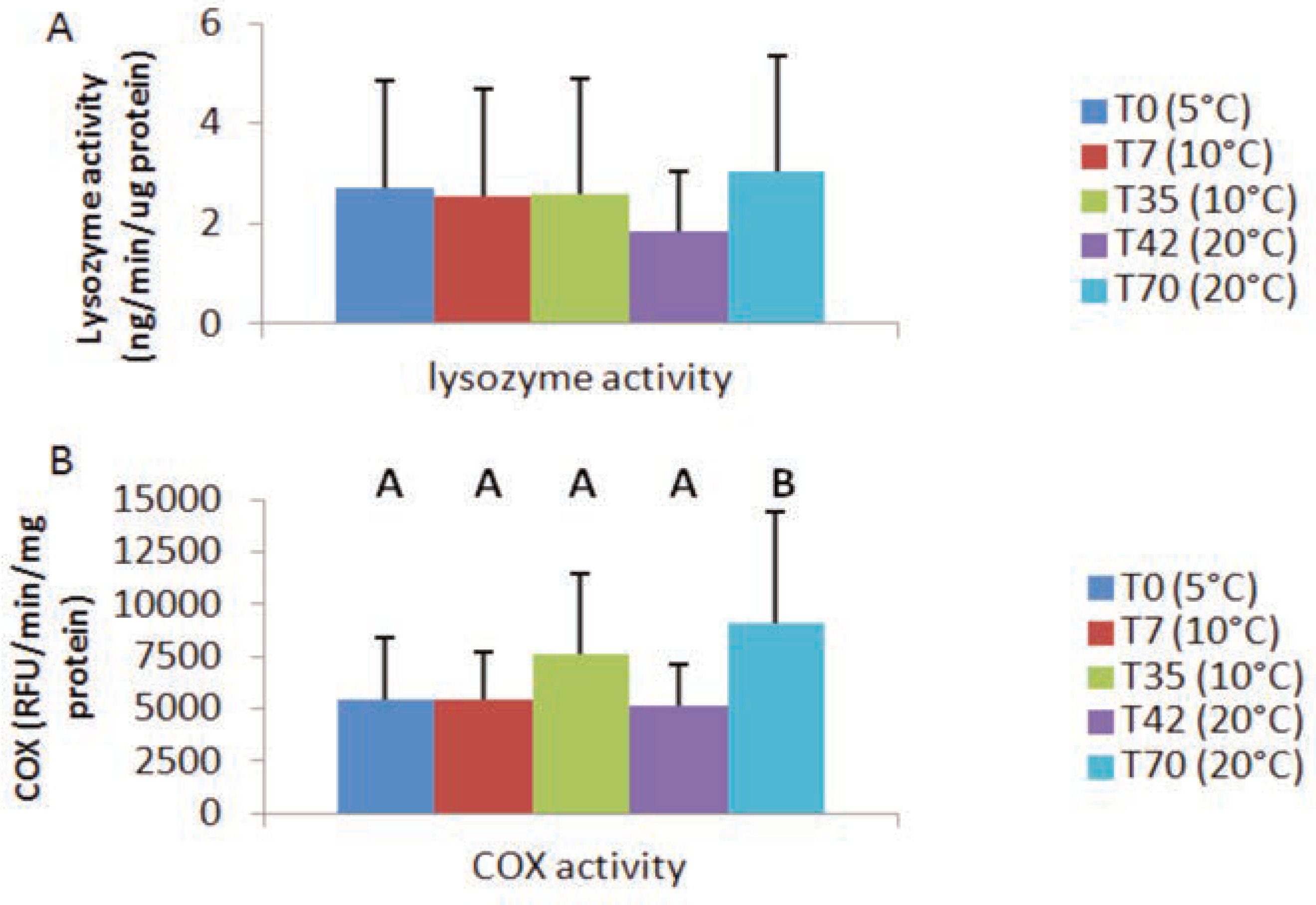
Lysozyme and cyclooxygenase activities
Discussion
Research highlights
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bussell, J.A.; Gidman, E.A.; Causton, D.R.; Bussella, J.A.; Gidmanb, E.A.; Caustonb, D.R.; et al. Changes in the immune response and metabolic fingerprint of the mussel, Mytilus edulis (Linnaeus) in response to lowered salinity and physical stress. J Exp Mar Biol Ecol 2008, 358, 78–85. [Google Scholar] [CrossRef]
- Alix, G.; Beaudry, A.; Brousseau-Fournier, C.; Fortier, M.; Auffret, M.; Fournier, M.; et al. Increase sensitivity to metals of hemocytes obtained from Mya arenaria collected at different distances from the shore. J Xenobiotics 2013, 3, 29–30. [Google Scholar] [CrossRef]
- Matozzo, V.; Chinellato, A.; Munari, M.; Finos, L.; Bressan, M.; Marin, M.G. First evidence of immunomodulation in bivalves under seawater acidification and increased temperature. Plos One 2012, 7, 1–14. [Google Scholar] [CrossRef]
- Mosca, F.; Narcisi, V.; Calzetta, A.; Gioia, L.; Finoia, M.G.; Latini, M.; et al. Effects of high temperature and exposure to air on mussel (Mytilus galloprovincialis, Lmk 1819) hemocyte phagocytosis: modulation of spreading and oxidative response. Tissue Cell 2013, 45, 198203. [Google Scholar] [CrossRef]
- Lemaire, N.; Pellerin, J.; Fournier, M.; Giraultc, L.; Tamigneauxc, E.; Cartiera, S.; et al. Seasonal variations of physiological parameters in the blue mussel Mytilus spp. from farm sites of eastern Quebec. Aquaculture 2006, 261, 729–751. [Google Scholar] [CrossRef]
- Gust, M.; Gélinas, M.; Fortier, M.; Fournier, M.; Gagné, F. In vitro immunotoxicity of environmentally representative antibiotics to the freshwater mussel Elliptio complanata. Environ Pollut 2012, 169, 50–58. [Google Scholar] [CrossRef]
- Mydlarz, L.D.; Jones, L.E.; Harvell, C.D. Innate immunity, environmental drivers, and disease ecology of marine and freshwater invertebrates. Annu Rev Ecol Syst 2006, 37, 251–288. [Google Scholar] [CrossRef]
- Nilsen, I.W.; Overbø, K.; Sandsdalen, E.; Sandaker, E.; Sletten, K.; Myrnes, B. Protein purification and gene isolation of chlamysin, a cold-active lysozyme-like enzyme with antibacterial activity. FEBS Lett 1999, 464, 153–158. [Google Scholar] [CrossRef]
- Lee, Y.C.; Yang, D. Determination of lysosome activities in a microplate format. Anal Biochem 2002, 310, 223–224. [Google Scholar] [CrossRef]
- Gagné, F.; Douville, M.; Fortier, M.; Fournier, M. Effects of a municipal effluent on freshwater mussel Elliptio complanata following challenge with Vibrio anguillarum. J Environ Sci 2015, 37, 91–99. [Google Scholar]
- Rowley, A.F.; Vogan, C.L.; Taylor, G.W.; Clare, A.S. Prostaglandins in non-insectan invertebrates: recent insights and unsolved problems. J Exp Biol 2005, 208, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Auffret, M. Bivalves as models for marine immunotoxicology. In Investigative immunotoxicology; Tryphonas, H., Fournier, M., Blakley, B.R., Smits, J.E.G., Brousseau, P., Eds.; Taylor and Francis: New York, NY, USA, 2005; pp. 29–48. [Google Scholar]
- Gagné, F.; Blaise, C.; Pellerin, J.; André, C. Neuroendocrine disruption in Mya arenaria clams during gametogenesis at sites under pollution stress. Mar Environ Res 2007, 64, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Seed, R. Ecology. In Marine mussels: their ecology and physiology; Bayne, B.L., Ed.; Cambridge University Press: New York, NY, USA, 1976; pp. 13–66. [Google Scholar]
- Maugmyint, U.; Tyler, P.A. Effects of temperature, nutritive and metal stressors on the reproductive biology of Mytilus edulis. Mar Biol 1982, 67, 209–223. [Google Scholar] [CrossRef]
- Thompson, R.J. The reproductive cycle and physiological ecology of the mussel Mytilus edulis in a subarctic, non-estuarine environment. Mar Biol 1984, 79, 277–288. [Google Scholar] [CrossRef]
- Cartier, S.; Pellerin, J.; Fournier, M.; Tamigneauxc, E.; Giraultc, L.; Lemairea, N. Use of an index based on the mussel (Mytilus edulis and Mytilus trossulus) digestive gland weight to assess the nutritional quality of mussel farm sites. Aquaculture 2004, 241, 633–654. [Google Scholar] [CrossRef]
- Monari, M.; Matozzo, V.; Foschi, J.; Cattani, O.; Serrazanetti, G.P.; Marin, M.G. Effects of high temperatures on functional responses of haemocytes in the clam Chamelea gallina. Fish Shellfish Immunol 2007, 22, 98–114. [Google Scholar] [CrossRef]
- Carballal, M.J.; Lopez, C.; Azevedo, C.; Villalba, A. In vitro study of phagocytic ability of Mytilus galloprovincialis hemocytes. Fish Shellfish Immunol 1997, 7, 403–416. [Google Scholar] [CrossRef]
- Li, H.; Parisi, M.G.; Toubiana, M.; Cammarata, M.; Roch, P. Lysozyme gene expression and hemocyte behavious in the Mediterranean mussel, Mytilus galloprovincialis, after injection of various bacteria or temperature stresses. Fish Shellfish Immunol 2008, 25, 143–152. [Google Scholar] [CrossRef]
- Gagnaire, B.; Frouin, H.; Moreau, K.; ThomasGuyon, H.; Renault, T. Effects of temperature and salinity on haemocyte activities of the Pacific oyster Crassostrea gigas (Thunberg). Fish Shellfish Immunol 2006, 20, 536–547. [Google Scholar] [CrossRef]
- Hernroth, B. The influence of temperature and dose on antibacterial peptide response against lipopolysaccharide in the blue mussel, Mytilus edulis. Fish Shellfish Immunol 2003, 14, 25–37. [Google Scholar] [CrossRef]
- Parry, H.E.; Pipe, R.K. Interactive effects of temperature and copper on immunocompetence and disease susceptibility in mussels (Mytilus edulis). Aquat Tox 2004, 69, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, Y.; Suarez, P.; Alonso, A.; Longo, E.; Villaverde, A.; San Juan, F. Environmental quality of mussel farms in Vigo estuary: pollution by PAHs, origin and effects on reproduction. Environ Pollut 2011, 159, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, J.; Fournier, M.; Gauthier-Clerc, S.; Blaise, C.; Garnerot, F.; Amiard, J.C.; Gagné, F. Qu’en est-il de l’état de santé des myes au Saguenay? Un bilan d’études sur plus d’une décennie. Rev Sci Eau 2009, 22, 271–289. [Google Scholar] [CrossRef][Green Version]
- Fujimoto, Y.; Sakuma, S.; Inoue, T.; Uno, E.; Fujita, T. The endocrine disruptor nonylphenol preferentially blocks cyclooxygenase-1. Life Sci 2002, 70, 2209–2214. [Google Scholar] [CrossRef]
- Gagné, F.; Blaise, C.; Pellerin, J.; Fournier, M.; Durand, M.J.; Talbot, A. Relationships between intertidal clam population and health status of the soft-shell clam Mya arenaria in the St. Lawrence Estuary and Saguenay Fjord (Québec, Canada). Environ Int 2008, 34, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M.; Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Clim Res 2008, 37, 181–201. [Google Scholar] [CrossRef]
- Fisher, S.W.; Tamplin, M. Environmental influence on activities and foreign-particles binding by hemocytes of American oysters, Crassostrea virginica. Can J Fish Aquat Sci 1988, 45, 1309–1315. [Google Scholar] [CrossRef]
- Fraser, M.; Rault, P.H.; Fortier, M.; Fortier, M.; André, C.; Brousseau, P.; et al. Decrease in phagocytosis capacity of hemocyte during spawning in Mytilus edulis: a pilot study. J Xenobiotics 2013, 3, 31–33. [Google Scholar] [CrossRef][Green Version]
 ; Stage 2 (development) =
; Stage 2 (development) =  ; Stage 3 (ripe) =
; Stage 3 (ripe) =  ; Stage 4 (mature) =
; Stage 4 (mature) =  ; Stage 5 (post-spawn) =
; Stage 5 (post-spawn) =  ].
].
 ; Stage 2 (development) =
; Stage 2 (development) =  ; Stage 3 (ripe) =
; Stage 3 (ripe) =  ; Stage 4 (mature) =
; Stage 4 (mature) =  ; Stage 5 (post-spawn) =
; Stage 5 (post-spawn) =  ].
].

© Copyright A. Beaudry et al., 2016 Licensee PAGEPress, Italy. This work is licensed under a Creative Commons Attribution NonCommercial 4.0 License (CC BY-NC 4.0).
Share and Cite
Beaudry, A.; Fortier, M.; Masson, S.; Auffret, M.; Brousseau, P.; Fournier, M. Effect of Temperature on Immunocompetence of the Blue Mussel (Mytilus edulis). J. Xenobiot. 2016, 6, 5889. https://doi.org/10.4081/xeno.2016.5889
Beaudry A, Fortier M, Masson S, Auffret M, Brousseau P, Fournier M. Effect of Temperature on Immunocompetence of the Blue Mussel (Mytilus edulis). Journal of Xenobiotics. 2016; 6(1):5889. https://doi.org/10.4081/xeno.2016.5889
Chicago/Turabian StyleBeaudry, Alexandre, Marlène Fortier, Stéphane Masson, Michel Auffret, Pauline Brousseau, and Michel Fournier. 2016. "Effect of Temperature on Immunocompetence of the Blue Mussel (Mytilus edulis)" Journal of Xenobiotics 6, no. 1: 5889. https://doi.org/10.4081/xeno.2016.5889
APA StyleBeaudry, A., Fortier, M., Masson, S., Auffret, M., Brousseau, P., & Fournier, M. (2016). Effect of Temperature on Immunocompetence of the Blue Mussel (Mytilus edulis). Journal of Xenobiotics, 6(1), 5889. https://doi.org/10.4081/xeno.2016.5889



