Association Between Antibiotic Exposure and the Risk of Male Infertility: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sample Size
2.3. Data Collection
2.4. Analysis of Urinary Antibiotics
2.5. Data Analysis
3. Results
3.1. Socio-Demographic Characteristics of the Study Population
3.2. Descriptive Analysis of the Sperm Parameters by Group
3.3. Descriptive Analysis of Antibiotics by Group
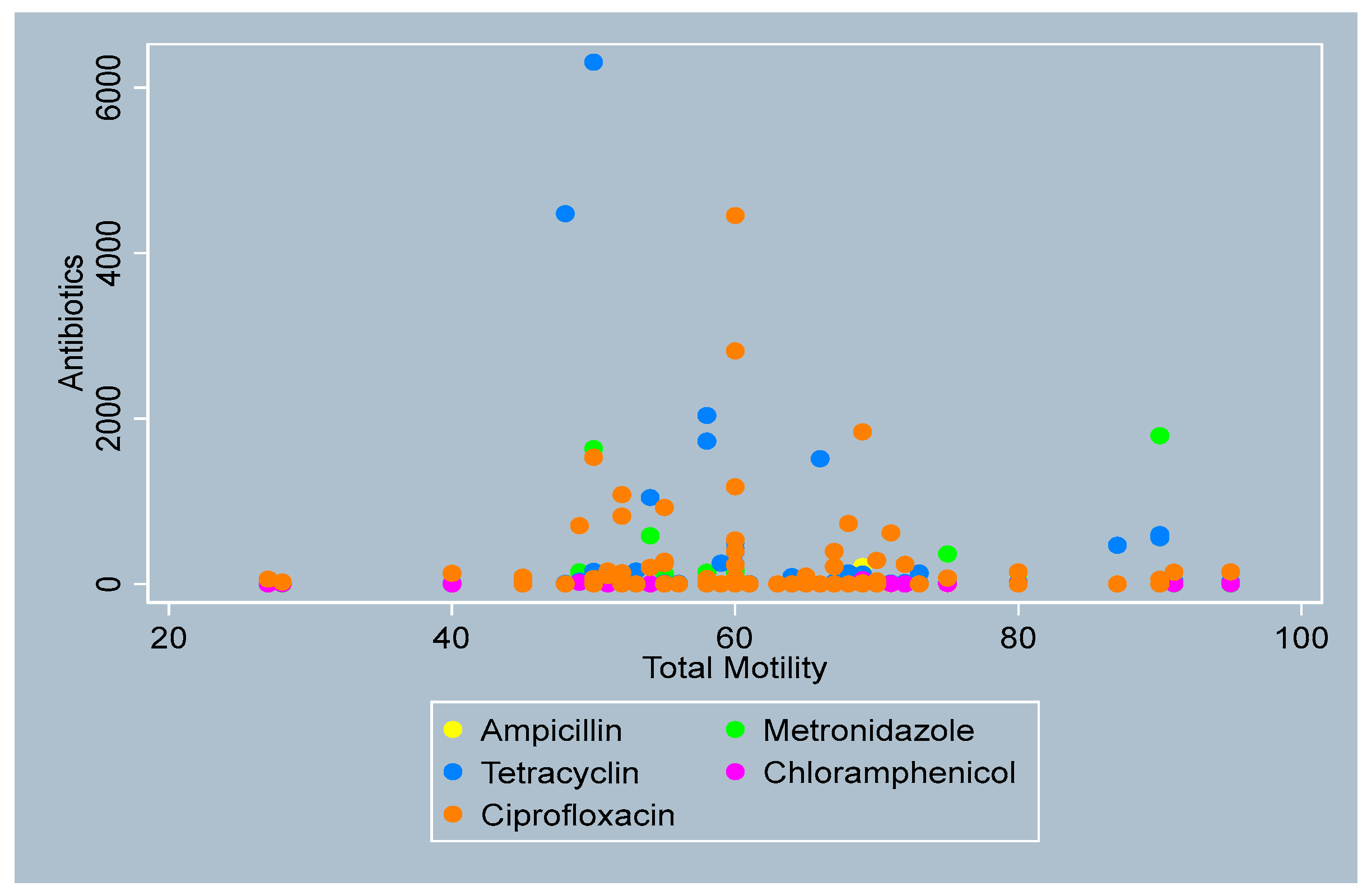
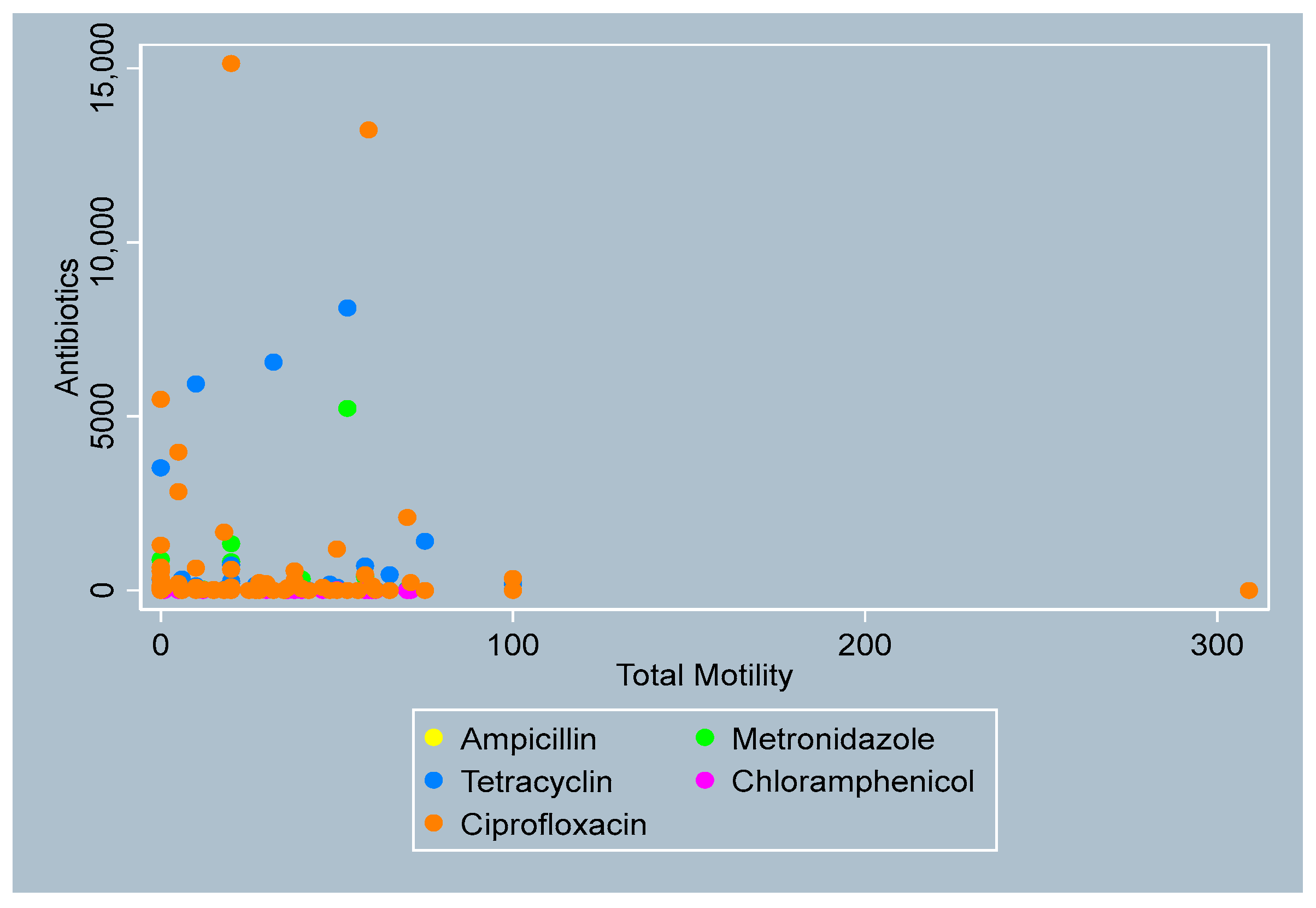
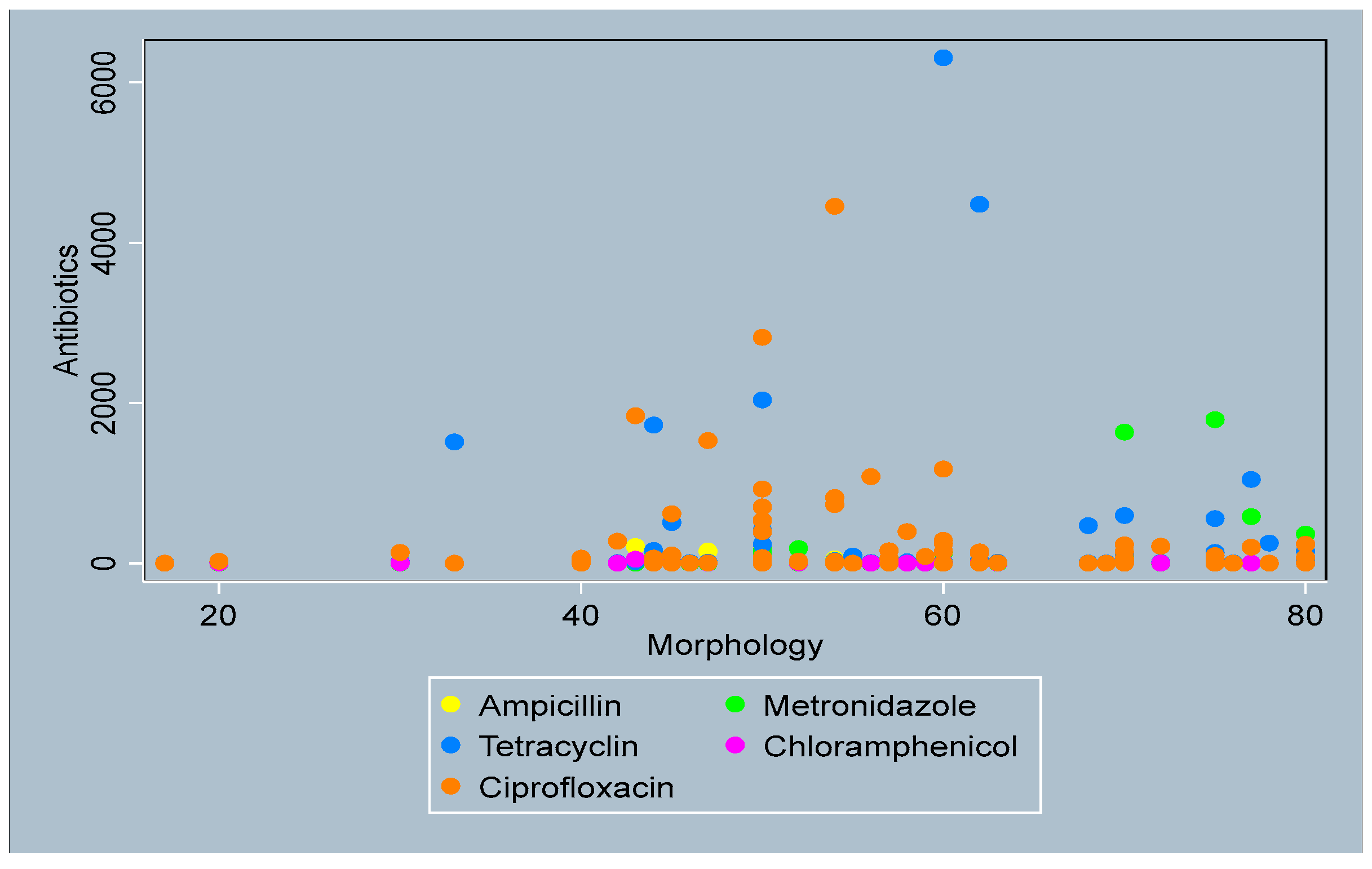
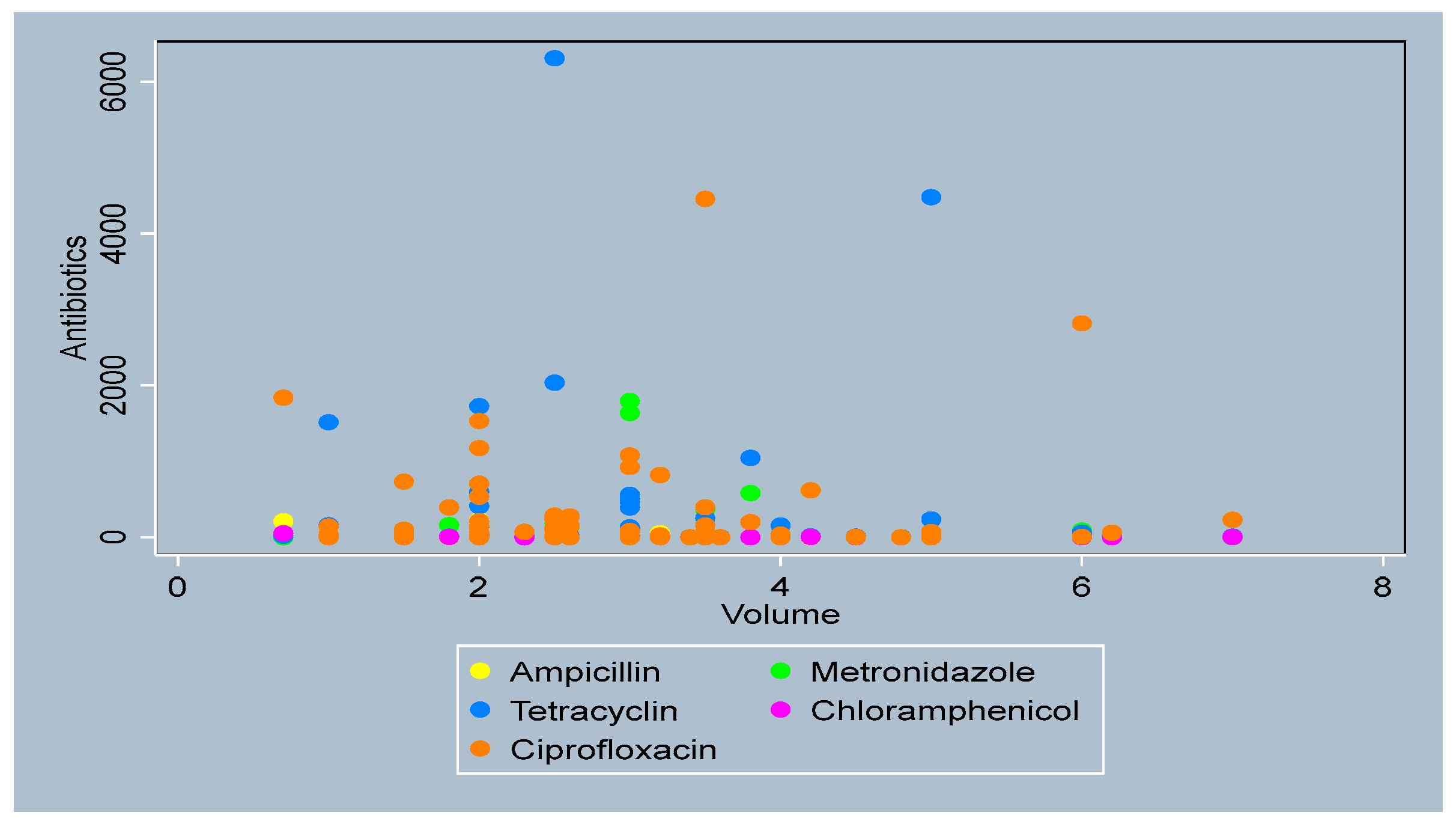
3.4. Distribution of Antibiotics by Sperm Parameters
3.5. Multivariable Analysis of the Marginal Effect of Antibiotics on Sperm Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LCMS/MS | Liquid Chromatography Mass Spectrometry/Mass Spectrometry |
| IQR | Inter-quartile Range |
| SD | Standard Deviation |
| SE | Standard Error |
Appendix A
- Sperm Count
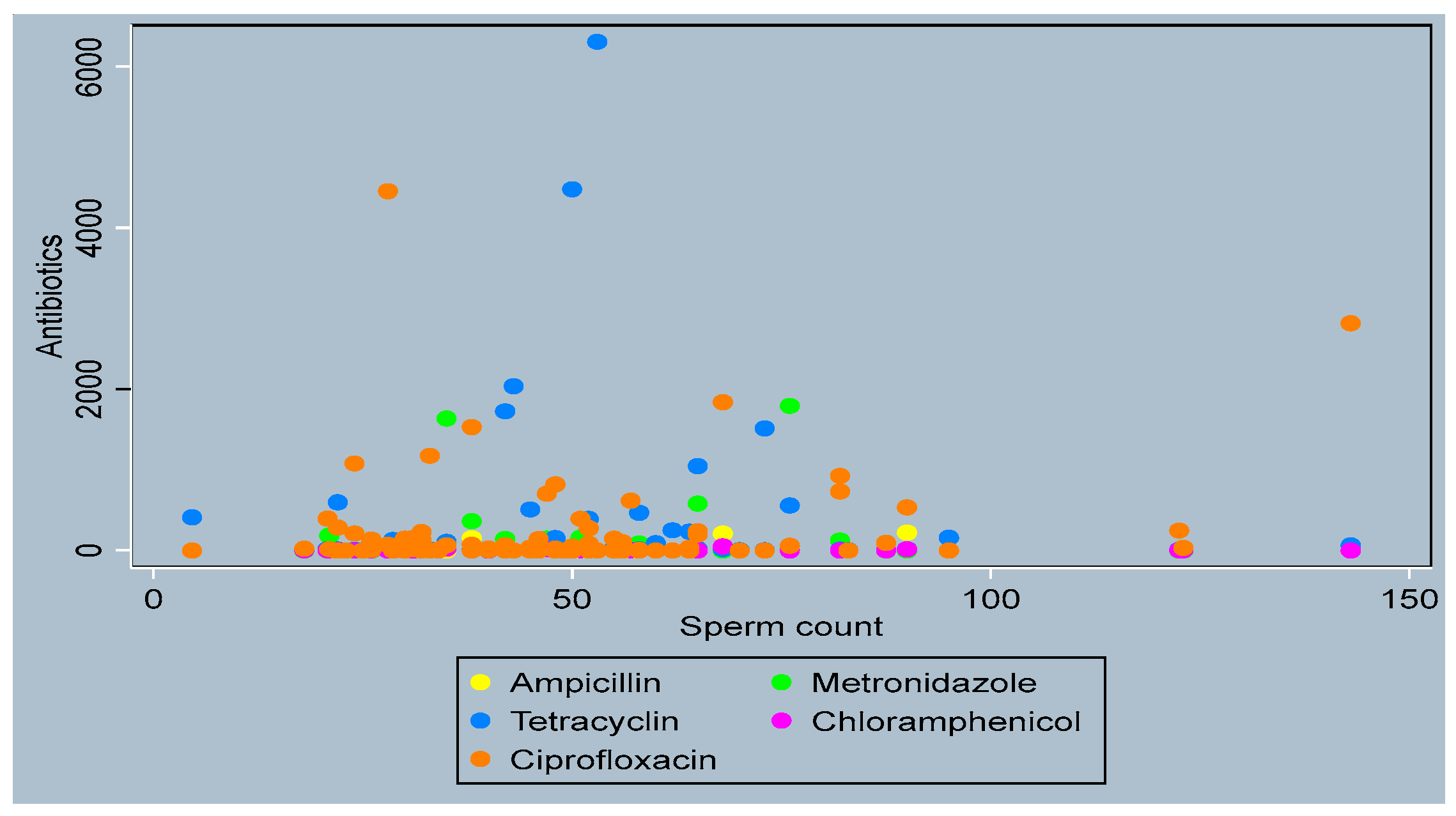
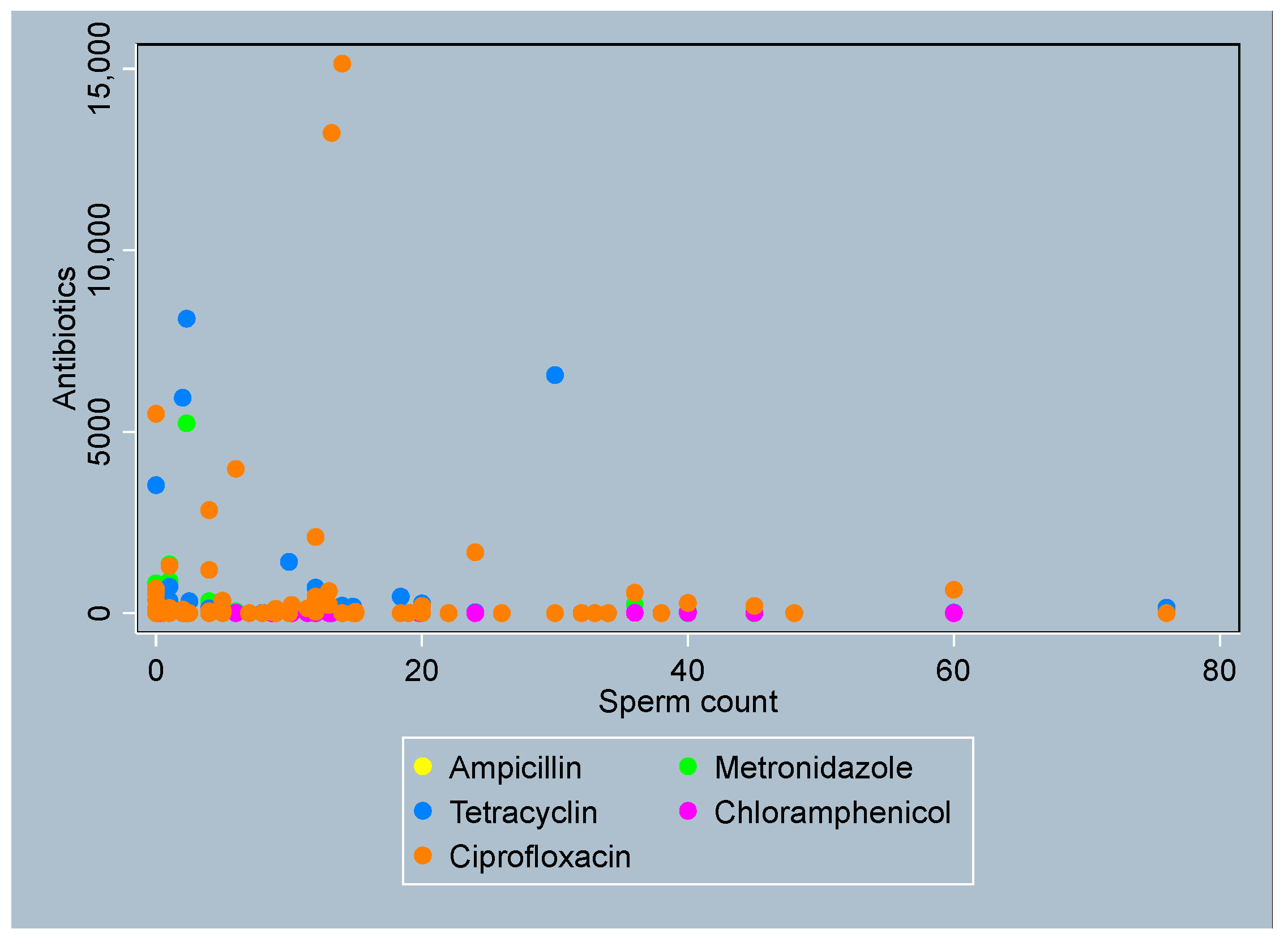
- Active Motility
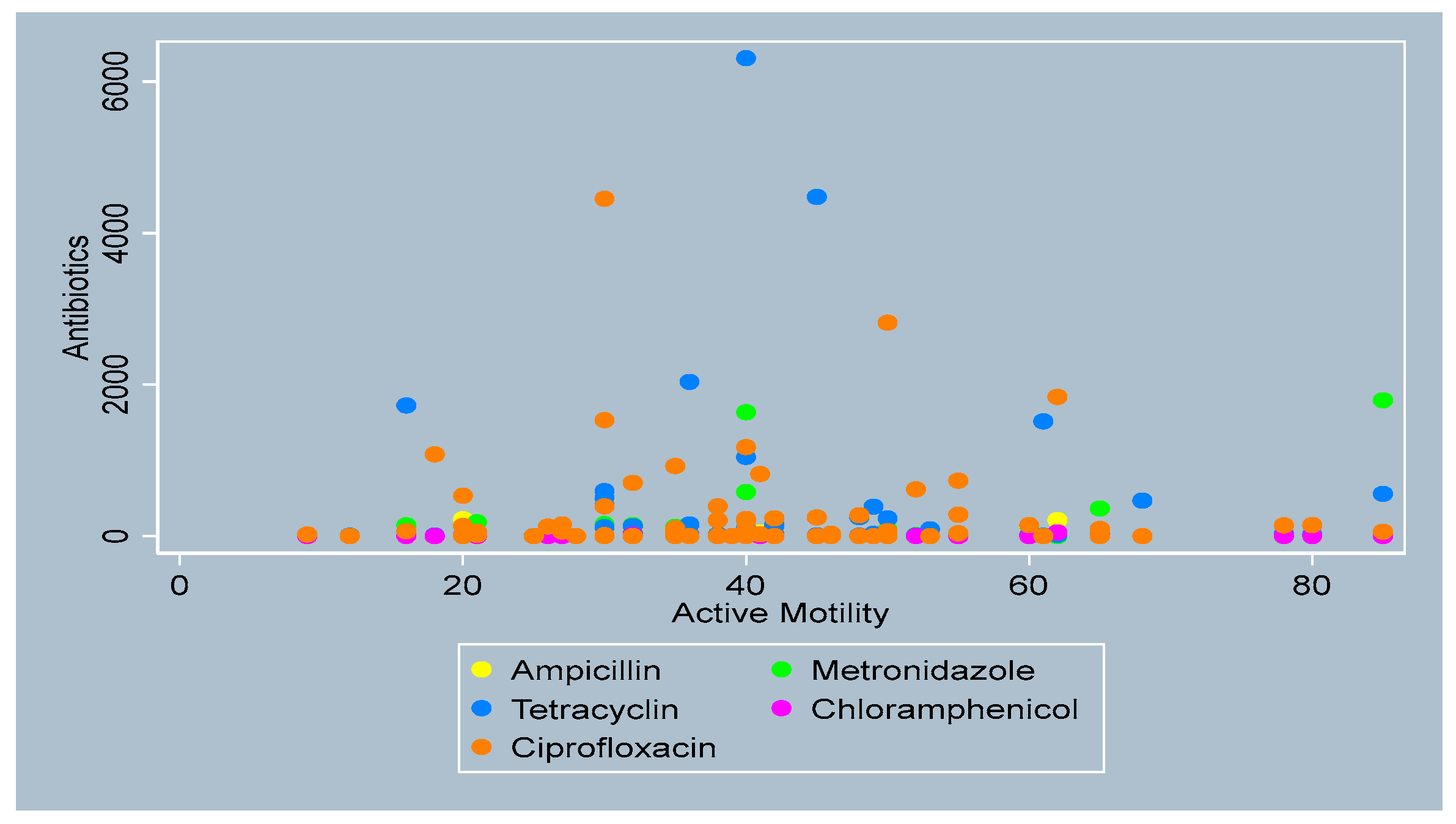
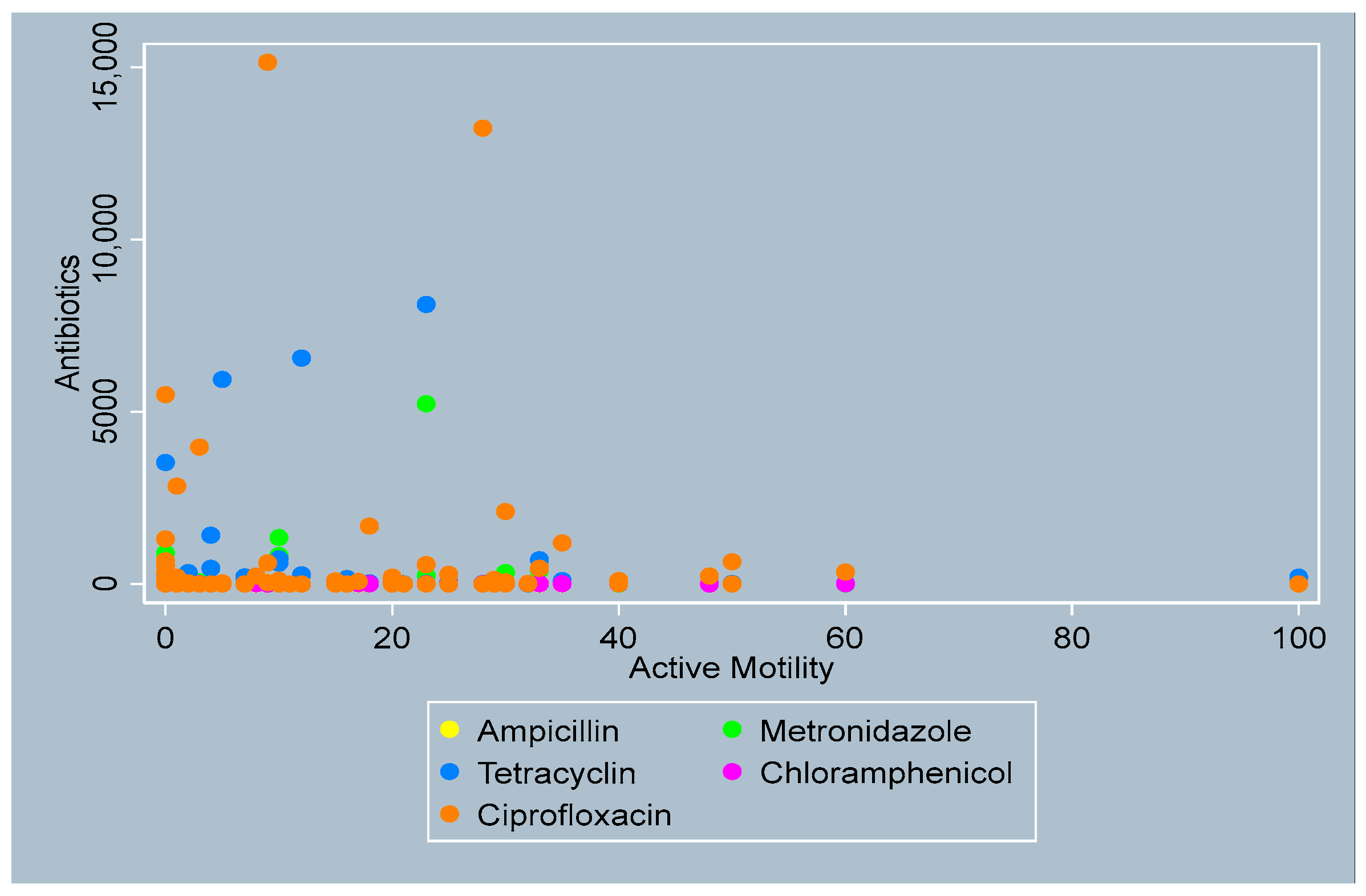
- Total Motility
- Sperm morphology

- Sperm volume

References
- Belsey, M.A. The Epidemiology of Infertility: A Review with Particular Reference to Sub-Saharan Africa. Bull. World Health Organ. 1976, 54, 319. [Google Scholar]
- Frank, O. Infertility in Sub-Saharan Africa: Estimates and Implications. Popul. Dev. Rev. 1983, 9, 137–144. [Google Scholar] [CrossRef]
- Okonofua, F.E.; Ntoimo, L.F.C.; Omonkhua, A.; Ayodeji, O.; Olafusi, C.; Unuabonah, E.; Ohenhen, V. Causes and Risk Factors for Male Infertility: A Scoping Review of Published Studies. Int. J. Gen. Med. 2022, 15, 5985–5997. [Google Scholar] [CrossRef]
- Abarikwu, S.O. Causes and Risk Factors for Male-Factor Infertility in Nigeria: A Review. Afr. J. Reprod. Health 2013, 17, 150–166. [Google Scholar]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.-M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef]
- Skau, P.A.; Folstad, I. Do Bacterial Infections Cause Reduced Ejaculate Quality? A Meta-Analysis of Antibiotic Treatment of Male Infertility. Behav. Ecol. 2003, 14, 40–47. [Google Scholar] [CrossRef]
- Gupta, H.; Maheshwari, K.K.; Kumar, N. Reversible Germ Cell Toxicity of Sulphasalazine and Ampicillin Combination in Male Rats. J. Reprod. Infertil. 2013, 14, 126. [Google Scholar]
- Khaki, A. Assessment on the Adverse Effects of Aminoglycosides and Flouroquinolone on Sperm Parameters and Male Reproductive Tissue: A Systematic Review. Iran. J. Reprod. Med. 2015, 13, 125. [Google Scholar]
- Fahmy, M.A.; Farghaly, A.A.; Omara, E.A.; Hassan, Z.M.; Aly, F.A.; Donya, S.M.; Ibrahim, A.A.; Bayoumy, E.M. Amoxicillin–Clavulanic Acid Induced Sperm Abnormalities and Histopathological Changes in Mice. Asian Pac. J. Trop. Biomed. 2017, 7, 809–816. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Chang, T.; Marshall, F.F. Antibiotics: Potential Hazards to Male Fertility. Fertil. Steril. 1991, 55, 235–242. [Google Scholar] [CrossRef]
- Okonofua, F.E.; Ntoimo, L.F.C.; Unuabonah, E.I.; Msagati, T.A.M.; Ayodeji, O.; Aziken, M.; Omonkhua, A.; Ohenhen, V.; Olafusi, C.; Alfred, M.O. Association of Urinary Mycotoxins with Sperm Quality: A Case-Control Study in Southern Nigeria. Toxins 2024, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Okonofua, F.E.; Ntoimo, L.F.C.; Unuabonah, E.; Msagati, T.A.M.; Ekwo, M.C.; Ayodeji, O.F.; Aziken, M.E.; Maduako, K.T.; Onoh, V.I.; Omonkhua, A. Association Between Urinary Parabens and Sperm Quality in Nigerian Men: A Case–Control Study. Int. J. Gen. Med. 2024, 17, 2767–2779. [Google Scholar] [CrossRef]
- Mohammedi, L.; Messaï, A.; Ouamane, H.; Bencharif, S.; Touazi, L.; Iguer-Ouada, M. In Vivo Effect of Ampicillin, Enrofloxacin, Colistin, and Sulfonamides on Sperm Parameters in Breeding Roosters. J. Microbiol. Biotechnol. Food Sci. 2024, 13, e9607. [Google Scholar] [CrossRef]
- Adesanya, A.; AWOBAJO, F. Fourteen Days Oral Administration of Therapeutic Dosage of Some Antibiotics Reduced Serum Testosterone in Male Rats. Niger. J. Health Biomed. Sci. 2006, 5, 17. [Google Scholar]
- Oyedeji, K.; Bolarinwa, A.; Dare, A. Effect of Tetracyclin on Reproductive Functions in Male Albino Rats. IOSR J. Dent. Med. Sci. 2013, 3, 55–60. [Google Scholar]
- Hargreaves, C.A.; Rogers, S.; Hills, F.; Rahman, F.; Howell, R.; Homa, S.T. Effects of Co-Trimoxazole, Erythromycin, Amoxycillin, Tetracycline and Chloroquine on Sperm Function In Vitro. Hum. Reprod. 1998, 13, 1878–1886. [Google Scholar] [CrossRef]
- Okonofua, F.; Menakaya, U.; Onemu, S.; Omo–Aghoja, L.; Bergstrom, S. A Cas–control Study of Risk Factors for Male Infertility in Nigeria. Asian J. Androl. 2005, 7, 351–361. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hoque, S.M.; Kawai, T.; Zeng, W.; Shimada, M. Gene Expression and Protein Synthesis in Mitochondria Enhance the Duration of High-Speed Linear Motility in Boar Sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Oyeyemi, M.O.; Adeniji, D.A. Morphological Characteristics and Haematological Studies in Wistar Rats Subjected to Prolonged Treatment of Chloramphenicol. Int. J. Morphol. 2009, 27, 7–11. [Google Scholar] [CrossRef]
- Twaina–Bechor, E.; Bartoov, B. The Relationship Between Ejaculated Ram Sperm Mitochondrial Protein Synthesis and Motility. Andrologia 1994, 26, 351–355. [Google Scholar] [CrossRef]
- Bisconti, M.; Leroy, B.; Gallagher, M.T.; Senet, C.; Martinet, B.; Arcolia, V.; Wattiez, R.; Kirkman-Brown, J.C.; Simon, J.-F.; Hennebert, E. The Ribosome Inhibitor Chloramphenicol Induces Motility Deficits in Human Spermatozoa: A Proteomic Approach Identifies Potentially Involved Proteins. Front. Cell Dev. Biol. 2022, 10, 965076. [Google Scholar] [CrossRef] [PubMed]
- Chukwuani, C.M.; Onifade, M.; Sumonu, K. Survey of Drug Use Practices and Antibiotic Prescribing Pattern at a General Hospital in Nigeria. Pharm. World Sci. 2002, 24, 188–195. [Google Scholar] [CrossRef]
- Kehinde, O.O.; Ogunnowo, B.E. The Pattern of Antibiotic Use in an Urban Slum in Lagos State, Nigeria. West Afr. J. Pharm. 2013, 24, 24–57. [Google Scholar]
- Chukwu, E.E.; Oladele, D.A.; Enwuru, C.A.; Gogwan, P.L.; Abuh, D.; Audu, R.A.; Ogunsola, F.T. Antimicrobial Resistance Awareness and Antibiotic Prescribing Behavior among Healthcare Workers in Nigeria: A National Survey. BMC Infect. Dis. 2021, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Ajibola, O.; Omisakin, O.A.; Eze, A.A.; Omoleke, S.A. Self-Medication with Antibiotics, Attitude and Knowledge of Antibiotic Resistance Among Community Residents and Undergraduate Students in Northwest Nigeria. Diseases 2018, 6, 32. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Fertile N = 92 | Infertile N = 87 | Total N = 179 | p-Value |
|---|---|---|---|---|
| Mean(SD) [Range] | Mean(SD) [Range] | Mean(SD) [Range] | ||
| Age | 40.9(7.6) [20–62] | 40.1(7.9) [18–64] | 40.5(7.7) [18–64] | 0.4823 |
| Weight(kg) (n = 177) | 80.2(15.2) [46–115] | 81.5(14.5) [51.2–122] | 80.8(14.8) [46–122] | 0.5661 |
| BMI (n = 176) | 26.7(5.4) [16–41.2] | 27.6(5.1) [16.2–43] | 27.2(5.3) [16–43] | 0.2698 |
| Height (meter) (n = 176) | 1.7(0.13) [1.3–1.9] | 1.6(0.11) [1.3–1.9] | 1.6(0.12) [1.3–1.9] | 0.8599 |
| No.(%) | No.(%) | No.(%) | ||
| BMI (n = 176) | 0.202 | |||
| Normal | 28(31.1) | 26(30.2) | 54(30.7) | |
| Thin | 6(6.7) | 1(1.2) | 7(4.0) | |
| Overweight | 32(35.6) | 28(32.6) | 60(34.1) | |
| Obese | 24(26.7) | 31(36) | 55(31.3) | |
| Marital Status | 0.878 | |||
| Single | 8(9.0) | 7(8.3) | 15(8.7) | |
| Married | 81(91.0) | 77(91.7) | 158(91.3) | |
| Religion | 0.725 | |||
| Islam | 6(6.5) | 4(4.6) | 10(5.6) | |
| Christian | 85(92.4) | 81(93.1) | 166(92.7) | |
| Traditional/Other | 1(1.1) | 2(2.3) | 3(1.7) | |
| Education | 0.808 | |||
| Primary | 7(7.6) | 4(4.6) | 11(6.1) | |
| Secondary | 21(22.8) | 24(27.6) | 45(25.1) | |
| Tertiary | 62(67.4) | 57(65.5) | 119(66.5) | |
| Other | 2(2.2) | 2(2.3) | 4(2.2) | |
| Occupation | 0.148 | |||
| Agriculture | 2(2.2) | 2(2.3) | 4(2.2) | |
| Business | 28(30.4) | 22(25.3) | 50(27.9) | |
| Skilled manual | 12(13.0) | 10(11.5) | 22(12.3) | |
| Blue collar | 18(19.6) | 8(9.2) | 26(14.5) | |
| Professional | 14(15.2) | 19(21.8) | 33(18.4) | |
| Civil servant | 12(13.0) | 11(12.6) | 23(12.8) | |
| Others | 6(6.5) | 15(17.2) | 21(11.7) | |
| Frequency of alcohol intake (n = 174) | 0.903 | |||
| Always | 3(3.4) | 4(4.7) | 7(4.0) | |
| Often | 9(10.1) | 9(10.6) | 18(10.3) | |
| Occasionally | 42(47.2) | 36(42.4) | 78(44.8) | |
| Do not take | 35(39.3) | 36(42.4) | 71(40.8) | |
| Frequency of cigarette smoking (n = 166) | 0.456 | |||
| Often | 1(1.2) | 4(4.8) | 5(3.0) | |
| Occasionally | 5(6.1) | 3(3.6) | 8(4.8) | |
| Do not take | 76(92.7) | 77(91.7) | 153(92.2) |
| Fertility Status | Sperm Count (million) | Active Motility (%) | Total Motility (%) | Morphology (%) | Volume (mls) |
|---|---|---|---|---|---|
| Case (median) | 6 | 10 | 20 | 20 | 2.2 |
| IQR | 14 | 24 | 36 | 40 | 1 |
| Min | 0 | 0 | 0 | 0 | 0.5 |
| Max | 76 | 100 | 309 | 80 | 5.2 |
| Control (median) | 46 | 40 | 60 | 57 | 2.6 |
| IQR | 26.5 | 19.5 | 15.5 | 20 | 1.5 |
| Min | 4.6 | 9 | 27 | 17 | 0.7 |
| Max | 143 | 85 | 95 | 80 | 7 |
| Total (median) | 26 | 30 | 50.5 | 50 | 2.5 |
| IQR | 42 | 31 | 40 | 42 | 1.1 |
| Min | 0 | 0 | 0 | 0 | 0.5 |
| Max | 143 | 100 | 309 | 80 | 7 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | 0.0284 |
| Fertility Status | Ampicillin (N = 179) Median (IQR) [Range] | Metronidazole (N = 179) Median (IQR) [Range] | Tetracycline (N = 179) Median (IQR) [Range] | Chloramphenicol (N = 179) Median (IQR) [Range] | Ciprofloxacin (N = 179) Median (IQR) [Range] |
|---|---|---|---|---|---|
| Cases (n = 87) | 0(0) [0–47] | 0(19) [0–5232.67] | 11(28) [0–8118] | 1(3) [0–57] | 11(199.47) [0–15,144.8] |
| Controls (n = 92) | 0(0) [0–223] | 0(0) [0–1793] | 0(0) [0–6307] | 0(0) [0–51] | 16(146.6) [0–4454.34] |
| Total | 0(0) [0–223] | 0(0) [0–5232.67] | 0(0) [0–8118] | 0(0) [0–57] | 14(156.28) [0–15,144.80] |
| p-value | 0.8078 | 0.0910 | 0.9069 | 0.3587 | 0.6989 |
| Antibiotics | Case | Control | ||
|---|---|---|---|---|
| Unadjusted Observed Estimate (SE) [95% CI] | Adjusted Observed Estimate (SE) [95% CI] | Unadjusted Observed Estimate (SE) [95% CI] | Adjusted Observed Estimate (SE) [95% CI] | |
| Sperm count | ||||
| Ampicillin | −0.980(1.501) [−3.923–1.963] | −3.455(1.438) * [−6.276–−0.635] | −0.409(3.304) [−6.885–6.067] | −0.230(2.868) [−5.852–5.392] |
| Metronidazole | −0.009(0.020) [−0.049–0.031] | −0.045(0.050) [−0.144–0.053] | −0.008(0.038) [−0.084–0.067] | −0.098(1.368) [−2.781–2.584] |
| Tetracycline | −0.004(0.004) [−0.014–0.004] | 0.004(0.017) [−0.029–0.038] | 0.008(0.013) [−0.018–0.036] | 0.162(0.148) [−0.128–0.453] |
| Chloramphenicol | −1.365(1.082) [−3.487–0.757] | −1.114(1.562) [−4.177–1.948] | 0.743(1.674) [−2.538–4.024] | 0.454(2.184) [−3.827–4.736] |
| Ciprofloxacin | 0.001(0.004) [−0.007–0.009] | 0.005(0.305) [−0.592–0.0603] | 0.018(0.021) [−0.023–0.060] | 0.026(0.028) [−0.028–0.082] |
| Active motility | ||||
| Ampicillin | 1.618(0.566) ** [0.507–2.729] | −0.391(3.613) [−7.474–6.690] | −0.342(9.888) [−19.723–19.039] | −0.180(13.025) [−25.709–25.348] |
| Metronidazole | −0.027(0.073) [−0.171–0.116] | 0.010(0.074) [−0.136–0.157] | 0.004(0.048) [−0.089–0.099] | 0.049(0.421) [−0.776–0.874] |
| Tetracycline | −0.001(0.011) [−0.023–0.020] | 0.081(0.034) * [0.014–0.148] | 0.015(0.013) [−0.010–0.042] | 0.022(0.027) [−0.032–0.076] |
| Chloramphenicol | −0.719(1.23) [−3.148–1.709] | −1.140(2.68) [−6.407–4.127] | −0.021(1.07) [−2.119–2.076] | −0.415(1.05) [−2.478–1.646] |
| Ciprofloxacin | 0.002(0.003) [−0.005–0.009] | 0.006(1.519) [−2.972–2.985] | 0.025(0.018) [−0.011–0.061] | −0.001(0.013) [−0.027–0.025] |
| Total Motility | ||||
| Ampicillin | 1.868(2.544) [−3.119–6.855] | −0.135(7.556) [−14.945–14.674] | −0.018(8.108) [−15.910–15.874] | −0.261(6.380) [−12.767–12.245] |
| Metronidazole | 0.025(0.075) [−0.122–0.173] | −0.173(0.594) −1.339–0.992] | 0.021(0.022) [−0.022–0.065] | 0.067(0.325) [−0.569–0.705] |
| Tetracycline | 0.002(0.021) [−0.039–0.043] | 0.068(0.023) ** [0.022–0.113] | 0.028(0.011) * [0.006–0.050] | 0.011(0.020) [−0.027–0.051] |
| Chloramphenicol | −2.888(1.872) [−6.559–0.781] | −3.994(1.726) * [−7.379–−0.610] | −0.351(0.860) [−2.037–1.335] | −0.609(0.896) [−2.365–1.146] |
| Ciprofloxacin | −0.007(0.009) [−0.025–0.010] | −0.002(0.443) [−0.872–0.867] | 0.006(0.008) [−0.010–0.023] | −0.001(0.009) [−0.020–0.017] |
| Morphology | ||||
| Ampicillin | 2.727(1.357) * [0.065–5.388] | 3.362(3.847) [−4.177–10.902] | −1.359(3.480) [−8.181–5.462] | −0.475(6.740) [−13.687–12.735] |
| Metronidazole | 0.046(0.044) [−0.041–0.134] | 0.044(0.101) [−0.153–0.242] | 0.103(0.563) [−1.001–1.207] | 0.001(0.145) [−0.283–0.285] |
| Tetracycline | 0.031(0.016) [−0.001–0.064] | 0.031(0.020) [−0.008–0.071] | 0.015(0.007) [−0.000–0.030] | −0.007(0.037) [−0.081–0.066] |
| Chloramphenicol | 0.372(1.147) [−1.875–2.620] | −0.928(2.148) [−5.138–3.282] | −1.737(0.850) * [−3.403–−0.070] | −2.163(1.077) * [−4.274–−0.0522] |
| Ciprofloxacin | 0.000(0.005) [−0.010–0.011] | 0.003(2.799) [−5.483–5.490] | −0.004(0.009) [−0.023–0.014] | −0.005(0.007) [−0.020–0.008] |
| Volume | ||||
| Ampicillin | −0.037(0.083) [−0.200–0.125] | −0.036(0.112) [−0.258–0.184] | 0.012(0.250) [−0.478–0.503] | 0.010(0.114) [−0.214–0.235] |
| Metronidazole | 0.003(0.002) [−0.002–0.009] | 0.004(0.007) [−0.009–0.018] | 0.029(0.101) [−0.169–0.229] | −0.004(0.080) [−0.163–0.153] |
| Tetracycline | 0.000(0.001) [−0.002–0.002] | −0.000(0.001) [−0.003–0.002] | 0.000(0.000) [−0.001–0.001] | −0.002(0.002) [−0.008–0.003] |
| Chloramphenicol | 0.015(0.067) [−0.115–0.147] | 0.094(0.168) [−0.235–0.424] | 0.012(0.100) [−0.183–0.208] | 0.023(0.116) [−0.204–0.250] |
| Ciprofloxacin | 0.000(0.000) [−0.000–0.001] | 0.000(0.122) [−0.240–0.241] | −0.000(0.001) [−0.002–0.001] | 0.000(0.002) [−0.004–0.004] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonofua, F.E.; Ntoimo, L.F.C.; Msagati, T.A.M.; Ayodeji, O.; Aziken, M.; Omonkhua, A.; Ohenhen, V.; Olafusi, C.; Alfred, M.O. Association Between Antibiotic Exposure and the Risk of Male Infertility: A Case–Control Study. J. Xenobiot. 2025, 15, 172. https://doi.org/10.3390/jox15050172
Okonofua FE, Ntoimo LFC, Msagati TAM, Ayodeji O, Aziken M, Omonkhua A, Ohenhen V, Olafusi C, Alfred MO. Association Between Antibiotic Exposure and the Risk of Male Infertility: A Case–Control Study. Journal of Xenobiotics. 2025; 15(5):172. https://doi.org/10.3390/jox15050172
Chicago/Turabian StyleOkonofua, Friday E., Lorretta Favour C. Ntoimo, Titus A. M. Msagati, Oladiran Ayodeji, Michael Aziken, Akhere Omonkhua, Victor Ohenhen, Celestina Olafusi, and Moses O. Alfred. 2025. "Association Between Antibiotic Exposure and the Risk of Male Infertility: A Case–Control Study" Journal of Xenobiotics 15, no. 5: 172. https://doi.org/10.3390/jox15050172
APA StyleOkonofua, F. E., Ntoimo, L. F. C., Msagati, T. A. M., Ayodeji, O., Aziken, M., Omonkhua, A., Ohenhen, V., Olafusi, C., & Alfred, M. O. (2025). Association Between Antibiotic Exposure and the Risk of Male Infertility: A Case–Control Study. Journal of Xenobiotics, 15(5), 172. https://doi.org/10.3390/jox15050172






