Abstract
The eutrophication of aquatic ecosystems often triggers the excessive growth of cyanobacteria, many of which release toxic metabolites such as microcystins (MCs). When irrigation water is contaminated by these compounds, adverse consequences may arise for plants as well as for animal and human health. In contrast, certain non-toxic cyanobacterial species like Limnospira platensis are increasingly regarded as valuable tools for sustainable agriculture, given their ability to enhance plant nutrition, growth, yield, and stress tolerance while also mitigating the detrimental impacts of MCs. The present work aimed to investigate the potential of L. platensis extract to enhance growth, physiological responses, and tolerance of radish (Raphanus sativus) plants stressed with Microcystis aeruginosa extract containing microcystins. Experiments were conducted in a hydroponic system under controlled environmental conditions, where radish seedlings were cultivated in perlite and exposed for 45 days to M. aeruginosa extract (10 and 40 µg/L of MCs) and L. platensis extract (0.1 and 1 g/L), applied either separately or in combination. The results showed that the application of L. platensis extract, especially at 1 g/L in combination with 40 µg/L of MCs, decreased the bioaccumulation of MCs from 8.81 to 5.35 µg/kg FW in the leaves and from 14.64 to 10.15 µg/kg FW in the taproots. In addition, it significantly stimulated radish growth and improved several biochemical parameters. In contrast, exposure to MCs at 10 and 40 µg/L negatively affected growth, chlorophyll pigments and protein contents while promoting the accumulation of malondialdehyde (MDA), polyphenols and sugars. The activities of peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) were also increased under MCs stress, suggesting activation of the antioxidant defense system in response to oxidative damage. Combinations of MCs with L. platensis extract, especially at 1 g/L, improved antioxidant enzyme activities by significantly reducing MDA levels, biometric parameters, chlorophyll pigment, and protein and sugar contents. These results indicate that the application of L. platensis extract as a biostimulant can improve radish development, growth, and tolerance to MC-induced stress.
1. Introduction
The issue of water availability and quality is becoming increasingly critical in semi-arid Mediterranean countries, where the incidence of cyanobacterial harmful algal blooms (cyano-HABs) has been exacerbated by the synergistic effects of eutrophication and climate change [1,2]. The majority of cyanobacteria involved in bloom formation are recognized as potential cyanotoxin producers. Among the most frequently encountered cyanotoxins are microcystins (MCs), part of the hepatotoxin group, generally produced by Microcystis aeruginosa [3]. To date, 279 structural variants have been identified, with microcystin-LR (MC-LR) reported as the most prevalent [4]. MCs act as strong inhibitors of protein phosphatases 1 and 2A, enzymes that play key roles in numerous physiological and molecular processes in both animals and higher plants [5]. Globally, the highest reported concentration of MCs (2,900,000 µg/L) was detected in cyanobacterial scums from a Microcystis sp. bloom in Pinto Lake, California [6]. In Morocco, specifically in the Lalla Takerskoust reservoir, concentrations of the extracellular and intracellular fractions of MCs in water can reach 281 µg/L and 10,257 µg/g, respectively [7]. This untreated water, containing high MC concentrations, is primarily used for irrigation without any regulation or monitoring. Therefore, this practice is likely to cause phytotoxic effects, including reduced seed germination, impaired growth and development, and decreased photosynthetic performance [8,9]. Such toxins are capable of altering plant metabolic processes, leading to increased lipid peroxidation, decreased protein levels, and triggering oxidative stress [9]. However, reactive oxygen species (ROS) are inevitably produced under stress conditions and can cause severe oxidative damage to proteins, lipids, and photosynthetic pigments. To counteract this, plants rely on an efficient antioxidant defense system, including enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD). SOD converts superoxide radicals into hydrogen peroxide, while CAT and POD subsequently detoxify hydrogen peroxide into water and oxygen, thereby maintaining cellular homeostasis and protecting the photosynthetic machinery [9,10].
Improving plant growth and nutritional quality in MC-contaminated environments requires effective management of irrigation water. Physical and chemical techniques, such as flocculation [11], ultrasonic treatment [12], and copper sulfate application [13], have been developed to control cyanobacterial blooms. However, these methods often present limitations, including high energy costs, limited long-term effectiveness, and potential environmental side effects. To address these issues, biological alternatives based on the use of microorganisms have attracted growing attention in recent years. Most research on the biodegradation of MCs has focused on microorganisms isolated from water sources that have experienced algal blooms [14]. In this context of biological solutions, the use of biostimulants derived from non-MC-producing cyanobacterial species, such as L. platensis, offers a sustainable approach to safely improve crop production [15]. Biostimulants have been shown to enhance rooting, nutrient uptake, seedling establishment, and plant tolerance to a wide range of biotic and abiotic stresses [16,17]. These beneficial effects are largely attributed to their content in growth regulators, including phytohormones, vitamins, amino acids, and polysaccharides, which are essential for plant development [18]. In this context, the present study investigates the potential of an aqueous extract of L. platensis as a biotechnological tool to stimulate growth and productivity while mitigating MC-induced stress in hydroponically grown radish (Raphanus sativus) under controlled conditions.
2. Materials and Methods
2.1. Preparation of M. aeruginosa and L. platensis Extracts
To obtain cyanobacterial extracts, 10 g of dried L. platensis biomass previously collected from Lake Chad was suspended in 100 mL of distilled water and agitated continuously at 60 °C for 15 min. The extract was filtered through a 0.22 µm Millipore membrane and stored at 4 °C. This stock solution, corresponding to 100 g dry weight (DW)/L, was subsequently diluted to prepare the working concentrations of 0.1 and 1 g (DW)/L. Samples of M. aeruginosa bloom were collected in October 2010 from the Lalla Takerkoust reservoir, Marrakech, Morocco (31°36′ N, 8°20′ W, 664 m), and freeze-dried. For extraction, 250 mg of freeze-dried material was ground in a mortar with 50 mL of distilled water and subjected to cold sonication (42 kHz, 5 min) to release intracellular microcystins (MCs). After centrifugation at 10,000× g for 15 min, the supernatant was collected, while the pellet was re-extracted twice under identical conditions. All supernatants were pooled and stored at −20 °C until analysis. The total MC content in the aqueous extract was determined using the serine/threonine protein phosphatase type 2A inhibition assay as described by Bouaïcha et al. [19]. In brief, the assay is based on the dephosphorylation of para-nitrophenylphosphate (p-NPP), generating para-nitrophenol, a colored compound. Enzymatic activity is quantified by monitoring the formation of this product at 405 nm, thereby enabling evaluation of the inhibitory effect of MCs on PP2A-mediated p-NPP dephosphorylation. Using this method, the concentration of MCs in the M. aeruginosa bloom extract was determined as 11.5 mg MC-LR equivalents per g DW. Complementary qualitative analysis performed with high-performance liquid chromatography (HPLC) coupled to mass spectrometry showed that MC-LR was the dominant variant, accounting for 98% of the total MCs [20]. Two MC concentrations (10 and 40 µg/L) were freshly prepared for each application in plant irrigation during the treatment period.
2.2. Biochemical Characterization of M. aeruginosa, L. platensis, and Raphanus sativus Tissue Samples
2.2.1. Total Soluble Sugars
To determine soluble sugar concentrations in M. aeruginosa, L. platensis, and Raphanus sativus tissues, 100 mg of sample material was extracted with 4 mL of 80% ethanol and centrifuged at 5000× g for 10 min. From the supernatant, 1 mL was combined with 1 mL of 5% phenol and 5 mL of concentrated sulfuric acid. After incubation for 5 min, the absorbance of the mixture was recorded at 485 nm. Glucose was used as the calibration standard [21], and soluble sugar content was expressed as mg glucose equivalents per g DW (M. aeruginosa and L. platensis) or per g FW (radish leaves, roots, and taproots).
2.2.2. Total Protein Contents
An aliquot of 500 mg of dried M. aeruginosa, L. platensis, and Raphanus sativus tissue samples was ground in liquid nitrogen in a cold mortar. The resulting powder was homogenized in 5 mL of buffer composed of 50 mM potassium phosphate (pH 7.0), 5% (w/v) polyvinylpolypyrrolidone (PVPP), and 0.1 mM EDTA. The homogenates were centrifuged at 12,500× g for 20 min at 4 °C, and the supernatants were collected for subsequent analyses of protein content and antioxidant enzyme activities following the method of Savicka et al. [22].
For proteins quantification, 100 µL of each extract was combined with 2 mL of Bradford reagent. The mixtures were incubated at room temperature for 5 min, after which absorbance was measured at 595 nm. Protein concentrations were calculated against a standard curve prepared with bovine serum albumin (BSA) [23]. Each measurement was performed in triplicate, and results were expressed as mg BSA equivalent per g DW (M. aeruginosa, L. platensis) or per g FW (radish leaves, roots, and taproots). The analysis of antioxidant enzyme activities on fresh leaves and roots samples is described in Section 2.6.4.
2.2.3. Total Polyphenols
The total polyphenol content of dried M. aeruginosa, L. platensis, and Raphanus sativus tissues was quantified using the colorimetric method described by Singleton et al. [24]. For the assay, 0.2 mL of each extract was mixed with 2.4 mL of distilled water and 0.4 mL of Folin–Ciocalteu reagent. After vortexing, the mixture was left to react for 3 min, followed by the addition of 1 mL of 20% (w/v) sodium carbonate (Na2CO3). The samples were vortexed again and incubated for 1 h at room temperature in the dark. Absorbance was subsequently measured at 765 nm. Quantification was performed using a calibration curve prepared with gallic acid (0–200 µg/mL). All measurements were carried out in triplicate, and results were expressed as µg gallic acid equivalents per g DW (M. aeruginosa and L. platensis) or per g FW (radish leaves, roots, and taproots).
2.2.4. Flavonoids
The total flavonoid contents in dried M. aeruginosa and L. platensis extract samples were determined using a colorimetric assay with aluminum trichloride (AlCl3) according to Kim et al. [25]. For each assay, 500 µL of aqueous extract were mixed with 1.5 mL of distilled water and 150 µL of 5% sodium nitrite (NaNO2) solution. After a 5-min incubation, 150 µL of 10% AlCl3 (prepared in methanol (MeOH)) was added. After 11 min of incubation, 500 µL of 1 M NaOH was introduced, and the mixture was vortexed. Absorbance was recorded immediately at 510 nm. Quantification was carried out using a calibration curve constructed with catechin standards (0–200 µg/mL). Each measurement was performed in triplicate, and results were expressed as µg catechin equivalents per g DW.
2.2.5. Auxins
Auxin levels in dried M. aeruginosa and L. platensis extracts were quantified using the Salkowski colorimetric method following Gang et al. [26]. For the assay, 3 mL of aqueous extract was mixed with 2 mL of Salkowski reagent, vortexed, and kept in the dark at room temperature for 30 min. The absorbance of the resulting solution was recorded at 536 nm. Concentrations were calculated from a calibration curve prepared with pure indole-3-acetic acid (IAA). Each determination was carried out in triplicate, and results were expressed as mg IAA equivalents per g DW.
2.3. Culture of Radish Plants and Treatment with Different Extracts
Certified seeds of Raphanus sativus (national variety) were obtained from BADRA S.A.R.L, Casablanca, Morocco. The seeds were rinsed with distilled water, and germinated individually in plastic cells filled with peat. After 10 days, when seedlings had developed two to three leaves, they were transplanted into 0.5 L plastic bags containing perlite, a substrate commonly used in hydroponics. Plants were cultivated for 45 days in a growth chamber under controlled conditions (20 ± 1 °C, light intensity of 200 µmol m−2 s−1, 13/11 h light/dark cycle, relative humidity 70–80%). The 54 pots were assigned to nine experimental treatments:
- C (control): irrigation with alternating water and nutrient solution,
- C1: irrigation with water containing 10 µg/L of MCs,
- C2: irrigation with water containing 40 µg/L of MCs,
- B1: irrigation with water containing 0.1 g/L L. platensis extract,
- B2: irrigation with water containing 1 g/L L. platensis extract,
- C1 + B1: irrigation with water containing 10 µg/L of MCs + 0.1 g/L L. platensis extract,
- C1 + B2: irrigation with water containing 10 µg/L of MCs + 1 g/L L. platensis extract,
- C2 + B1: irrigation with water containing 40 µg/L of MCs + 0.1 g/L L. platensis extract,
- C2 + B2: irrigation with water containing 40 µg/L of MCs + 1 g/L L. platensis extract.
To minimize positional effects, pots were randomly rearranged every 7 days. The experiment was conducted with six replicates per treatment (one plant per pot). Plants were irrigated with 75 mL of Hoagland’s nutrient solution [27] alternated with water containing L. platensis and/or MC extracts at two-day intervals. After 45 days, biomass was harvested to assess growth traits. Plant tissues (leaves, roots, and taproots) were separated, stored at −20 °C for biochemical analyses, and selected samples (leaves and taproots) were reserved for MCs detection.
2.4. Determination of MCs in Radish Tissues
To evaluate the bioaccumulation of MCs in radish tissues, 1 g of leaves or 2 g of taproots was ground in liquid nitrogen and freeze-dried. Samples were homogenized in 5 mL (leaves) or 10 mL (taproots) of 75% (v/v) aqueous methanol. Following centrifugation at 4000× g for 10 min at 4 °C, the supernatants were collected, and the residues were re-extracted twice under the same conditions. The combined supernatants were then purified using C18 solid-phase extraction (SPE). After, the cartridges were conditioned with 5 mL methanol followed by 5 mL deionized water. Elution of MCs was performed with 5 mL methanol/formic acid (95:5, v/v). The eluates were evaporated to dryness in a rotary evaporator, and the residues were resuspended in 1 mL deionized water before storage at −80 °C until ELISA analysis. MC concentrations in radish leaves and taproots were expressed as µg MC-LR equivalents per kg FW [28].
2.5. Determination of Biometric Parameters and Yield Parameters of Radish Plants
After 45 days of exposure with the different treatments, plant biomass was harvested (at fruit stage). The taproots and aerial sections were separated to determine various growth metrics. To evaluate the effect of different treatments on radish plant growth, total root length, aerial part height and yield parameters (taproots) were measured using a ruler and caliper. The results were expressed in centimeters (cm). Furthermore, the number of leaves per plant was counted for all treatments. For leaf area determination, leaves were sliced, put on a white sheet with a scale, scanned using a digital scanner, and the area was determined using the Mesurum software version 3.4.4.0 [29]. For biomass determination, aerial parts, roots, and taproots were carefully rinsed with water to eliminate perlite residues, blotted dry with absorbent paper, and weighed using a precision balance, and the results were expressed in grams (g).
2.6. Determination of Biochemical Parameters in Radish Tissues
2.6.1. Pigment Content in Leaves
After 45 days of treatment, radish plants were harvested and their leaves were collected for the determination of photosynthetic pigments, including chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll, and carotenoids. For pigment extraction, 0.5 g of fresh leaf tissue was homogenized in 5 mL of 95.5% acetone. The samples were incubated in darkness for 48 h, after which absorbance was recorded at 470, 644, and 662 nm, following the procedure described in [30]. Pigment concentrations (mg/g FW) were then calculated using the standard equations.
Chl a = 9.784 (OD 662) − 0.99 (OD 644)
Chl b = 21.42 (OD 644) − 4.65 (OD 662)
Total chlorophyll = Chl a + Chl b
Carotenoids = 1000 (OD470) − 1.90 Chl a − 63.14 Chl b/214
2.6.2. Vitamin C Content in Taproots
After 45 days of treatment, radish plants were harvested and the taproots were used for the determination of ascorbic acid content through a titrimetric assay. 0.5 g of fresh taproot tissue was ground in a mortar and extracted with 3 mL of 2% HCl, followed by a 10 min incubation. The homogenate was centrifuged at 5000× g for 10 min at 4 °C, and the supernatant was diluted with 3 mL of distilled water. A few drops of 0.5% starch solution were added as an indicator, and the mixture was titrated with 0.01 N iodine solution until the appearance of the characteristic dark blue-black color, which marked the endpoint [31]. The concentration of vitamin C was calculated and expressed as mg ascorbic acid equivalent per 100 g FW.
2.6.3. Inorganic Ions in Taproots
The collected taproots were analyzed to determine the concentrations of inorganic ions, including calcium (Ca), magnesium (Mg), zinc (Zn), potassium (K), and iron (Fe). For this purpose, 0.25 g of freeze-dried material was subjected to calcination in a muffle furnace at 550 °C for 6 h. The resulting ash was solubilized in 3 mL of 6 N HCl, then evaporated at 250 °C under a fume hood. The residue was dissolved in 3 mL of preheated distilled water (95 °C) and filtered through 0.45 μm Whatman paper. Each sample was subsequently brought to a final volume of 50 mL with hot distilled water (95 °C). Mineral concentrations (Ca, Mg, Zn, K, and Fe) were quantified using inductively coupled plasma (ICP) analysis according to [32], while total phosphorus content was determined following the method of Olsen and Sommers [33]. Results were expressed as mg/100 g DW.
2.6.4. Antioxidant Enzyme Activities in Radish Tissues
After 45 days of exposure to the different treatments, radish plants were collected and separated into leaves and roots to assess the activities of antioxidant enzymes: peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT). POD activity was determined using a reaction mixture consisting of 100 µL of leaf or root extracts (prepared in 10 mM phosphate buffer, pH 6), 0.25% guaiacol, and 20 mM H2O2. The oxidation of guaiacol was monitored at 470 nm following the method of [34], and the activity was expressed as Unit/mg protein. For SOD activity was assayed according to [35] with slight modifications. The reaction mixture contained 200 µL of extracts prepared in 100 mM phosphate buffer (pH 7.8), 0.75 mM nitroblue tetrazolium, 0.1 mM riboflavin, and 55 mM methionine. After incubation at 25 °C for 20 min, the absorbance was measured at 560 nm, and results were expressed as Unit/mg protein. CAT activity was quantified using extracts prepared in 50 mM phosphate buffer (pH 7.0). The reaction mixture (200 µL of extract, 15 mM H2O2, and 2.6 mL of buffer) was used to measure the degradation of hydrogen peroxide at 240 nm, as described by [36]. The activity was expressed as µmol H2O2 decomposed/min/mg protein.
2.6.5. Determination of Malondialdehyde (MDA) in Radish Tissues
The level of malondialdehyde (MDA), an indicator of lipid peroxidation, was assessed using the thiobarbituric acid (TBA) assay as outlined in [22]. Fresh leaf and root tissues (0.2 g) were homogenized in 2 mL of 0.1% trichloroacetic acid (TCA), and the homogenate was centrifuged at 14,000× g for 15 min. To 1 mL of the obtained supernatant, 2.5 mL of 20% TCA containing 0.5% TBA was added. The mixture was heated at 95 °C for 30 min, then immediately cooled in an ice bath. After a second centrifugation at 10,000× g for 30 min, absorbance of the clear supernatant was recorded at 532 and 600 nm. MDA concentration was calculated and expressed as µmol/g fresh weight (FW).
2.7. Statistical Analysis
All data were tested for normality (Shapiro–Wilk test) and homogeneity of variances (Levene’s test) prior to analysis. Measurements were expressed as mean ± standard error (SE). Statistical differences among treatments were assessed using one-way ANOVA. When significant differences were detected, multiple comparisons were performed using Tukey’s HSD post hoc test. Groups marked with different letters were considered significantly different at p < 0.05. All analyses were conducted using IBM SPSS Statistics software, version 26.0 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Characterization of M. aeruginosa and L. platensis Extracts
Table 1 presents the biochemical properties of the aqueous extracts of L. platensis and M. aeruginosa. The extract of L. platensis contains high contents of soluble sugars (15.23 mg/g DW), proteins (39.67 mg/g DW) and auxins (44.69 mg/g DW), as well as low amounts of total phenols (0.2 µg Gallic acid/g DW) and flavonoids (0.3 µg Catechin/g DW). In contrast, the extract of M. aeruginosa contains higher soluble sugars (25.62 mg/g DW) and a little less proteins (30.23 mg/g DW), while total flavonoids, auxins, and phenols are not detected in this extract. These findings highlight clear differences in the biochemical profiles of the two cyanobacterial extracts, with some bioactive compounds—including auxins, phenols, and flavonoids—being detected exclusively in L. platensis extract (Table 1).

Table 1.
Biochemical properties of M. aeruginosa and L. platensis extracts. The values are denoted as mean ± standard error. Nd: not detected.
3.2. Bioaccumulation of MCs in Radish Tissues
Table 2 presents the levels of MCs accumulated in radish leaves and taproots, as determined by the ELISA assay. After 45 days of exposure to water contaminated with MCs (10 and 40 µg/L) showed an increase in the bioaccumulation of MCs in both organs compared to the control. The concentrations ranged from 10.13 to 14.64 µg equiv. MC-LR/kg FW in taproots and from 7.33 to 8.81 µg equiv. MC-LR/kg FW in leaves (Table 2). However, when radish plants were simultaneously exposed to both cyanobacterial extracts, treatment with L. platensis at 0.1 or 1 g/L resulted in a modest, though statistically non-significant, reduction in MC accumulation in both leaves and taproots.

Table 2.
Concentrations of MCs (µg equiv. MC-LR/kg FW) detected in radish (Raphanus sativus) tissues (leaves and taproots) after 45 days of exposure to L. platensis, MC extracts, and their combinations. Values are mean ± standard error (n = 3). Different letters indicate significant differences at p < 0.05 (Anova, Tukey’s HSD test). Nd: not detected.
3.3. Effects of L. platensis Extract on Radish Growth Under MC-Induced Stress
Table 3 summarizes the impact of the different treatments on radish growth parameters after 45 days of exposure to MC-induced stress. Application of MCs alone, at either tested concentration, did not significantly affect leaf number (LN), leaf area (LA), leaf fresh weight (LFW), or root fresh weight (RFW) compared with the control. By contrast, supplementation with L. platensis extract (0.1 and 1 g/L) led to a significant increase (p < 0.001) in LN relative to the control. Moreover, co-application of 10 µg/L MCs + 1 g/L L. platensis or 40 µg/L MCs + 1 g/L L. platensis significantly enhanced LA compared with plants treated with MCs alone. A notable increase in LFW was observed in plants receiving 40 µg/L MCs combined with 1 g/L L. platensis compared with the control. Similarly, RFW was significantly higher (p < 0.001) in plants treated with 1 g/L of L. platensis, 10 µg/L MCs + 1 g/L of L. platensis and 40 µg/L MCs + 1 g/L L. platensis, compared with the control (Table 3). Treatment of radish plants with either MCs or L. platensis extracts alone did not significantly affect taproot length (TL) (p = 0.276) or diameter (TD) (p < 0.001) (Table 3). A significant increase in taproot fresh weight (TFW) and dry weight (TDW) was observed only with the higher concentration of L. platensis extract (1 g/L), whereas exposure to 40 µg/L MCs led to a significant reduction (p < 0.001) in taproot dry weight (TDW) compared with control. In contrast, combined application of MCs and L. platensis extracts, at the tested concentrations, produced no significant changes in taproot yield (Table 3).

Table 3.
Effects of L. platensis (LP), MC extracts, and their combinations at different concentrations on radish growth and yield parameters after 45 days of treatment. Values are mean ± standard error (n = 3). Different letters indicate significant differences at p < 0.05 (Anova, Tukey’s HSD test). LN: Leaves Number; LA: Leaf area (cm2); APL: Aerial part Length (cm); RL: Root Length (cm); RFW: Root Fresh Weight (g); LFW: Leaf fresh weight (g); TL: Taproot length (cm); TFW: Taproot fresh weight (g); TDW: Taproot dry weight (g); TD: Taproot diameter (cm).
3.4. Effect of L. platensis Extract on the Biochemical Parameters of Radish-Stressed Tissues by MCs
3.4.1. Photosynthetic Pigment Content
Figure 1 illustrates the effects of the different treatments on photosynthetic pigment contents in radish plants exposed to MC-induced stress. No significant differences (p = 0.872) in chlorophyll a were detected among treatments with MCs, L. platensis extracts, or their combinations compared with the control (Figure 1a). In contrast, chlorophyll b significantly increased (p < 0.001) in response to both concentrations of L. platensis extract (0.1 and 1 g/L), whereas MCs alone (10 and 40 µg/L) had no effect (Figure 1b). Co-treatments combining MCs with 1 g/L of L. platensis also enhanced chlorophyll b, although to a lesser extent than the extract applied alone. A similar pattern was observed for total chlorophyll content (Figure 1c). For carotenoids, neither L. platensis extract nor 10 µg/L MCs significantly altered (p < 0.001) levels, while the highest MC concentration (40 µg/L) and all co-treatments caused a significant reduction compared with the control (Figure 1d).
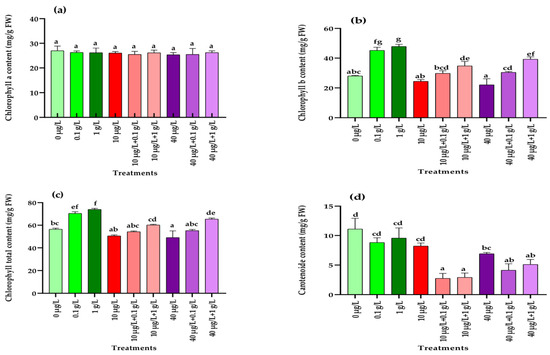
Figure 1.
Photosynthetic pigment contents (mg/g FW) in radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. (a) Chlorophyll a, (b) chlorophyll b, (c) total chlorophyll, and (d) carotenoids. Bars represent mean values ± standard deviation (SD). Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.4.2. Total Soluble Sugar Content
The impact of MCs and L. platensis extracts, applied either individually or in combination, on soluble sugar levels in radish leaves and roots is illustrated in Figure 2. In the leaves, all treatments resulted in a significant increase (p < 0.001) in soluble sugar content compared with the control, with the strongest induction observed in plants treated with both concentrations of L. platensis extract alone (Figure 2a). The combination of MC and L. platensis extract showed also a significant increase (p < 0.001) of total sugar contents compared to the control and to the treatment with MCs extract alone, but remain significantly low than those induced by the L. platensis extract alone. The same trend of results was also observed for the roots (Figure 2b).
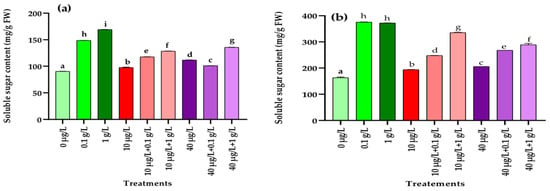
Figure 2.
Total sugar content (mg/g FW) in leaves (a) and roots (b) of the radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. Bars represent mean values ± SD. Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.4.3. Total Proteins Content
Total proteins content obtained from radish leaves and roots treated with the different extracts alone or in combination are presented in Figure 3. In the leaves, exposure to MCs at both concentrations (10 and 40 µg/L) caused a dose-dependent decline in total protein content compared with the control (Figure 3a). However, co-application of L. platensis extract with MCs led to a significant recovery (p < 0.001) of protein levels, restoring them close to the control values (Figure 3a). In contrast, no significant (p = 0.122) variations were observed in root protein content across the different treatments compared with the control (Figure 3b).
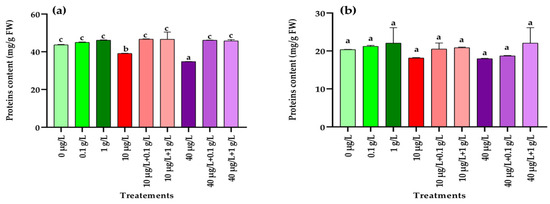
Figure 3.
Protein contents (mg/g FW) in leaves (a) and roots (b) of the radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. Bars represent mean values ± SD. Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.5. Effect of L. platensis Extract on Stress Markers of Radish-Stressed by MCs
3.5.1. Total Polyphenols Content
Exposure to MCs at both concentrations (10 and 40 µg/L) resulted in a significant increase (p < 0.001) in polyphenol levels in radish leaves and roots compared with the control (Figure 4a,b). In contrast, treatment with L. platensis extract at both concentrations (0.1 and 1 g/L) caused a significant reduction (p < 0.001) in leaf polyphenols, while in roots this effect was observed only at the higher concentration (1 g/L) (Figure 4b). Under combined treatments, a significant decrease (p < 0.001) in leaf polyphenols compared to the control was detected only when MCs were applied together with the highest concentration (1 g/L) of L. platensis extract (Figure 4a). In roots, co-treatment with 10 µg/L of MCs and both concentrations of L. platensis extract significantly reduced (p < 0.001) polyphenol content, whereas the combination of 40 µg/L of MCs with both L. platensis concentrations significantly increased (p < 0.001) it compared with the control (Figure 4b).
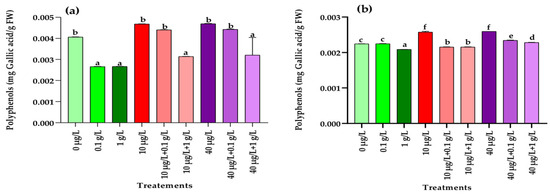
Figure 4.
Polyphenol contents (mg Gallic acid/g FW) in leaves (a) and roots (b) of the radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. Bars represent mean values ± SD. Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.5.2. Determination of Malondialdehyde (MDA)
As shown in Figure 5, a significant (p < 0.001) rise in malondialdehyde (MDA) levels compared to the control was detected only in radish roots treated with the lower concentration (0.1 g/L) of L. platensis extract (Figure 5b). In contrast, both MC concentrations (10 and 40 µg/L) caused a marked increase in MDA content in leaves and roots (Figure 5a,b). However, when plants were co-exposed to MCs and L. platensis extracts, the presence of L. platensis, at both concentrations, restored MDA levels in both tissues to values comparable to the control (Figure 5a,b).
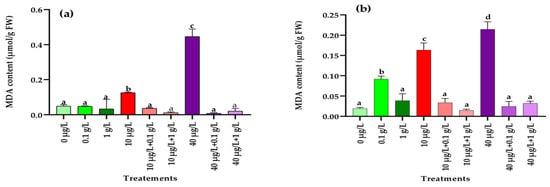
Figure 5.
Malondialdehyde content (MDA) (µmol/g FW) in leaves (a) and roots (b) of the radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. Bars represent mean values ± SD. Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.5.3. Peroxidase (POD) Activity
Application of L. platensis extract at both tested concentrations (0.1 and 1 g/L) significantly enhanced POD activity in radish leaves and roots compared with the control. Similarly, exposure to the highest MC concentration (40 µg/L) also led to a marked increase in this enzymatic activity in both tissues, whereas the lower dose (10 µg/L) significantly stimulated POD activity only in roots (Figure 6a,b). Interestingly, co-application of L. platensis and MCs extracts further amplified POD activity in both leaves and roots, exceeding not only the control values but also those recorded under single treatments (Figure 6a,b).
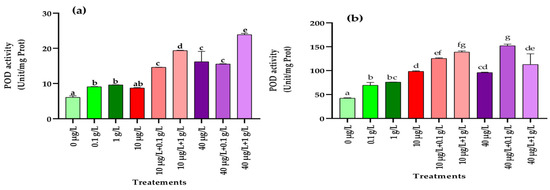
Figure 6.
Peroxidase (POD) activity (Unit/mg Prot.) in leaves (a) and roots (b) of the radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. Bars represent mean values ± SD. Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.5.4. Superoxide Dismutase (SOD) Activity
As shown in Figure 7, exposure to both L. platensis and MC extracts significantly enhanced (p < 0.001) superoxide dismutase (SOD) activity in radish leaves and roots compared with the control. The increase was even more pronounced under combined treatments, where co-application of the two extracts resulted in higher SOD activity than in the control or plants treated with L. platensis extract alone.
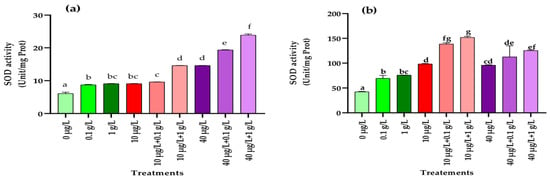
Figure 7.
Superoxidase dismutase (SOD) (Unit/mg Prot.) in leaves (a) and roots (b) of the radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. Bars represent mean values ± SD. Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.5.5. Catalase (CAT) Activity
As illustrated in Figure 8a, a significant increase (p < 0.001) in CAT activity in leaves was detected only at the highest concentration of MC extract (40 µg/L) compared with the control. In contrast, in roots, CAT activity was significantly enhanced (p < 0.001) when plants were exposed to both L. platensis and MC extracts (Figure 8b). Co-application of the lowest MC concentration (10 µg/L) with either 0.1 or 1 g/L of L. platensis extract strongly stimulated CAT activity in both leaves and roots, surpassing the control and the effects of each extract applied separately. For the highest MC concentration (40 µg/L), co-treatment with L. platensis extracts also resulted in higher CAT activity compared to the control, but the strongest induction compared with MC extract alone was observed only in the roots with the highest L. platensis concentration (1 g/L) (Figure 8b).
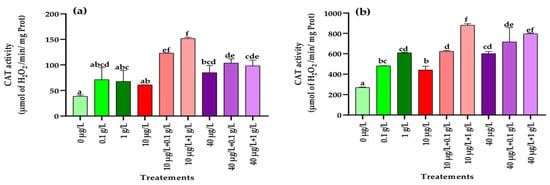
Figure 8.
Catalase (CAT) (µmol of H2O2/min/mg Prot.) in leaves (a) and roots (b) of the radish leaves after 45 days of treatment with 0 µg/L (water and nutrient solution), L. platensis extract (0.1 and 1 g/L), MCs (10 and 40 µg/L), and their combinations. Bars represent mean values ± SD. Different letters indicate significant differences (p < 0.05, Tukey’s HSD test).
3.6. Effects of L. platensis Extract on Biochemical Parameters of Radish Taproots Under MC-Induced Stress
Regarding the biochemical parameters of radish taproots, the results showed that soluble sugar, proteins, and polyphenols levels exhibited no significant effects in all applied treatments compared to the control. However, the vitamin C content was decreased notably under MCs extract (10 and 40 µg/L) exposure in comparison to the control. Controversy, it exhibited a significant increase with L. platensis extracts (0.1 and 1 g/L) compared to the control. A significant increase (p < 0.001) of the vitamin C level was also recorded for treatments with 0.1 g/L, 1 g/L of the L. platensis extract, and 10 µg/L MCs + 0.1 of the L. platensis extract compared to the control (Table 4). In contrast, when the radish plant was co-treated with the high concentration of MC extract (40 µg/L) and both concentrations (0.1 and 1 g/L) of L. platensis extract, a significant increase (p < 0.001) in vitamin C level was also recorded compared to the treatment with 40 µg/L MC (Table 4).

Table 4.
Biochemical characteristics of radish taproots after 45 days of exposure to different concentrations of L. platensis and MC extracts, applied individually or in combination. Values are mean ± standard error (n = 3). Different letters indicate significant differences at p < 0.05 (Anova, Tukey’s HSD test).
3.7. Effects of L. platensis Extract on Nutrient Content of Radish Taproots Under MC-Induced Stress
The results presented in Table 5 indicate that the taproots of plants treated with 40 µg/L of MC extract or with the different combinations between MC and L. platensis showed a significant reduction (p < 0.001) in potassium (K) content compared to the control. In contrast, treatment with the biostimulant concentrations (0.1 and 1 g/L) of L. platensis extract resulted in a significant increase (p < 0.001) in K levels compared to treatment with MC alone. Regarding magnesium (Mg), both concentrations of L. platensis extract induced a significant decrease compared to the control, a reduction that was also evident under treatment with the high concentration (40 µg/L) of MC extract. Similarly, co-treatments with both 10 and 40 µg/L MC extracts and 1 g/L L. platensis extract led to a significant decrease (p < 0.001) in Mg content compared to the control. Treatment of radish plants with both concentrations of MC extract resulted in a significant reduction (p < 0.001) of zinc (Zn) content in taproots compared to the control. Conversely, application of both concentrations of L. platensis extract significantly increased (p < 0.001) in Zn levels. In co-treatment, the combination of the lowest concentration of MC extract (10 µg/L) with either concentration of L. platensis extract significantly increased the Zn content compared to the treatment with both concentrations of MC (10 and 40 µg/L). However, when the highest concentration of MC extract was applied in combination, the Zn content remained significantly lower (p < 0.001) than that of the control.

Table 5.
Effects of different treatments on nutrient contents of radish taproots after 45 days of treatment with L. platensis and MC extracts and their combinations at various concentrations. Values are mean ± standard error (n = 3). Different letters indicate significant differences at p < 0.05 (Anova, Tukey’s HSD test). Fe: Iron; K: Potassium; Mg: Magnesium; Zn: Zinc; Ca: Calcium; P: Phosphorus.
Taproots of radish plants treated with both concentrations of MCs (10 and 40 µg/L) as well as with L. platensis extracts (0.1 and 1 g/L) showed a significant reduction (p < 0.001) in calcium (Ca) content relative to the control. Similarly, co-treatment with MCs and L. platensis extracts also resulted in a significant decrease (p < 0.001) in Ca levels. By contrast, application of L. platensis extract alone (0.1 and 1 g/L) significantly (p < 0.001) enhanced phosphorus (p) content, whereas the highest concentration of MCs extract (40 µg/L) caused a significant reduction compared with control. Interestingly, co-treatment with all combinations of MC extract and L. platensis extract showed a significant increase (p < 0.001) in P content compared to the control and the treatment with MC alone.
4. Discussion
The findings of this study demonstrate that the presence of MCs in irrigation water, even at environmentally relevant concentrations (10 and 40 µg/L), exerts dose-dependent effects on the growth, oxidative stress markers, and nutritional quality of radish under hydroponic conditions. Morphologically, exposure to MCs led to a significant decline in leaf number, leaf area, plant height, and fresh biomass. These observations are in line with previous reports describing growth inhibition in plants exposed to MCs. For instance, Haida et al. [9] reported a reduction in fresh biomass, leaf number, and root length in Fragaria vulgaris exposed to 20 µg/L of MCs. Similarly, irrigation with water containing 1–20 µg/L of MCs led to a decrease in fresh weight in Lactuca sativa and Lepidium sativum [37,38], inhibition of root growth in Medicago sativa and Lactuca sativa [39], as well as reduced root elongation, crown root initiation, and lateral root development in Brassica rapa [37]. These results can be explained that under hydroponic conditions, MCs are likely more bioavailable, allowing direct contact with the root system, which may explain the strong effects observed even at low doses. The toxic action of MCs is mainly attributed to their strong inhibitory effect on serine/threonine protein phosphatases, key enzymes regulating essential processes such as metabolism, cell division, development, and gene transcription and translation in both animals and higher plants [40,41]. Supporting this, Saqrane et al. [42] demonstrated that the inhibition of these phosphatases in plants could lead to leaf malformations, histological alterations, and delays in root organ differentiation and vascular cylinder formation, accompanied by inhibition of lateral root primordia development.
In contrast, when Raphanus sativus L. was cultivated under the same hydroponic conditions but supplemented with an aqueous extract of L. platensis (0.1 and 1 g/L), a cyanobacterium that does not produce MCs, the plants displayed a significant improvement in several growth traits, including leaf number, leaf area, and fresh biomass of both roots and shoots (Table 3). These findings are consistent with previous research reporting that hydrolysates or extracts of L. platensis, applied at different concentrations (1–9 g/L), enhance plant growth and development. Such biostimulant activity has been reflected in increased plant biomass and height, greater leaf production, earlier flowering, higher floral biomass, more flowers, and increased stem number in several plant species, including Lactuca sativa [43], Eruca sativa [44], Beta vulgaris [45], Amaranthus gangeticus, and Brassica rapa [46], Petunia x hybrida [47], and Solanum lycopersicum [48]. The analysis of physiological traits revealed that the aqueous extract of L. platensis is particularly rich in total sugars, proteins, and auxins. These findings are consistent with previous reports highlighting the abundance of proteins, carbohydrates, macro- and micronutrients, polyamines, vitamins, enzymes, and hormone-like compounds in L. platensis [47,49]. Among these components, polysaccharides represent a major fraction of microalgal extracts and are known to play a key role in promoting plant growth [50]. Likewise, auxins, as central phytohormones, regulate fundamental processes such as cell division and elongation, tissue differentiation, apical dominance, abscission, and flowering [51].
The significantly higher growth rates, chlorophyll pigment levels, and soluble sugar contents recorded in radish plants cultured hydroponically with the application of L. platensis aqueous extract (0.1 and 1 g/L) (Figure 1 and Figure 2) raised an important question: can this extract confer protection against the phytotoxic effects induced by MCs? To address this, radish plants were exposed either to MCs alone (10 and 40 µg/L) or in combination with L. platensis extract (0.1 and 1 g/L), and their responses were assessed at the physiological, biochemical, and nutritional levels. Application of the aqueous extract at 1 g/L markedly alleviated the negative effects of MC exposure, leading to improvements in key growth parameters, including leaf number, leaf area, shoot and root length, and fresh biomass of both aerial and root tissues (Table 3). These findings align with earlier reports indicating that L. platensis-based biostimulants can mitigate the detrimental impact of various abiotic stresses on plants [52]. For instance, L. platensis suspension enhanced growth and productivity in salt-stressed Vicia faba L., an effect attributed to osmoregulatory metabolites such as proline, which are known to counteract reactive oxygen species (ROS) [53]. In the present study, growth inhibition observed after chronic exposure (45 days) to MCs is likely linked to disruptions in photosynthetic activity. This was reflected in reduced levels of photosynthetic pigments, notably chlorophylls and carotenoids, which in turn slowed plant development (Figure 1). Saqrane et al. [42], who found that irrigation with MC-contaminated water decreased chlorophyll a and b in the leaves of Zea mays, Lens esculenta, and Triticum aestivum, reported similar observations. Since these pigments are essential for light capture and energy transfer, their depletion directly hampers photosynthetic efficiency and plant performance [54]. Interestingly, supplementation with L. platensis extract (0.1 or 1 g/L), a non-MC-producing species, significantly enhanced chlorophyll b and total chlorophyll levels compared to the control, although no effect was recorded for carotenoids. These results corroborate previous findings where spirulina extracts boosted chlorophyll a and b in Lupinus luteus L. [55], and increased pigment levels when applied as foliar sprays on Beta vulgaris [39], or as supplements in sprouted radish leaves [44]. The stimulatory effect of spirulina is thought to be mediated by improved cell membrane permeability and nutrient uptake efficiency, particularly nitrogen, which is directly linked to chlorophyll biosynthesis [56]. Co-treatment of radish plants with MCs (10 and 40 µg/L) and L. platensis extract (1 g/L) resulted in a significant increase in chlorophyll b compared to MCs exposure alone, although levels remained lower than those obtained with L. platensis extract alone. This indicates a partial protective effect of the extract against MC-induced inhibition of chlorophyll b biosynthesis. However, even at the highest concentration (1 g/L), the extract failed to mitigate the MC-induced decline in carotenoid contents (Figure 1c).
At the biochemical level, exposure of radish plants to MCs (10 and 40 µg/L) significantly increased soluble sugar contents in both leaves and roots compared to the control. However, the increase was more pronounced in plants treated with L. platensis aqueous extract. Similar observations have been reported in Beta vulgaris and Eruca sativa, where foliar application of L. platensis extracts enhanced sugar accumulation [44,45]. In the present study, co-treatment with MCs and L. platensis extract (0.1 and 1 g/L) led to significantly higher sugar levels in both leaves and roots compared to MC exposure alone, though values remained lower than those obtained with L. platensis extract alone. Sugars are known to act as protective metabolites by stabilizing cellular membranes and functioning as antioxidants, thereby improving plant tolerance to stress [56]. Regarding proteins, both MC concentrations (10 and 40 µg/L) caused a significant reduction in leaf protein content relative to the control, while no effect was detected in roots (Figure 2). A similar decrease in protein levels was reported in Lactuca sativa leaves exposed to high concentrations of MC-LR (0.5–10.0 µg/L), MC-RR (0.15–3 µg/L), and total MCs (0.65–13.0 µg/L) [57]. In contrast, Haida et al. [9] observed reductions in protein content in both roots and leaves of F. vulgaris irrigated with MC-contaminated water (20 µg/L), whereas El Khalloufi et al. [8] reported increased protein levels in Medicago sativa exposed to 1–10 µg/L of MCs. These discrepancies likely reflect differences in the ability of MCs to inhibit protein synthesis while simultaneously stimulating the biosynthesis of antioxidant enzymes as a protective strategy against ROS [58]. In the current study, supplementation with L. platensis extract (0.1 and 1 g/L) effectively mitigated the MC-induced reduction in leaf protein content, restoring values to control levels. This protective effect may be attributed to the presence of vitamins and other bioactive compounds in spirulina extracts that promote protein and sugar synthesis [59].
In addition to inhibiting serine/threonine protein phosphatases [40], MCs can induce oxidative stress through mechanisms such as free radical generation, glutathione depletion, and lipid peroxidation, which represent another key pathway underlying their toxicity in both animal and plant cells [60]. Phenolic compounds are central to plant defense mechanisms against abiotic and biotic stresses [61]. Their accumulation is often regarded as an adaptive strategy, as they act as potent antioxidants involved in detoxifying free radicals, thereby maintaining essential plant functions under cyanotoxin-induced stress [62,63]. In the present study, treatment with MCs at both tested concentrations (10 and 40 µg/L) significantly increased polyphenol contents in radish leaves and roots compared to the control (Figure 4a,b). These findings align with previous studies reporting enhanced accumulation of phenolic compounds in various plant species exposed to MCs, including Medicago sativa (11.12 and 22.24 µg/mL) [22], Fragaria vulgaris (1–20 µg/L) [9], Vicia faba (50 and 100 µg/L) [64], and both Raphanus sativus and Daucus carota (1.5–33 µg/L) [65]. However, in this study we observed that treatment of the radish plant with the Limnospira extract at 1 g/L, the L. plattensis extract alone, and with the low concentration (10 µg/L) of MC extract and both concentrations of the L. plattensis extract induced a significant decrease in polyphenols content in leaves and root tissues, suggesting the protective potential against oxidative stress of this aqueous extract.
One of the primary events associated with oxidative stress in plants is the excessive production of ROS, which can overwhelm the antioxidant defense system and impair the plant’s ability to cope with contaminant-induced stress, ultimately leading to reduced biomass accumulation [66]. This defense system comprises non-enzymatic components (vitamins, polyphenols, carotenoids, glutathione) as well as enzymatic antioxidants such as catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD). In the present study, in addition to the significant reduction in carotenoid levels in leaves (Figure 1) and the accumulation of polyphenols in leaves and roots (Figure 5), exposure of radish plants to environmentally relevant concentrations of MCs (10 and 40 µg/L) significantly altered the activities of SOD, POD, and CAT, enzymes that play a central role in maintaining redox balance by scavenging ROS. In leaf tissues, a significant increase in the activities of POD, SOD, and CAT was observed only at the higher MCs concentration (40 µg/L) compared to the control (Figure 6, Figure 7 and Figure 8). By contrast, in root tissues, all three enzymatic activities were significantly enhanced at both MC concentrations relative to the control. These findings are consistent with previous reports in other plant species. For instance, significant increases in POD and CAT activities were observed in the leaves and roots of Medicago sativa and Vicia faba exposed to 10–100 µg/L of MCs [8,64]. Similarly, elevated activities of CAT, SOD, and POD were reported in seedling cells of Cucumis sativus and Oryza sativa after 14 days of exposure to 100 µg/L of MCs [67]. Enhanced POD activity was also recorded in Eruca sativa after 15 days of exposure to 75–100 µg/L of MCs [68], while SOD and CAT activities were increased in Lactuca sativa and Brassica rapa subjected to higher MC concentrations (30–6400 µg/L) [69]. The upregulation of these antioxidant enzymes likely represents a crucial adaptive mechanism by which plants resist abiotic stress induced by MCs and regulate ROS homeostasis [69,70,71]. Together with polyphenols and carotenoids, the increased activities of POD, SOD, and CAT contribute to scavenging toxic free radicals and provide partial protection against lipid peroxidation during both acute and chronic MCs exposure.
Exposure of radish plants to both concentrations (0.1 and 1 g/L) of the aqueous extract of L. platensis significantly increased the activities of SOD, POD, and CAT in root tissues, while in leaf tissues only SOD and POD activities were enhanced (Figure 6, Figure 7 and Figure 8). Under MC-induced stress conditions, the aqueous extract of L. platensis further stimulated the activities of all three antioxidant enzymes (SOD, POD, and CAT) in both leaves and roots. This upregulation of enzymatic activities clearly indicates that the aqueous extract of L. platensis strengthens the enzymatic defense system of radish plants exposed to MCs, thereby improving their capacity to scavenge ROS. These findings are in agreement with previous studies showing that L. platensis can stimulate antioxidant enzymes such as CAT, SOD, and POD under various stress conditions, thereby attenuating oxidative damage through efficient detoxification of ROS [72,73,74,75], and mitigating salt stress in salt-tolerant tomato species [76]. Mechanistically, SOD catalyzes the conversion of superoxide anion radicals (O2−) into H2O2 and O2, while CAT decomposes H2O2 into non-toxic H2O and O2. Consequently, SOD and CAT are often induced simultaneously during oxidative stress to maintain redox homeostasis [77]. Therefore, this study highlighted the beneficial effect of the biostimulant extract based on L. platensis on strengthening and improving the antioxidant potential of radish plants. This can be explained by the fact that L. platensis naturally contains antioxidant elements such as polyphenols and carotenoids and also by its role in enhancing antioxidant activities, which helps plants to rapidly eliminate toxic ROS and thus reduce their damage to metabolic processes [78].
Lipid peroxidation is a well-known mechanism of cellular injury and is widely used as an indicator of oxidative stress in plant and animal tissues [79,80]. Malondialdehyde (MDA), a stable end-product of lipid peroxidation, is commonly used as a biomarker to assess oxidative damage under stress conditions [74]. In the present study, exposure of radish plants to MCs led to a significant increase in MDA levels in both leaves and roots after 45 days of treatment with environmentally relevant concentrations (10 and 40 µg/L). These findings are consistent with previous work reporting elevated MDA levels in Medicago sativa exposed to MCs at concentrations as low as 0.5 μg/L [61]. The observed rise in MDA content reflects the inability of the antioxidant defense system to fully counteract excessive ROS production, resulting in cellular oxidative damage [81]. Interestingly, when radish plants were co-treated with the aqueous extract of L. platensis, MDA levels significantly decreased, reaching values comparable to those of the control group. This demonstrates the protective potential of the extract in mitigating lipid peroxidation and maintaining membrane integrity under MC-induced stress. Similar protective effects were reported by Gharib et al. [82], who observed that L. platensis at 0.1 g/L significantly reduced lipid peroxidation and lowered MDA content in Rosmarinus officinalis.
Several studies have demonstrated that the presence of MCs in irrigation water not only affects plant growth, biochemical parameters, and oxidative stress markers but also compromises taproot yield and nutritional quality [83,84,85]. In the present study, exposure of radish plants to environmentally relevant concentrations of MCs (10 and 40 µg/L) led to a significant reduction in taproot biometric parameters and yield, as well as in their nutritional quality (Table 4 and Table 5). For instance, treatment with 40 µg/L of MCs caused a marked decrease in taproot dry weight (TDW) compared to the control (Table 3). Although co-treatment with 40 µg/L of MCs and the aqueous extract of L. platensis (0.1 or 1 g/L) partially restored TDW, the values remained significantly lower than those of the control. Moreover, vitamin C levels in taproots significantly decreased following exposure to both concentrations of MCs, while co-treatment with L. platensis extract restored these levels significantly. A similar protective effect of L. platensis was also observed for Zn and P contents in radish taproots (Table 5).
5. Conclusions
MCs can be bioaccumulated in both leaves and edible parts (taproots), with the highest concentration measured in taproots. Co-exposure of the plant with the L. platensis extract induced a slight but non-significant decrease in MCs’ bioaccumulation in both tissues. Bioaccumulation of MCs in both edible parts and leaves negatively affected taproot quality and yield, as well as plant biochemical and morphological characteristics by inducing oxidative stress marked by a significant increase in MDA level in both roots and leaves. However, treatment with 1 g/L of L. platensis extract improved the defense mechanisms of the radish plant by significantly reducing the MDA level in leaves and roots to the control value. Moreover, the L. platensis extract enhanced the nutritional quality of the plant that was affected by MCs by significantly increasing the levels of vitamin C and some minerals like zinc and phosphorus in the edible part (taproots). The promising results of the biostimulant and protective potential of the L. platensis extract against the negative effects of MCs would deserve to be further explored in field studies with different crops.
Author Contributions
Conceptualization, M.H., B.E. and B.O.; methodology, M.H., B.E., Z.H. and Y.E.; software, R.M.; validation, B.O., A.H. and N.B.; formal analysis, M.H., M.L. and R.M.; investigation, B.O.; resources, B.O.; data curation, M.H.; writing—original draft preparation, M.H. and B.E.; writing—review and editing, M.H.; visualization, F.E.K.; supervision, B.O., A.H., F.E.K. and N.B.; project administration, N.B.; funding acquisition, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
“This research received funding from the Moroccan Ministry of Higher Education via the budget allocated to the accredited “Aquatic Sciences, Microbial Biotechnology and Sustainability of Natural Resources Laboratory”; formerly “EAUBIODICC laboratory”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would also like to express their thanks and gratitude to Suleiman Muhammat Nakour for providing the natural Limnospira material from lake Chad used in this work and to the anonymous reviewers for their helpful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins–occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.J.; Borthwick, E.B.; Morrison, L.F.; Codd, G.A.; Cohen, P.T.W. Inhibition of several protein phosphatases by a non-covalently interacting microcystin and a novel cyanobacterial peptide, nostocyclin. Biochim. Biophys. Acta Gen. Subj. 2005, 1726, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C. Evidence for a novel marine harmful algal bloom: Cyanotoxin (microcystin) transfer from land to sea otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef]
- Mugani, R.; El Khalloufi, F.; Kasada, M.; Haida, M.; Aba, R.P.; Essadki, Y.; Zerrifi, S.E.A.; Herter, S.-O.; Hejjaj, A.; Aziz, F. Monitoring of toxic cyanobacterial blooms in Lalla Takerkoust reservoir by satellite imagery and microcystin transfer to surrounding farms. Harmful Algae 2024, 135, 102631. [Google Scholar] [CrossRef]
- El Khalloufi, F.; Oufdou, K.; Lahrouni, M.; El Ghazali, I.; Saqrane, S.; Vasconcelos, V.; Oudra, B. Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa-Rhizobia symbiosis. Ecotoxicol. Environ. Saf. 2011, 74, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Haida, M.; El Khalloufi, F.; Mugani, R.; Redouane, E.M.; Campos, A.; Vasconcelos, V.; Oudra, B. Effects of Irrigation with Microcystin-Containing Water on Growth, Physiology, and Antioxidant Defense in Strawberry Fragaria vulgaris under Hydroponic Culture. Toxins 2022, 14, 198. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; Oudra, B. Occurrence of cyanobacteria producing toxins in irrigation freshwaters: Which impacts on crop quality and public health? J. Mater. Environ. Sci. 2015, 6, 2986–3001. [Google Scholar]
- Peng, K.; Liu, X.; Cheng, H.; Xu, M.; Liu, Y.; Yang, H.; Liu, P.; Yang, S. Characterization of driving factors for the long-term succession of bloom-forming cyanobacterial genera in Lake Erhai, southwest China. J. Environ. Manag. 2024, 351, 119729. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H. Removal of cyanobacterial and algal cells from water by ultrasonic waves—A review. J. Mol. Liq. 2016, 222, 1109–1114. [Google Scholar] [CrossRef]
- Anderson, C.R.; Sellner, K.G.; Anderson, D.M. Bloom prevention and control. In Harmful Algal Blooms (HABs) and Desalination: A Guide to Impacts, Monitoring and Management; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2017; pp. 205–222. [Google Scholar]
- Beasley, V.R.; Cook, W.O.; Dahlem, A.M.; Hooser, S.B.; Lovell, R.A.; Valentine, W.M. Algae intoxication in livestock and waterfowl. Vet. Clin. N. Am. Food Anim. Pract. 1989, 5, 345–361. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Microalgae: New source of plant biostimulants. Agronomy 2020, 10, 1240. [Google Scholar] [CrossRef]
- El-Sayed, S.A.A. Effect of potassium fertilization levels and algae extract on growth, bulb yield and quality of onion (Allium cepa L.). Middle East J. 2018, 7, 625–638. [Google Scholar]
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754. [Google Scholar] [CrossRef] [PubMed]
- Dmytryk, A.; Chojnacka, K. Algae as fertilizers, biostimulants, and regulators of plant growth. In Algae Biomass: Characteristics And Applications: Towards Algae-Based Products; Springer: Cham, Switzerland, 2018; pp. 115–122. [Google Scholar]
- Bouaıcha, N.; Chézeau, A.; Turquet, J.; Quod, J.-P.; Puiseux-Dao, S. Morphological and toxicological variability of Prorocentrum lima clones isolated from four locations in the south-west Indian Ocean. Toxicon 2001, 39, 1195–1202. [Google Scholar] [CrossRef]
- El Khalloufi, F.; Oufdou, K.; Lahrouni, M.; Faghire, M.; Peix, A.; Ramírez-Bahena, M.H.; Vasconcelos, V.; Oudra, B. Physiological and antioxidant responses of Medicago sativa-rhizobia symbiosis to cyanobacterial toxins (Microcystins) exposure. Toxicon 2013, 76, 167–177. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Savicka, M.; Škute, N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 2010, 56, 26–33. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, p. 152. [Google Scholar]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of indole-3-acetic acid (IAA) production in Klebsiella by LC-MS/MS and the Salkowski method. Bio-Protocol 2019, 9, e3230. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circular 1950, 347, 32. [Google Scholar]
- Corbel, S.; Mougin, C.; Nélieu, S.; Delarue, G.; Bouaïcha, N. Evaluation of the transfer and the accumulation of microcystins in tomato (Solanum lycopersicum cultivar MicroTom) tissues using a cyanobacterial extract containing microcystins and the radiolabeled microcystin-LR (14C-MC-LR). Sci. Total Environ. 2016, 541, 1052–1058. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Carol, P.; Mouradi, M.; Savoure, A.; Farissi, M. Silicon improves physiological, biochemical, and morphological adaptations of alfalfa (Medicago sativa L.) during salinity stress. Symbiosis 2021, 85, 305–324. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Pame, P. Lead phyto-toxicity induced by accumulation and uptake potentially inhibits morpho-physiological depression and alterations in an aquatic model plant, Eichhornia crassipes. EurAsian J. Biosci. 2019, 13, 1565–1573. [Google Scholar]
- Pathy, K. Process for preparation of vitamin C and method for determination of vitamin c in tablets. SF J. Chem. Res. 2018, 2, 2. [Google Scholar] [CrossRef]
- Pequerul, A.; Pérez, C.; Madero, P.; Val, J.; Monge, E. A rapid wet digestion method for plant analysis. In Optimization of Plant Nutrition: Refereed Papers from the Eighth International Colloquium for the Optimization of Plant Nutrition, Portugal; Springer: Berlin/Heidelberg, Germany, 1993; pp. 3–6. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Nitrogen-total. In Methods of Soil Analysis. Part; ASA; SSSA: Madison, WI, USA, 1982; Volume 2, pp. 403–430. [Google Scholar]
- Fidalgo, F.; Azenha, M.; Silva, A.F.; de Sousa, A.; Santiago, A.; Ferraz, P.; Teixeira, J. Copper-induced stress in Solanum nigrum L. and antioxidant defense system responses. Food Energy Secur. 2013, 2, 70–80. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Gehringer, M.M.; Kewada, V.; Coates, N.; Downing, T.G. The use of Lepidium sativum in a plant bioassay system for the detection of microcystin-LR. Toxicon 2003, 41, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Azevedo, J.; Pinto, E.; Neves, J.; Campos, A.; Vasconcelos, V. Effects of microcystin-LR, cylindrospermopsin and a microcystin-LR/cylindrospermopsin mixture on growth, oxidative stress and mineral content in lettuce plants (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2015, 116, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhong, Y.M.; Zhang, H.Q.; Shi, Z.Q. Nitrate reductase-dependent nitric oxide production is involved in microcystin-LR-induced oxidative stress in brassica rapa. Water Air Soil Pollut. 2012, 223, 4141–4152. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kurki-Helasmo, K.; Meriluoto, J. Microcystin uptake inhibits growth and protein phosphatase activity in mustard (Sinapis alba L.) seedlings. Toxicon 1998, 36, 1921–1926. [Google Scholar] [CrossRef]
- Saqrane, S.; El Ghazali, I.; Oudra, B.; Bouarab, L.; Vasconcelos, V. Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: A laboratory experimental approach. Toxicon 2009, 53, 786–796. [Google Scholar] [CrossRef]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.F. Anticancer Activity, Antioxidant Activity, Mineral Contents, Vegetative and Yield of Eruca sativa Using Foliar Application of Autoclaved Cellular Extract of Spirulina platensis Extract, Comparing to NPK Fertilizers. J. Plant Prod. 2017, 8, 529–536. [Google Scholar] [CrossRef]
- Enan, S.; El-Saady, A.M.; El-Sayed, A.B. Impact of foliar feeding with alga extract and boron on yield and quality of sugar beet grown in sandy soil. Egypt. J. Agronematol. 2016, 38, 319–336. [Google Scholar]
- Wang, A.; Hu, J.; Huang, X.; Li, X.; Zhou, G.; Yan, Z. Comparative transcriptome analysis reveals heat-responsive genes in Chinese cabbage (Brassica rapa ssp. chinensis). Front. Plant Sci. 2016, 7, 939. [Google Scholar] [CrossRef]
- Plaza, I.; García, J.L.; Villarroel, M. Effect of spirulina (Arthrospira platensis) supplementation on tilapia (Oreochromis niloticus) growth and stress responsiveness under hypoxia. Span. J. Agric. Res. 2018, 16, e0606. [Google Scholar] [CrossRef]
- Rachidi, F.; Benhima, R.; Sbabou, L.; El Arroussi, H. Microalgae polysaccharides bio-stimulating effect on tomato plants: Growth and metabolic distribution. Biotechnol. Rep. 2020, 25, e00426. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.S.; Esawy, M.A. Evaluation of Spirulina platensis as bio stimulator for organic farming systems. J. Genet. Eng. Biotechnol. 2008, 6, 1–7. [Google Scholar]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baz, F.K.; El Baroty, G.S. Enhancing Antioxidant Availability in Wheat Grains from Plants Grown under Seawater Stress in Response to Microalgae Extract Treatments. J. Sci. Food Agric. 2010, 90, 299–303. [Google Scholar] [CrossRef]
- Selem, E. Physiological effects of Spirulina platensis in salt stressed Vicia faba L. plants. Egypt. J. Bot. 2019, 59, 185–194. [Google Scholar] [CrossRef]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Shedeed, Z.A.; Gheda, S.; Elsanadily, S.; Alharbi, K.; Osman, M.E.H. Spirulina platensis biofertilization for enhancing growth, photosynthetic capacity and yield of Lupinus luteus. Agriculture 2022, 12, 781. [Google Scholar] [CrossRef]
- Arahou, F.; Lijassi, I.; Wahby, A.; Rhazi, L.; Arahou, M.; Wahby, I. Spirulina-based biostimulants for sustainable agriculture: Yield improvement and market trends. BioEnergy Res. 2023, 16, 1401–1416. [Google Scholar] [CrossRef]
- Ben Abdallah, N.; Ben Salem, M. Parametres morphophysiologiques de selection pour la resistance a la secheresse des cereales. In Tolerance a la Secheresse des Cereales en Zone Mediterraneenne; Diversité génétique et amélioration variétale; INRA: Paris, France, 1993; pp. 4216–4222. [Google Scholar]
- do Bittencourt-Oliveira, M.C.; Cordeiro-Araújo, M.K.; Chia, M.A.; de Arruda-Neto, J.D.T.; de Oliveira, Ê.T.; dos Santos, F. Lettuce irrigated with contaminated water: Photosynthetic effects, antioxidative response and bioaccumulation of microcystin congeners. Ecotoxicol. Environ. Saf. 2016, 128, 83–90. [Google Scholar] [CrossRef]
- Marrs, K.A. The functions and regulation of glutathione s-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 127–158. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N. Productivity and quality of kohlrabi grown in a newly reclaimed sandy soil using organic and mineral-N fertilizer regimes with or without spraying of Spirulina platensis extract. Egypt. J. Hortic. 2019, 46, 169–178. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Aulhorn, M.; Grimm, B. Influence of a cyanobacterial crude extract containing microcystin-LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol. 2007, 175, 482–489. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P.; Stavroulaki, V.; Sumbele, S. Carbon gain vs. water saving, growth vs. defence: Two dilemmas with soluble phenolics as a joker. Plant Sci. 2014, 227, 21–27. [Google Scholar] [CrossRef]
- Ksouri, R.; Wided, M.; Ahmed, D.; Hanen, F.; Claude, G.; Chedly, A. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile Maritima. Plant Physiol. Biochem. PPB 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; El Khalloufi, F.; Baz, M.; Lafuente, A.; Dary, M.; Pajuelo, E.; Oudra, B. Physiological and biochemical defense reactions of Vicia faba L.-Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ. Sci. Pollut. Res. 2013, 20, 5405–5415. [Google Scholar] [CrossRef] [PubMed]
- Levizou, E.; Papadimitriou, T.; Papavasileiou, E.; Papadimitriou, N.; Kormas, K.A. Root vegetables bioaccumulate microcystins-LR in a developmental stage-dependent manner under realistic exposure scenario: The case of carrot and radish. Agric. Water Manag. 2020, 240, 106274. [Google Scholar] [CrossRef]
- Turkan, I. ROS and RNS: Key signalling molecules in plants. J. Exp. Bot. 2018, 69, 3313–3315. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro-Araújo, M.K.; Chia, M.A.; de Arruda-Neto, J.D.T.; Tornisielo, V.L.; Vilca, F.Z.; do Bittencourt-Oliveira, M.C. Microcystin-LR bioaccumulation and depuration kinetics in lettuce and arugula: Human health risk assessment. Sci. Total Environ. 2016, 566–567, 1379–1386. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, B.; Song, L.; Wu, X.; Zhang, J.; Wang, C. Effects of microcystin-LR, linear alkylbenzene sulfonate and their mixture on lettuce (Lactuca sativa L.) seeds and seedlings. Ecotoxicology 2011, 20, 803–814. [Google Scholar] [CrossRef]
- Gómez, R.; Vicino, P.; Carrillo, N.; Lodeyro, A.F. Manipulation of oxidative stress responses as a strategy to generate stress-tolerant crops. From damage to signaling to tolerance. Crit. Rev. Biotechnol. 2019, 39, 693–708. [Google Scholar] [CrossRef]
- Kumar, D.; Yusuf, M.A.; Singh, P.; Sardar, M.; Sarin, N.B. Modulation of antioxidant machinery in α-tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions. Protoplasma 2013, 250, 1079–1089. [Google Scholar] [CrossRef]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N., Jr.; Vargas, L. Defenses against ROS in crops and weeds: The effects of interference and herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-UI-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.G.M.S.U.; Polash, M.A.S.; Hossain, M.T.; Sohel, M.A.T.; Brestic, M. Differential response of sugar beet to long-term mild to severe salinity in a soil–pot culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.-J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Mittova, V.; Tal, M.; Volokita, M.; Guy, M. Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 2003, 26, 845–856. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE. 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kobayashi, Y.; Matsumoto, H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Gharib, F.A.E.L.; Ahmed, E.Z. Spirulina platensis improves growth, oil content, and antioxidant activitiy of rosemary plant under cadmium and lead stress. Sci. Rep. 2023, 13, 8008. [Google Scholar] [CrossRef] [PubMed]
- Haida, M.; El Khalloufi, F.; Essadki, Y.; Alexandrino, D.A.M.; Mugani, R.; Hejjaj, A.; Campos, A.; Vasconcelos, V.; Carvalho, M.F.; Díez-Quijada, L. Microcystin-degrading bacteria reduce bioaccumulation in Fragaria vulgaris and enhance fruit yield and quality. Environ. Sci. Pollut. Res. 2024, 31, 54502–54524. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ma, X.; Liu, H. Effect of microcystins at different rice growth stages on its yield, quality, and safety. Environ. Sci. Pollut. Res. 2021, 28, 13942–13954. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, X.; Liu, H.; Liang, C. Effect of irrigation with microcystins-contaminated water on growth and fruit quality of Cucumis sativus L. and the health risk. Agric. Water Manag. 2018, 204, 91–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).