Surfactant Protein D Mediates the Association Between Smoking and Type 2 Diabetes Mellitus Incidence in the Spanish Adult Population: Di@bet.es Study
Abstract
1. Introduction
2. Materials and Methods
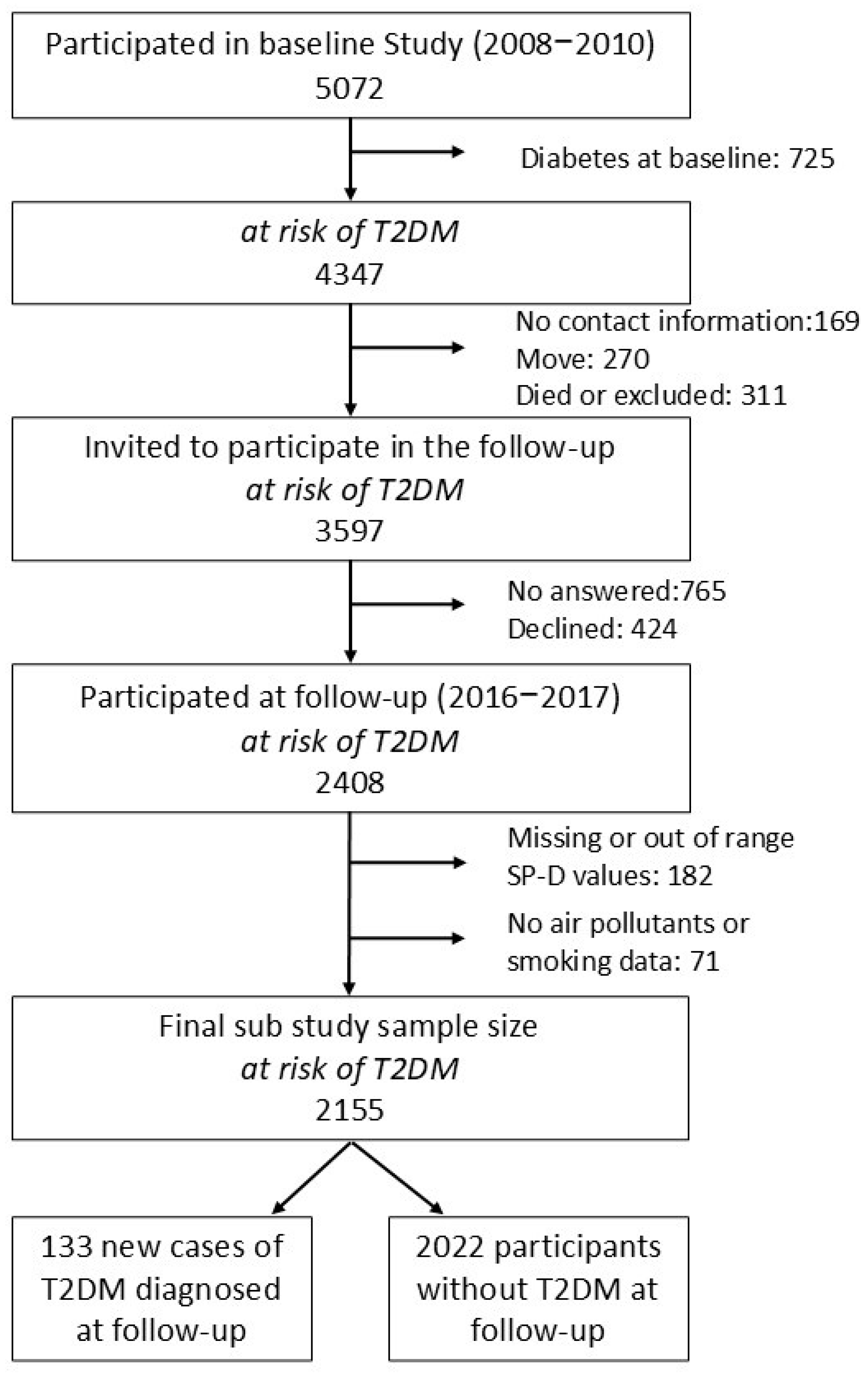
2.1. Study Population
2.2. Variables and Procedures
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Population at Risk of T2DM According to SP-D Categories
3.2. Association Between Serum SP-D Levels and Environmental Exposure Variables
3.3. T2DM Incidence After 7.5 Years According to SP-D Categories
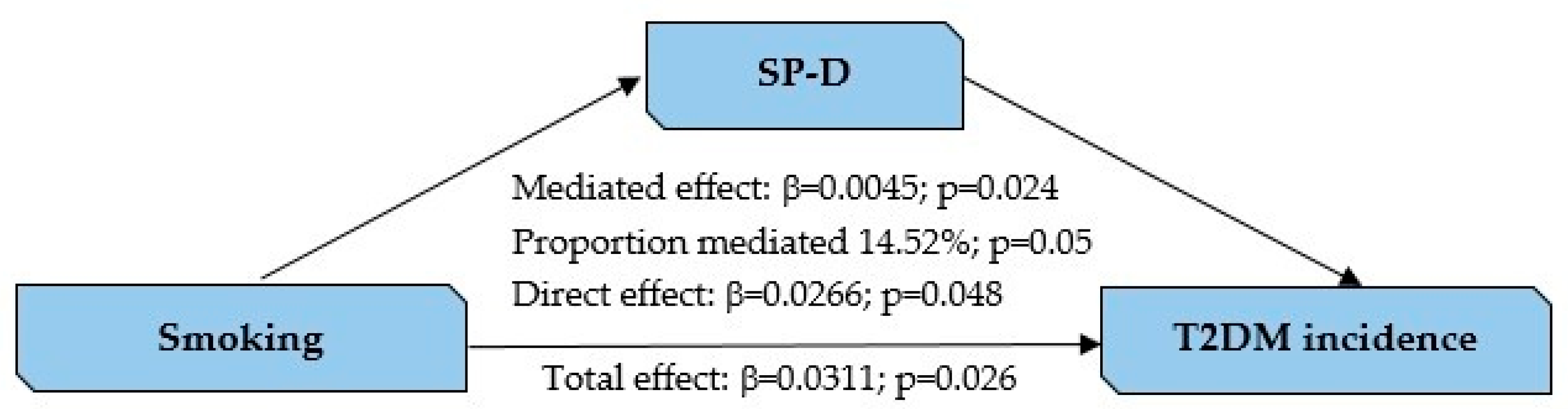
3.4. SP-D as a Mediator Between Smoking and T2DM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 Diabetes Mellitus |
| SP-D | Surfactant Protein D |
| HOMA | Homeostasis Model Assessment |
| BMI | Body Mass Index |
| LDL | Low Density Lipoproteins |
| HDL | High Density Lipoproteins |
| PM10 | Particle Matter < 10µ |
| PM2.5 | Particle Matter < 2.5μ |
| SO2 | Sulfur Dioxide |
| CO | Carbon Monoxide |
| NO2 | Nitrogen Dioxide |
References
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025; Available online: https://Diabetesatlas.Org (accessed on 25 June 2025).
- Smith, K.; Deutsch, A.J.; McGrail, C.; Kim, H.; Hsu, S.; Huerta-Chagoya, A.; Mandla, R.; Schroeder, P.H.; Westerman, K.E.; Szczerbinski, L.; et al. Multi-Ancestry Polygenic Mechanisms of Type 2 Diabetes. Nat. Med. 2024, 30, 1065–1074. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.F.; Chen, J.; Xia, L.; Cao, A.; Zhang, Y.; Wang, J.; Li, H.; Yang, K.; Guo, K.; et al. Combined Lifestyle Factors and Risk of Incident Type 2 Diabetes and Prognosis among Individuals with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Diabetologia 2020, 63, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Martin, S. Environmental/Lifestyle Factors in the Pathogenesis and Prevention of Type 2 Diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; Pinho, M.G.M.; Abreu, T.C.; den Braver, N.R.; Lam, T.M.; Huss, A.; Vlaanderen, J.; Sonnenschein, T.; Siddiqui, N.Z.; Yuan, Z.; et al. Environmental Risk Factors of Type 2 Diabetes—An Exposome Approach. Diabetologia 2022, 65, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, Q.; Li, Y.; He, J. The Function of Xenobiotic Receptors in Metabolic Diseases. Drug Metab. Dispos. 2023, 51, 237–248. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Carcinogenic Components of Tobacco and Tobacco Smoke: A 2022 Update. Food Chem. Toxicol. 2022, 165, 113179. [Google Scholar] [CrossRef]
- Cachon, B.F.; Firmin, S.; Verdin, A.; Ayi-Fanou, L.; Billet, S.; Cazier, F.; Martin, P.J.; Aissi, F.; Courcot, D.; Sanni, A.; et al. Proinflammatory Effects and Oxidative Stress within Human Bronchial Epithelial Cells Exposed to Atmospheric Particulate Matter (PM2.5 and PM>2.5) Collected from Cotonou, Benin. Environ. Pollut. 2014, 185, 340–351. [Google Scholar] [CrossRef]
- Hu, Y.; Naito, S.; Kobayashi, N.; Hasatani, M. CO2, NOx and SO2 Emissions from the Combustion of Coal with High Oxygen Concentration Gases. Fuel 2000, 79, 1925–1932. [Google Scholar] [CrossRef]
- Alberg, A.J.; Shopland, D.R.; Cummings, K.M. The 2014 Surgeon General’s Report: Commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and Updating the Evidence on the Health Consequences of Cigarette Smoking. Am. J. Epidemiol. 2014, 179, 403–412. [Google Scholar] [CrossRef]
- Akter, S.; Goto, A.; Mizoue, T. Smoking and the Risk of Type 2 Diabetes in Japan: A Systematic Review and Meta-Analysis. J. Epidemiol. 2017, 27, 553–561. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, S.; Qian, S.E.; Cai, M.; Li, H.; Wang, C.; Zou, H.; Chen, L.; Vaughn, M.G.; McMillin, S.E.; et al. Ambient Air Pollution Associated with Incidence and Dynamic Progression of Type 2 Diabetes: A Trajectory Analysis of a Population-Based Cohort. BMC Med. 2022, 20, 375. [Google Scholar] [CrossRef]
- Li, Y.L.; Chuang, T.W.; Chang, P.; Lin, L.Y.; Su, C.T.; Chien, L.N.; Chiou, H.Y. Long-Term Exposure to Ozone and Sulfur Dioxide Increases the Incidence of Type 2 Diabetes Mellitus among Aged 30 to 50 Adult Population. Environ. Res. 2021, 194, 110624. [Google Scholar] [CrossRef]
- Shan, A.; Zhang, Y.; Zhang, L.; Chen, X.; Li, X.; Wu, H.; Yan, M.; Li, Y.; Xian, P.; Ma, Z.; et al. Associations between the Incidence and Mortality Rates of Type 2 Diabetes Mellitus and Long-Term Exposure to Ambient Air Pollution: A 12-Year Cohort Study in Northern China. Environ. Res. 2020, 186, 109551. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, H.; Zhang, C.; Yuan, S.; Zhao, S.; Han, C. Association of PM2.5 with Hypertension, Type 2 Diabetes, and Multimorbidity: Evidence from the China Migrants Dynamic Survey of 165,075 Individuals. Int. J. Environ. Health Res. 2025, 1–14. [Google Scholar] [CrossRef]
- Yang, B.Y.; Fan, S.; Thiering, E.; Seissler, J.; Nowak, D.; Dong, G.H.; Heinrich, J. Ambient Air Pollution and Diabetes: A Systematic Review and Meta-Analysis. Environ. Res. 2020, 180, 108817. [Google Scholar] [CrossRef]
- Parasin, N.; Amnuaylojaroen, T.; Saokaew, S.; Sittichai, N.; Tabkhan, N.; Dilokthornsakul, P. Outdoor Air Pollution Exposure and the Risk of Type 2 Diabetes Mellitus: A Systematic Umbrella Review and Meta-Analysis. Environ. Res. 2025, 269, 120885. [Google Scholar] [CrossRef]
- Wang, B.; Xu, D.; Jing, Z.; Liu, D.; Yan, S.; Wang, Y. Effect of Long-Term Exposure to Air Pollution on Type 2 Diabetes Mellitus Risk: A Systemic Review and Meta-Analysis of Cohort Studies. Eur. J. Endocrinol. 2014, 171, R173–R182. [Google Scholar] [CrossRef]
- Sorensen, G.L. Surfactant Protein D in Respiratory and Non-Respiratory Diseases. Front. Med. 2018, 5, 18. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Valdés, S.; Manco, M.; Chico, B.; Botas, P.; Campo, A.; Casamitjana, R.; Delgado, E.; Salvador, J.; Fruhbeck, G.; et al. Surfactant Protein d, a Marker of Lung Innate Immunity, Is Positively Associated with Insulin Sensitivity. Diabetes Care 2010, 33, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Mohamed, M.; Altaf, B.; Ghazali, W.S.W. Surfactant Protein-D, Diabetes Mellitus, Oxidative Stress, Infections and Inflammation on the Crossroad: A Systematic Review. J. Pak. Med. Assoc. 2024, 74, 534–543. [Google Scholar] [CrossRef]
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of Diabetes Mellitus and Impaired Glucose Regulation in Spain: The Di@bet.Es Study. Diabetologia 2011, 55, 88. [Google Scholar] [CrossRef]
- Rojo-Martínez, G.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia, I.; Pérez, V.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Incidence of Diabetes Mellitus in Spain as Results of the Nation-Wide Cohort Di@bet.Es Study. Sci. Rep. 2020, 10, 2765. [Google Scholar] [CrossRef]
- WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Participants–WMA–The World Medical Association. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ (accessed on 9 June 2025).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Menut, L.; Bessagnet, B.; Khvorostyanov, D.; Beekmann, M.; Blond, N.; Colette, A.; Coll, I.; Curci, G.; Foret, G.; Hodzic, A.; et al. CHIMERE 2013: A Model for Regional Atmospheric Composition Modelling. Geosci. Model Dev. 2013, 6, 981–1028. [Google Scholar] [CrossRef]
- Vivanco, M.G.; Palomino, I.; Vautard, R.; Bessagnet, B.; Martín, F.; Menut, L.; Jiménez, S. Multi-Year Assessment of Photochemical Air Quality Simulation over Spain. Environ. Model. Softw. 2009, 24, 63–73. [Google Scholar] [CrossRef]
- Vivanco, M.G.; Azula, O.; Palomino, I.; Martín, F. Evaluating the Impact of Resolution on the Predictions of an Air Quality Model over Madrid Area (Spain). Stud. Comput. Intell. 2011, 348, 145–162. [Google Scholar] [CrossRef]
- Martín, F.; Palomino, I.; Vivanco, M.G. Combination of Measured and Modelling Data in Air Quality Assessment in Spain. Int. J. Environ. Pollut. 2012, 49, 36–44. [Google Scholar] [CrossRef]
- Committee, A.D.A.P.P.; ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Ebekozien, O.; Echouffo-Tcheugui, J.B.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Sorensen, G.L.; Husby, S.; Holmskov, U. Surfactant Protein A and Surfactant Protein D Variation in Pulmonary Disease. Immunobiology 2007, 212, 381–416. [Google Scholar] [CrossRef]
- Sorensen, G.L.; Hjelmborg, J.V.B.; Leth-Larsen, R.; Schmidt, V.; Fenger, M.; Poulain, F.; Hawgood, S.; Sørensen, T.I.A.; Kyvik, K.O.; Holmskov, U. Surfactant Protein D of the Innate Immune Defence Is Inversely Associated with Human Obesity and SP-D Deficiency Infers Increased Body Weight in Mice. Scand. J. Immunol. 2006, 64, 633–638. [Google Scholar] [CrossRef]
- Leth-Larsen, R.; Nordenbaek, C.; Tornoe, I.; Moeller, V.; Schlosser, A.; Koch, C.; Teisner, B.; Junker, P.; Holmskov, U. Surfactant Protein D (SP-D) Serum Levels in Patients with Community-Acquired Pneumonia. Clin. Immunol. 2003, 108, 29–37. [Google Scholar] [CrossRef]
- López-Cano, C.; Lecube, A.; García-Ramírez, M.; Muñoz, X.; Sánchez, E.; Seminario, A.; Hernández, M.; Ciudin, A.; Gutiérrez, L.; Hernández, C.; et al. Serum Surfactant Protein D as a Biomarker for Measuring Lung Involvement in Obese Patients With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2017, 102, 4109–4116. [Google Scholar] [CrossRef]
- Akasaka, H.; Ohnishi, H.; Narita, Y.; Kameda, M.; Miki, T.; Takahashi, H.; Yamamoto, W.; Sohma, H.; Masumori, N.; Miura, T.; et al. The Serum Level of KL-6 Is Associated with the Risk of Insulin Resistance and New-Onset Diabetes Mellitus: The Tanno-Sobetsu Study. Intern. Med. 2017, 56, 3009–3018. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wada, J.; Makino, H. Innate Immunity in Diabetes and Diabetic Nephropathy. Nat. Rev. Nephrol. 2016, 12, 13–26. [Google Scholar] [CrossRef]
- Barrow, A.D.; Palarasah, Y.; Bugatti, M.; Holehouse, A.S.; Byers, D.E.; Holtzman, M.J.; Vermi, W.; Skjødt, K.; Crouch, E.; Colonna, M. OSCAR Is a Receptor for Surfactant Protein D That Activates TNF-α Release from Human CCR2+ Inflammatory Monocytes. J. Immunol. 2015, 194, 3317. [Google Scholar] [CrossRef]
- Madsen, J.; Kliem, A.; Tornøe, I.; Skjødt, K.; Koch, C.; Holmskov, U. Localization of Lung Surfactant Protein D on Mucosal Surfaces in Human Tissues. J. Immunol. 2000, 164, 5866–5870. [Google Scholar] [CrossRef]
- Stahlman, M.T.; Gray, M.E.; Hull, W.M.; Whitsett, J.A. Immunolocalization of Surfactant Protein-D (SP-D) in Human Fetal, Newborn, and Adult Tissues. J. Histochem. Cytochem. 2002, 50, 651–660. [Google Scholar] [CrossRef]
- Mo, Y.K.; Kankavi, O.; Masci, P.P.; Mellick, G.D.; Whitehouse, M.W.; Boyle, G.M.; Parsons, P.G.; Roberts, M.S.; Cross, S.E. Surfactant Protein Expression in Human Skin: Evidence and Implications. J. Investig. Dermatol. 2007, 127, 381–386. [Google Scholar] [CrossRef]
- Dalgård, C.; Wang, F.; Titlestad, I.L.; Kyvik, K.O.; Vestbo, J.; Sorensen, G.L. Increased Serum SP-D in Identification of High-Risk Smokers at High Risk of COPD. Am. J. Physiol.–Lung Cell. Mol. Physiol. 2021, 320, L1005–L1010. [Google Scholar] [CrossRef] [PubMed]
- Rouland, A.; Thuillier, P.; Al-Salameh, A.; Benzerouk, F.; Bahougne, T.; Tramunt, B.; Berlin, I.; Clair, C.; Thomas, D.; Le Faou, A.L.; et al. Smoking and Diabetes. Ann. Endocrinol. 2024, 85, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Seo, M.H.; Cho, J.H.; Kwon, H.; Kim, Y.H.; Han, K.-D.; Jung, J.H.; Park, Y.G.; Rhee, E.J.; Lee, W.Y. Dose-Dependent Effect of Smoking on Risk of Diabetes Remains after Smoking Cessation: A Nationwide Population-Based Cohort Study in Korea. Diabetes Metab. J. 2021, 45, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.P.; Wang, X.; Liu, F.; Cheng, Y.; Hu, Z.W.; Zhang, L.N.; Xia, G.G.; Zhang, C.; Ma, J.; Wang, G.F. Serum Surfactant Protein D, Lung Function Decline, and Incident Chronic Obstructive Pulmonary Disease: A Longitudinal Study in Beijing. J. Thorac. Dis. 2021, 13, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, M.; Wang, Y.; Wang, T.; Wu, N.; Zheng, W.; Duan, H. Air Particulate Matter Pollution and Circulating Surfactant Protein: A Systemic Review and Meta-Analysis. Chemosphere 2021, 272, 129564. [Google Scholar] [CrossRef]
- Borge, R.; Requia, W.J.; Yagüe, C.; Jhun, I.; Koutrakis, P. Impact of Weather Changes on Air Quality and Related Mortality in Spain over a 25 year Period [1993–2017]. Environ. Int. 2019, 133, 105272. [Google Scholar] [CrossRef]
- General Subdirectorate of Air Quality Industrial Environment Directorate of Quality and Environmental Assessment and Natural Environment Secretariat of State for the Environment. Spain Air Quality Evaluation Report 2016; General Subdirectorate of Air Quality Industrial Environment Directorate of Quality and Environmental Assessment and Natural Environment Secretariat of State for the Environment: Madrid, Spain, 2016.
- Targher, G.; Alberiche, M.; Zenere, M.B.; Bonadonna, R.C.; Muggeo, M.; Bonora, E. Cigarette Smoking and Insulin Resistance in Patients with Noninsulin-Dependent Diabetes Mellitus. J. Clin. Endocrinol. Metab. 1997, 82, 3619–3624. [Google Scholar] [CrossRef]
- Bajaj, M. Nicotine and Insulin Resistance: When the Smoke Clears. Diabetes 2012, 61, 3078–3080. [Google Scholar] [CrossRef]
- Chiolero, A.; Faeh, D.; Paccaud, F.; Cornuz, J. Consequences of Smoking for Body Weight, Body Fat Distribution, and Insulin Resistance. Am. J. Clin. Nutr. 2008, 87, 801–809. [Google Scholar] [CrossRef]
- Bergman, B.C.; Perreault, L.; Hunerdosse, D.; Kerege, A.; Playdon, M.; Samek, A.M.; Eckel, R.H. Novel and Reversible Mechanisms of Smoking-Induced Insulin Resistance in Humans. Diabetes 2012, 61, 3156–3166. [Google Scholar] [CrossRef]
- Cha, S.-R.; Jang, J.; Park, S.-M.; Ryu, S.M.; Cho, S.-J.; Yang, S.-R. Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers. Antioxidants 2023, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide [Internet]–PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34662007/ (accessed on 14 July 2025).
| Variable | Overall Population (n = 2155) | Low SP-D (n = 587) | High SP-D (n = 1568) | p Value |
|---|---|---|---|---|
| Age, years | 48.00 (37.00–60.00) | 45.00 (36.00–57.00) | 48.00 (37.00–60.00) | p < 0.01 |
| Sex, N (%) | ||||
| Men | 838 (38.9%) | 190 (32.4%) | 648 (41.3%) | p < 0.001 |
| Women | 1317 (61.1%) | 397 (67.6%) | 920 (58.7%) | |
| BMI, kg/m2 | 27.10 (24.18–30.18) | 27.07 (24.13–29.88) | 27.11 (24.21–30.31) | 0.32 |
| Fasting serum glucose, mg/dL | 91.80 (84.24–99.36) | 91.26 (83.16–98.28) | 92.16 (84.87–99.54) | 0.03 |
| HOMA | 1.63 (1.14–2.41) | 1.59 (1.15–2.41) | 1.63 (1.13–2.41) | 0.76 |
| Total cholesterol, mg/dL | 197.22 (171.11–222.35) | 194.90 (167.83–219.64) | 197.60 (172.47–223.12) | 0.22 |
| LDL cholesterol, mg/dL | 104.80 (84.69–125.29) | 102.86 (83.14–123.74) | 105.18 (85.07–125.68) | 0.13 |
| HDL cholesterol, mg/dL | 51.82 (44.47–60.71) | 52.59 (45.24–61.48) | 51.43 (43.70–60.71) | 0.02 |
| Triglycerides, mg/dL | 97.43 (72.63–132.86) | 96.55 (69.97–129.32) | 98.32 (74.40–133.75) | p < 0.01 |
| Systolic blood Pressure, mmHg | 126.50 (115.00–140.00) | 124.33 (112.00–138.00) | 127.50 (115.50–140.50) | p < 0.001 |
| Diastolic blood Pressure, mmHg | 75.50 (69.00–82.50) | 74.50 (68.50–81.67) | 76.00 (69.33–83.00) | 0.02 |
| Smoking habit, N (%) | ||||
| Smokers | 555 (25.8%) | 89 (15.2%) | 466 (29.7%) | p < 0.001 |
| Former/never smokers | 1600 (74.2%) | 498 (84.8%) | 1102 (70.3%) | |
| Family history of T2DM, N (%) | 1069 (49.6%) | 297 (50.6%) | 772 (49.2%) | 0.61 |
| Insulin resistance, N (%) | 463 (22.3%) | 126 (22.4%) | 337 (22.3%) | 1.00 |
| PM10 exposure, µg/m3 | 23.73 (19.61–27.29) | 23.08 (19.81–27.03) | 23.73 (19.61–27.29) | 0.64 |
| PM2.5 exposure, µg/m3 | 12.19 (10.50–14.98) | 11.98 (10.36–15.02) | 12.19 (10.56–14.98) | 0.57 |
| SO2 exposure, µg/m3 | 3.78 (2.83–5.47) | 3.61 (2.69–5.44) | 3.80 (2.90–5.47) | 0.06 |
| CO exposure, µg/m3 | 0.23 (0.19–0.30) | 0.23 (0.19–0.30) | 0.22 (0.19–0.30) | 0.95 |
| NO2 exposure, µg/m3 | 16.20 (12.16–24.47) | 15.47 (10.81–22.31) | 16.39 (12.47–24.63) | p < 0.01 |
| Variable | Rho | p Value |
|---|---|---|
| Tobacco exposure | ||
| Smoking years | 0.17 | p < 0.001 |
| Cigarettes per day | 0.07 | 0.02 |
| Total cigarettes | 0.16 | p < 0.001 |
| Air pollutants exposure | ||
| PM10 | 0.02 | 0.24 |
| PM2.5 | 0.02 | 0.42 |
| SO2 | 0.04 | 0.07 |
| CO | 0.01 | 0.66 |
| NO2 | 0.05 | 0.02 |
| OR (95% CI) | p Value | |
|---|---|---|
| SP-D (high) | 1.85 (1.08–3.15) | 0.02 |
| Age | 1.04 (1.02–1.06) | p < 0.001 |
| Sex (women) | 0.81 (0.54–1.20) | 0.28 |
| BMI | 1.09 (1.04–1.14) | p < 0.001 |
| Fasting serum glucose | 1.07 (1.05–1.09) | p < 0.001 |
| Family history T2DM | 1.90 (1.26–2.86) | p < 0.01 |
| Insulin resistance | 1.30 (0.83–2.03) | 0.25 |
| Smoking status (current smokers) | 1.62 (1.01–2.61) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oualla-Bachiri, W.; Lago-Sampedro, A.; García-Escobar, E.; Maldonado-Araque, C.; Doulatram-Gamgaram, V.; García-Vivanco, M.; Martín-Llorente, F.; Garrido, J.L.; Delgado, E.; Chaves, F.J.; et al. Surfactant Protein D Mediates the Association Between Smoking and Type 2 Diabetes Mellitus Incidence in the Spanish Adult Population: Di@bet.es Study. J. Xenobiot. 2025, 15, 184. https://doi.org/10.3390/jox15060184
Oualla-Bachiri W, Lago-Sampedro A, García-Escobar E, Maldonado-Araque C, Doulatram-Gamgaram V, García-Vivanco M, Martín-Llorente F, Garrido JL, Delgado E, Chaves FJ, et al. Surfactant Protein D Mediates the Association Between Smoking and Type 2 Diabetes Mellitus Incidence in the Spanish Adult Population: Di@bet.es Study. Journal of Xenobiotics. 2025; 15(6):184. https://doi.org/10.3390/jox15060184
Chicago/Turabian StyleOualla-Bachiri, Wasima, Ana Lago-Sampedro, Eva García-Escobar, Cristina Maldonado-Araque, Viyey Doulatram-Gamgaram, Marta García-Vivanco, Fernando Martín-Llorente, Juan Luis Garrido, Elías Delgado, Felipe J. Chaves, and et al. 2025. "Surfactant Protein D Mediates the Association Between Smoking and Type 2 Diabetes Mellitus Incidence in the Spanish Adult Population: Di@bet.es Study" Journal of Xenobiotics 15, no. 6: 184. https://doi.org/10.3390/jox15060184
APA StyleOualla-Bachiri, W., Lago-Sampedro, A., García-Escobar, E., Maldonado-Araque, C., Doulatram-Gamgaram, V., García-Vivanco, M., Martín-Llorente, F., Garrido, J. L., Delgado, E., Chaves, F. J., Castaño, L., Calle-Pascual, A., Franch-Nadal, J., Olveira, G., Valdés, S., & Rojo-Martínez, G. (2025). Surfactant Protein D Mediates the Association Between Smoking and Type 2 Diabetes Mellitus Incidence in the Spanish Adult Population: Di@bet.es Study. Journal of Xenobiotics, 15(6), 184. https://doi.org/10.3390/jox15060184










