Temporal Trends, Multiple Residue Incidence, and Chronic Health Risk of Pesticides in Egyptian Onions: A Four-Year Market Surveillance
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Chemicals, and Standards
2.2. Standard Solution Preparation
2.3. Gathering of Samples
2.4. Preparation of Samples
2.5. Chromatographic Analysis
2.5.1. LC-MS/MS Analysis
2.5.2. GC-MS/MS Analysis
2.6. Method Validation
2.7. Quality Control and Quality Assurance
2.8. Evaluation of Potential Health Hazards Associated with Onions Contaminated with Pesticides
3. Results and Discussion
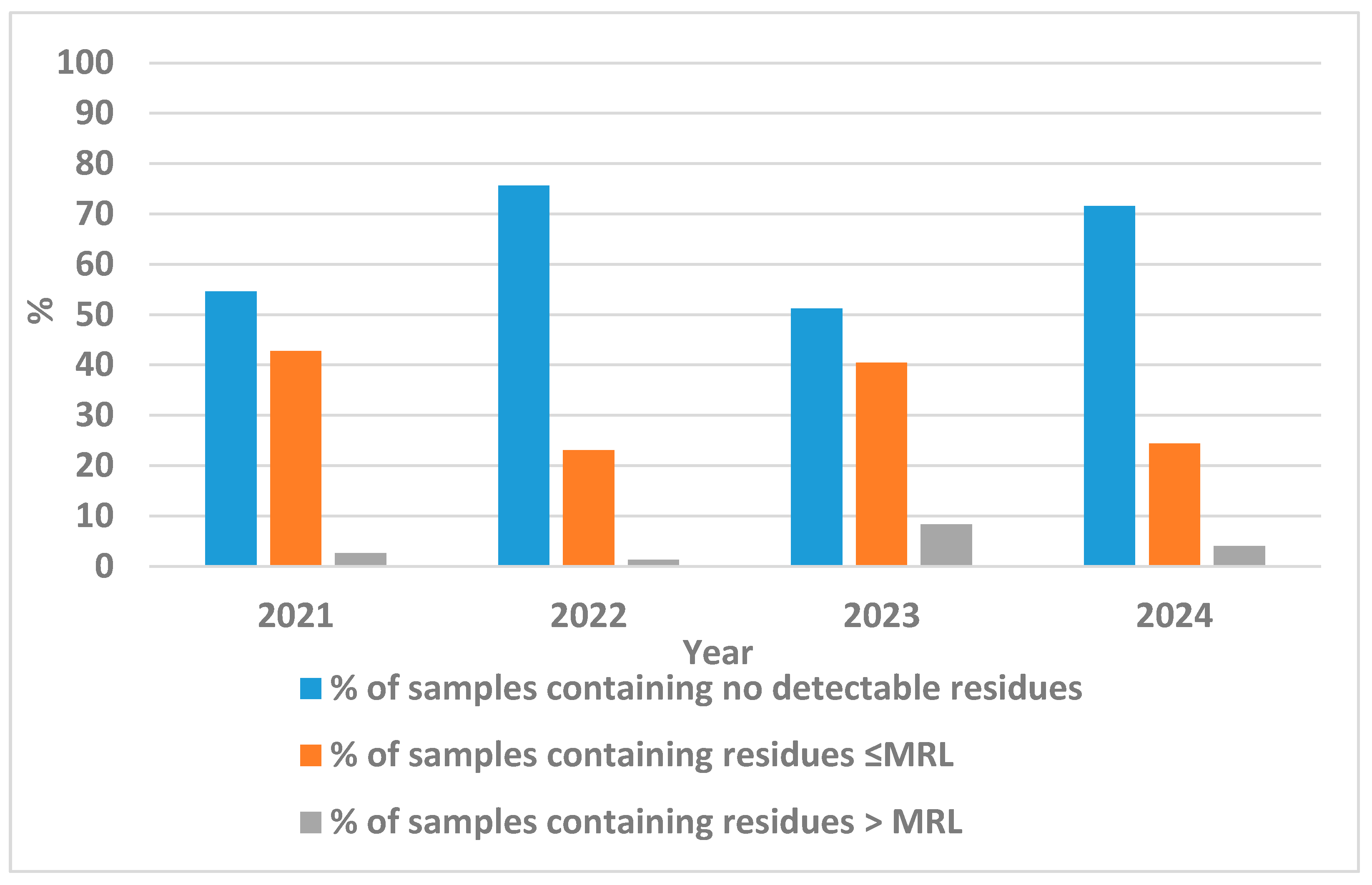
3.1. Determination of Pesticide Residues in Onion Samples and Evaluation of Adherence to EU MRLs
3.2. The Ten Pesticides Most Commonly Detected in Onion Samples
3.3. Incidence of Single and Multiple Residue Detections in Onion Samples
3.4. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoica, F.; Rațu, R.N.; Veleșcu, I.D.; Stănciuc, N.; Râpeanu, G. A comprehensive review on bioactive compounds, health benefits, and potential food applications of onion (Allium cepa L.) skin waste. Trends Food Sci. Technol. 2023, 141, 104173. [Google Scholar] [CrossRef]
- Gupta, A.J.; Kaldate, S.; Volaguthala, S.; Mahajan, V. Onion nutritional and nutraceutical composition and therapeutic potential of its phytochemicals assessed through preclinical and clinical studies. J. Funct. Foods 2025, 129, 106889. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 30 September 2025).
- Ahmed, M.F.A.; Amin, M.M.; Roshdy, F.H. Impact of eco-friendly treatments on managing onion downy mildew. J. Plant Food Sci. 2024, 2, 142–148. [Google Scholar] [CrossRef]
- Khalifa, M.; Mahmoud, N.; Abou-Zeid, N. Management of onion bulb rots during storage using pre-and post-harvest control treatments. Egypt. J. Phytopathol. 2016, 44, 1–16. [Google Scholar] [CrossRef]
- Eissa, F.; Zidan, N.E.H.; Sebaei, A.S.; Mohamed, M.E.B. Pesticide residues in fruits and vegetables: Analysis and risk assessment of EU RASFF notifications between 1999 and 2022. J. Food Compos. Anal. 2024, 134, 106556. [Google Scholar] [CrossRef]
- Malhat, F.; Abdel-Megeed, M.; Saber, E.S.; Shokr, S.A.S.; Eissa, F. Assessment of pesticide residue patterns and multiple residue occurrence in Egyptian medicinal and aromatic plants: A comprehensive market screening. Int. J. Environ. Anal. Chem. 2025, 1–16. [Google Scholar] [CrossRef]
- Bai, A.; Chen, A.; Chen, W.; Luo, X.; Liu, S.; Zhang, M.; Liu, Y.; Zhang, D. Study on degradation behaviour, residue distribution, and dietary risk assessment of propiconazole in celery and onion under field application. J. Sci. Food Agric. 2021, 101, 1998–2005. [Google Scholar] [CrossRef]
- Chu, N.; Shu, X.; Meng, X.; Zhang, X.; Yang, J.; Li, B. Determination and dietary exposure assessment of 79 pesticide residues in Chinese onion (Allium fistulosum L.). CyTA-J. Food 2023, 21, 41–48. [Google Scholar] [CrossRef]
- Guan, W.; Li, C.; Liu, X.; Zhou, S.; Ma, Y. Graphene as dispersive solid-phase extraction materials for pesticides LC-MS/MS multi-residue analysis in leek, onion and garlic. Food Addit. Contam. Part A 2014, 31, 250–261. [Google Scholar] [CrossRef]
- Ahn, J.W.; Jeon, Y.H.; Hwang, J.I.; Kim, H.Y.; Kim, J.H.; Chung, D.H.; Kim, J.E. Monitoring of pesticide residues and risk assessment for fruit vegetables and root vegetables of environment-friendly certified and general agricultural products. Korean J. Environ. Agric. 2012, 31, 164–169. [Google Scholar] [CrossRef][Green Version]
- Gordan, H.; Mahdavi, V.; Behbahan, A.K. Pesticides residue analysis and associated human health risk assessment in tomato and onion. Environ. Monit. Assess. 2025, 197, 718. [Google Scholar] [CrossRef]
- Chau, N.D.G.; Van Hop, N.; Long, H.T.; Duyen, N.T.M.; Raber, G. Multi-residue analytical method for trace detection of new-generation pesticides in vegetables using gas chromatography–tandem mass spectrometry. J. Environ. Sci. Health Part B 2020, 55, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Horská, T.; Kocourek, F.; Stará, J.; Holý, K.; Mráz, P.; Krátký, F.; Kocourek, V.; Hajšlová, J. Evaluation of pesticide residue dynamics in lettuce, onion, leek, carrot and parsley. Foods 2020, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Veiga-del-Baño, J.M.; Oliva, J.; Cámara, M.Á.; Andreo-Martínez, P.; Motas, M. Quick Monitoring of Tomato and Onion Samples During Routine Regulatory Analysis of Pesticide Residues. Arch. Environ. Contam. Toxicol. 2025, 89, 23–33. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al Sallagi, M.S.; Dhanasekaran, D.K.; Odeh, W.A.B.; Al Ali, H.J.; Al Ali, A.A.; Radwan, H.; Obaid, R.S.; Holley, R. Pesticide residues in fresh vegetables imported into the United Arab Emirates. Food Control 2022, 133, 108663. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Abdel-Hamid, M.M.; Altorgoman, M.M.; AlGaramah, H.A.; Alawi, M.A.; Shati, A.A.; Shweeta, H.A.; Awwad, N.S. Evaluation of pesticide residues in vegetables from the Asir Region, Saudi Arabia. Molecules 2020, 25, 205. [Google Scholar] [CrossRef]
- Skovgaard, M.; Renjel Encinas, S.; Jensen, O.C.; Andersen, J.H.; Condarco, G.; Jørs, E. Pesticide residues in commercial lettuce, onion, and potato samples from Bolivia—A threat to public health? Environ. Health Insights 2017, 11, 1178630217704194. [Google Scholar] [CrossRef]
- Tadesse, B.; Asrat, M.; Zewge, B.; Tesfaw, A. Quantitative Analysis of Pesticides in Vegetables: A Study on Tomatoes and Onions from Ethiopian Farms. Meas. Food 2025, 19, 100242. [Google Scholar] [CrossRef]
- Jardim, A.N.O.; Caldas, E.D. Pesticide residues in food of plant origin commercialized in Brazil from 2010 to 2020—An update from the two national monitoring programs. Food Control 2024, 165, 110674. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Marchese, E.; Medina Pastor, P. The 2022 European Union report on pesticide residues in food. EFSA J. 2024, 22, e8753. [Google Scholar] [CrossRef]
- Malhat, F.; Saber, A.; Saber, E.S.; Shokr, S.A.S.; Abdel-Megeed, M. Nationwide surveillance and cumulative risk assessment of pesticide residues in Egyptian vegetables: Results from 2018 to 2021. Separations 2024, 11, 318. [Google Scholar] [CrossRef]
- Malhat, F.; Saber, E.S.; Shokr, S.A.S.; Eissa, F. Pesticide residues in Egyptian vegetables: A comprehensive analysis of compliance, co-occurrence of multiple residues, and health risk assessment. J. Food Compos. Anal. 2025, 143, 107634. [Google Scholar] [CrossRef]
- El-Sheikh, E.S.A.; Ramadan, M.M.; El-Sobki, A.E.; Shalaby, A.A.; McCoy, M.R.; Hamed, I.A.; Ashour, M.B.; Hammock, B.D. Pesticide residues in vegetables and fruits from farmer markets and associated dietary risks. Molecules 2022, 27, 8072. [Google Scholar] [CrossRef] [PubMed]
- CAC. Codex Alimentarius Commission Joint FAO/WHO Food Standards Program. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 30 September 2025).
- EN 15662: 2018; Foods of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE. Modular QuEChERS-Method. BSI Standards Publication: London, UK, 2018.
- Pihlström, T.; Fernández-Alba, A.R.; Amate, C.F.; Poulsen, M.E.; Hardebusch, B.; Anastassiades, M.; Lippold, R.; Cabrera, L.C.; de Kok, A.; ORegan, F. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed SANTE 11312/2021. Sante 2021, 11312. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 30 September 2025).
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories (3rd ed.). International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/files/live/sites/isoorg/files/store/en/PUB100424.pdf (accessed on 10 September 2025).
- European Food Safety Authority (EFSA); Anastassiadou, M.; Brancato, A.; Carrasco Cabrera, L.; Ferreira, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; et al. Pesticide Residue Intake Model-EFSA PRIMo revision 3.1: (update of EFSA PRIMo revision 3). EFSA Supporting Publ. 2019, 16, 1605E. [Google Scholar] [CrossRef]
- Malhat, F.; Saber, E.S.; Shokr, S.A.S.; Eissa, F. Pesticide residues in Egyptian strawberries over a four-year period: Occurrence, adherence to maximum residue limits, co-occurrence, and effects on consumer health. J. Food Compos. Anal. 2025, 146, 107978. [Google Scholar] [CrossRef]
- Malhat, F.; Saber, E.S.; Shokr, S.A.S.; Eissa, F. Pesticide residue profiles and health risk assessment of domestically grown and imported peaches in Egyptian markets. J. Food Compos. Anal. 2025, 147, 108004. [Google Scholar] [CrossRef]
- Zidan, N.A.; Abdel-Halim, K.Y.; Eissa, F. Climate Change Impacts on Pesticide Use and Fate in the Environment. In Climate Change Impacts on Toxins and Health Effects; Saad-Hussein, A., El-Tawil, O., El-Sheikh, E.S.A., Eds.; Springer: Singapore, 2025; pp. 149–170. [Google Scholar] [CrossRef]
- Khaled, O.; Ryad, L.; Eissa, F. Determination of tetracycline residues in potatoes and soil by LC-MS/MS: Method development, validation, and risk assessment. Food Chem. 2024, 461, 140841. [Google Scholar] [CrossRef]
- Gamal, A.; Abdel-Megeed, M.; Mahmoud, H.; Soliman, M.; Al-Anany, M.; Eissa, F. Pesticide residues in green, roasted, and capsule coffee from the Egyptian market: Occurrence, processing effects, and health risk assessment. Food Chem. 2025, 486, 144671. [Google Scholar] [CrossRef]

| 2021 (n = 262) | 2022 (n = 2458) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pesticide | Frequency% | Mean (mg/kg) | Range (Min.–Max.) | EU MRL (mg/kg) | Pesticide | Frequency% | Mean (mg/kg) | Range (Min.–Max.) | EU MRL (mg/kg) |
| Carbendazim | 20.99 | 0.006 | <LOQ–0.025 | 0.10 | Carbendazim | 10.09 | 0.011 | <LOQ–0.197 | 0.10 |
| Azoxystrobin | 20.99 | 0.005 | <LOQ–0.013 | 10.00 | Metalaxyl | 5.57 | 0.005 | <LOQ–0.025 | 0.03 |
| Metalaxyl | 10.69 | 0.005 | <LOQ–0.027 | 0.03 | Azoxystrobin | 3.86 | 0.006 | <LOQ–0.030 | 10.00 |
| Boscalid | 4.96 | 0.005 | <LOQ–0.009 | 5.00 | Cypermethrin | 2.97 | 0.007 | <LOQ–0.057 | 0.10 |
| Dimethomorph | 2.67 | 0.005 | <LOQ–0.006 | 0.60 | Chlorpyrifos | 2.32 | 0.008 | <LOQ–0.064 | 0.01 |
| Difenoconazole | 2.29 | 0.005 | <LOQ–0.005 | 0.50 | Thiophanate-methyl | 2.24 | 0.020 | <LOQ–0.231 | 0.10 |
| Lambda-Cyhalothrin | 2.29 | 0.005 | <LOQ–0.006 | 0.20 | Difenoconazole | 1.79 | 0.007 | <LOQ–0.049 | 0.50 |
| Imazalil | 2.29 | 0.029 | <LOQ–0.054 | 0.01 | Dimethomorph | 1.51 | 0.008 | <LOQ–0.042 | 0.60 |
| Profenofos | 2.29 | 0.005 | <LOQ–0.005 | 0.02 | Clothianidin | 1.30 | 0.005 | <LOQ–0.026 | 0.01 |
| Clothianidin | 2.29 | 0.005 | <LOQ–0.005 | 0.01 | Chlorpropham | 1.10 | 0.014 | <LOQ–0.049 | 0.01 |
| 2023 (n = 756) | 2024 (n = 2255) | ||||||||
| Pesticide | Frequency% | Mean (mg/kg) | Range (Min.–Max.) | EU MRL (mg/kg) | Pesticide | Frequency% | Mean (mg/kg) | Range (Min.–Max.) | EU MRL (mg/kg) |
| Azoxystrobin | 18.78 | 0.018 | <LOQ–0.213 | 10.00 | Azoxystrobin | 11.97 | 0.030 | <LOQ–2.204 | 10.00 |
| Metalaxyl | 17.33 | 0.019 | <LOQ–0.277 | 0.03 | Metalaxyl | 8.07 | 0.011 | <LOQ–0.284 | 0.03 |
| Dimethomorph | 16.67 | 0.037 | <LOQ–0.297 | 0.60 | Dimethomorph | 7.23 | 0.046 | <LOQ–1.102 | 0.60 |
| Difenoconazole | 9.26 | 0.011 | <LOQ–0.079 | 0.50 | Difenoconazole | 6.08 | 0.022 | <LOQ–0.559 | 0.50 |
| Chlorpyrifos | 6.08 | 0.030 | <LOQ–0.297 | 0.01 | Profenofos | 3.59 | 0.032 | <LOQ–0.336 | 0.02 |
| Carbendazim | 5.56 | 0.020 | <LOQ–0.281 | 0.10 | Lambda-Cyhalothrin | 2.75 | 0.015 | <LOQ–0.225 | 0.20 |
| Boscalid | 5.42 | 0.020 | <LOQ–0.102 | 5.00 | Carbendazim | 2.35 | 0.011 | <LOQ–0.070 | 0.10 |
| Cypermethrin | 5.29 | 0.022 | <LOQ–0.183 | 0.10 | Chlorpropham | 2.17 | 0.021 | <LOQ–0.125 | 0.01 |
| Lambda-Cyhalothrin | 4.89 | 0.024 | <LOQ–0.205 | 0.20 | Cypermethrin | 1.95 | 0.010 | <LOQ–0.064 | 0.10 |
| Imidacloprid | 4.89 | 0.015 | <LOQ–0.089 | 0.01 | Imidacloprid | 1.95 | 0.040 | <LOQ–0.304 | 0.01 |
| Year | 2021 | 2022 | 2023 | 2024 |
|---|---|---|---|---|
| Multiple Pesticides | % | % | % | % |
| 9 pesticide residues | 0.00 | 0.04 | 0.40 | 0.09 |
| 8 pesticide residues | 0.00 | 0.04 | 0.40 | 0.13 |
| 7 pesticide residues | 0.38 | 0.00 | 1.06 | 0.40 |
| 6 pesticide residues | 0.00 | 0.08 | 2.91 | 0.67 |
| 5 pesticide residues | 1.15 | 0.33 | 3.84 | 1.11 |
| 4 pesticide residues | 4.20 | 0.98 | 4.63 | 1.86 |
| 3 pesticide residues | 6.49 | 2.44 | 6.22 | 3.46 |
| 2 pesticide residues | 10.3 | 5.90 | 10.32 | 6.52 |
| 1 pesticide residue | 22.9 | 14.7 | 17.9 | 14.1 |
| Pesticide (ADI) | MS Diet | GEMS/Food G06 | Romania General | GEMS/Food G10 | GEMS/Food G15 | GEMS/Food G08 | Sweden General | Finland 3-Year-Old Children | GEMS/Food G07 | Portugal General |
|---|---|---|---|---|---|---|---|---|---|---|
| Azoxystrobin (0.2) | IEDI | 2.27 × 10−2 | 2.25 × 10−2 | 1.75 × 10−2 | 1.56 × 10−2 | 1.53 × 10−2 | 1.35 × 10−2 | 1.17 × 10−2 | 1.06 × 10−2 | 1.06 × 10−2 |
| % of ADI | 1.14 × 10−2 | 1.13 × 10−2 | 8.77 × 10−3 | 7.78 × 10−3 | 7.64 × 10−3 | 6.75 × 10−3 | 5.86 × 10−3 | 5.31 × 10−3 | 5.28 × 10−3 | |
| Boscalid (0.04) | IEDI | 1.51 × 10−2 | 1.50 × 10−2 | 1.17 × 10−2 | 1.04 × 10−2 | 1.02 × 10−2 | 9.00 × 10−3 | 7.82 × 10−3 | 7.08 × 10−3 | 7.03 × 10−3 |
| % of ADI | 3.78 × 10−2 | 3.75 × 10−2 | 2.92 × 10−2 | 2.59 × 10−2 | 2.55 × 10−2 | 2.25 × 10−2 | 1.95 × 10−2 | 1.77 × 10−2 | 1.76 × 10−2 | |
| Carbendazim (0.02) | IEDI | 1.51 × 10−2 | 1.50 × 10−2 | 1.17 × 10−2 | 1.04 × 10−2 | 1.02 × 10−2 | 9.00 × 10−3 | 7.82 × 10−3 | 7.08 × 10−3 | 7.03 × 10−3 |
| % of ADI | 7.57 × 10−2 | 7.50 × 10−2 | 5.85 × 10−2 | 5.18 × 10−2 | 5.10 × 10−2 | 4.50 × 10−2 | 3.91 × 10−2 | 3.54 × 10−2 | 3.52 × 10−2 | |
| Chlorpropham (0.05) | IEDI | 1.59 × 10−2 | 1.58 × 10−2 | 1.23 × 10−2 | 1.09 × 10−2 | 1.07 × 10−2 | 9.45 × 10−3 | 8.21 × 10−3 | 7.43 × 10−3 | 7.39 × 10−3 |
| % of ADI | 3.18 × 10−2 | 3.15 × 10−2 | 2.46 × 10−2 | 2.18 × 10−2 | 2.14 × 10−2 | 1.89 × 10−2 | 1.64 × 10−2 | 1.49 × 10−2 | 1.48 × 10−2 | |
| Chlorpyrifos (0.001) | IEDI | 2.27 × 10−2 | 2.25 × 10−2 | 1.75 × 10−2 | 1.56 × 10−2 | 1.53 × 10−2 | 1.35 × 10−2 | 1.17 × 10−2 | 1.06 × 10−2 | 1.06 × 10−2 |
| % of ADI | 2.27 × 100 | 2.25× 100 | 1.75× 100 | 1.56× 100 | 1.53× 100 | 1.35× 100 | 1.17× 100 | 1.06× 100 | 1.06× 100 | |
| Clothianidin (0.097) | IEDI | 3.78 × 10−3 | 3.75 × 10−3 | 2.92 × 10−3 | 2.59 × 10−3 | 2.55 × 10−3 | 2.25 × 10−3 | 1.95 × 10−3 | 1.77 × 10−3 | 1.76 × 10−3 |
| % of ADI | 3.90 × 10−3 | 3.87 × 10−3 | 3.01 × 10−3 | 2.67 × 10−3 | 2.63 × 10−3 | 2.32 × 10−3 | 2.01 × 10−3 | 1.82 × 10−3 | 1.81 × 10−3 | |
| Cypermethrin (0.005) | IEDI | 1.67 × 10−2 | 1.65 × 10−2 | 1.29 × 10−2 | 1.14 × 10−2 | 1.12 × 10−2 | 9.90 × 10−3 | 8.60 × 10−3 | 7.79 × 10−3 | 7.74 × 10−3 |
| % of ADI | 3.33 × 10−1 | 3.30 × 10−1 | 2.57 × 10−1 | 2.28 × 10−1 | 2.24 × 10−1 | 1.98 × 10−1 | 1.72 × 10−1 | 1.56 × 10−1 | 1.55 × 10−1 | |
| Difenoconazole (0.01) | IEDI | 1.67 × 10−2 | 1.65 × 10−2 | 1.29 × 10−2 | 1.14 × 10−2 | 1.12 × 10−2 | 9.90 × 10−3 | 8.60 × 10−3 | 7.79 × 10−3 | 7.74 × 10−3 |
| % of ADI | 1.67 × 10−1 | 1.65 × 10−1 | 1.29 × 10−1 | 1.14 × 10−1 | 1.12 × 10−1 | 9.90 × 10−2 | 8.60 × 10−2 | 7.79 × 10−2 | 7.74 × 10−2 | |
| Dimethomorph (0.05) | IEDI | 3.48 × 10−2 | 3.45 × 10−2 | 2.69 × 10−2 | 2.38 × 10−2 | 2.34 × 10−2 | 2.07 × 10−2 | 1.80 × 10−2 | 1.63 × 10−2 | 1.62 × 10−2 |
| % of ADI | 6.96 × 10−2 | 6.90 × 10−2 | 5.38 × 10−2 | 4.77 × 10−2 | 4.69 × 10−2 | 4.14 × 10−2 | 3.60 × 10−2 | 3.26 × 10−2 | 3.24 × 10−2 | |
| Imazalil (0.025) | IEDI | 2.19 × 10−2 | 2.18 × 10−2 | 1.70 × 10−2 | 1.50 × 10−2 | 1.48 × 10−2 | 1.31 × 10−2 | 1.13 × 10−2 | 1.03 × 10−2 | 1.02 × 10−2 |
| % of ADI | 8.78 × 10−2 | 8.70 × 10−2 | 6.78 × 10−2 | 6.01 × 10−2 | 5.91 × 10−2 | 5.22 × 10−2 | 4.53 × 10−2 | 4.11 × 10−2 | 4.08 × 10−2 | |
| Imidacloprid (0.06) | IEDI | 3.03 × 10−2 | 3.00 × 10−2 | 2.34 × 10−2 | 2.07 × 10−2 | 2.04 × 10−2 | 1.80 × 10−2 | 1.56 × 10−2 | 1.42 × 10−2 | 1.41 × 10−2 |
| % of ADI | 5.05 × 10−2 | 5.00 × 10−2 | 3.90 × 10−2 | 3.46 × 10−2 | 3.40 × 10−2 | 3.00 × 10−2 | 2.61 × 10−2 | 2.36 × 10−2 | 2.34 × 10−2 | |
| Lambda-Cyhalothrin (0.0025) | IEDI | 1.82 × 10−2 | 1.80 × 10−2 | 1.40 × 10−2 | 1.24 × 10−2 | 1.22 × 10−2 | 1.08 × 10−2 | 9.38 × 10−3 | 8.50 × 10−3 | 8.44 × 10−3 |
| % of ADI | 7.27 × 10−1 | 7.20 × 10−1 | 5.61 × 10−1 | 4.98 × 10−1 | 4.89 × 10−1 | 4.32 × 10−1 | 3.75 × 10−1 | 3.40 × 10−1 | 3.38 × 10−1 | |
| Metalaxyl (0.08) | IEDI | 1.44 × 10−2 | 1.43 × 10−2 | 1.11 × 10−2 | 9.85 × 10−3 | 9.68 × 10−3 | 8.55 × 10−3 | 7.43 × 10−3 | 6.73 × 10−3 | 6.68 × 10−3 |
| % of ADI | 1.80 × 10−2 | 1.78 × 10−2 | 1.39 × 10−2 | 1.23 × 10−2 | 1.21 × 10−2 | 1.07 × 10−2 | 9.28 × 10−3 | 8.41 × 10−3 | 8.35 × 10−3 | |
| Profenofos (0.03) | IEDI | 2.42 × 10−2 | 2.40 × 10−2 | 1.87 × 10−2 | 1.66 × 10−2 | 1.63 × 10−2 | 1.44 × 10−2 | 1.25 × 10−2 | 1.13 × 10−2 | 1.13 × 10−2 |
| % of ADI | 8.07 × 10−2 | 8.00 × 10−2 | 6.24 × 10−2 | 5.53 × 10−2 | 5.43 × 10−2 | 4.80 × 10−2 | 4.17 × 10−2 | 3.78 × 10−2 | 3.75 × 10−2 | |
| Thiophanate-methyl (0.02) | IEDI | 1.51 × 10−2 | 1.50 × 10−2 | 1.17 × 10−2 | 1.04 × 10−2 | 1.02 × 10−2 | 9.00 × 10−3 | 7.82 × 10−3 | 7.08 × 10−3 | 7.03 × 10−3 |
| % of ADI | 7.57 × 10−2 | 7.50 × 10−2 | 5.85 × 10−2 | 5.18 × 10−2 | 5.10 × 10−2 | 4.50 × 10−2 | 3.91 × 10−2 | 3.54 × 10−2 | 3.52 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malhat, F.; Shokr, S.; Heikal, S.; Zidan, N.E.-H. Temporal Trends, Multiple Residue Incidence, and Chronic Health Risk of Pesticides in Egyptian Onions: A Four-Year Market Surveillance. J. Xenobiot. 2025, 15, 192. https://doi.org/10.3390/jox15060192
Malhat F, Shokr S, Heikal S, Zidan NE-H. Temporal Trends, Multiple Residue Incidence, and Chronic Health Risk of Pesticides in Egyptian Onions: A Four-Year Market Surveillance. Journal of Xenobiotics. 2025; 15(6):192. https://doi.org/10.3390/jox15060192
Chicago/Turabian StyleMalhat, Farag, Shokr Shokr, Sara Heikal, and Nour El-Hoda Zidan. 2025. "Temporal Trends, Multiple Residue Incidence, and Chronic Health Risk of Pesticides in Egyptian Onions: A Four-Year Market Surveillance" Journal of Xenobiotics 15, no. 6: 192. https://doi.org/10.3390/jox15060192
APA StyleMalhat, F., Shokr, S., Heikal, S., & Zidan, N. E.-H. (2025). Temporal Trends, Multiple Residue Incidence, and Chronic Health Risk of Pesticides in Egyptian Onions: A Four-Year Market Surveillance. Journal of Xenobiotics, 15(6), 192. https://doi.org/10.3390/jox15060192








