Sub-Toxic Exposure to DEPs and PM2.5 Impairs Dendritic Cell Function Through Intracellular Particle Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of DEPs and PM2.5 Suspensions
2.3. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
2.4. Assessment of DEPs and PM2.5 Uptake by Flow Cytometry and Morphological Observation
2.5. Assessment of Phagocytic Activity Following DEP or PM2.5 Exposure Using Fluorescent Particles
2.6. Quantitative RT-PCR Analysis of Interferon Gene Expression Following Toll-like Receptor (TLR) Stimulation
3. Results
3.1. Short-Term Exposure to DEPs and PM2.5 Only Induces Subtoxic Cytotoxicity in PMDC05 Cells
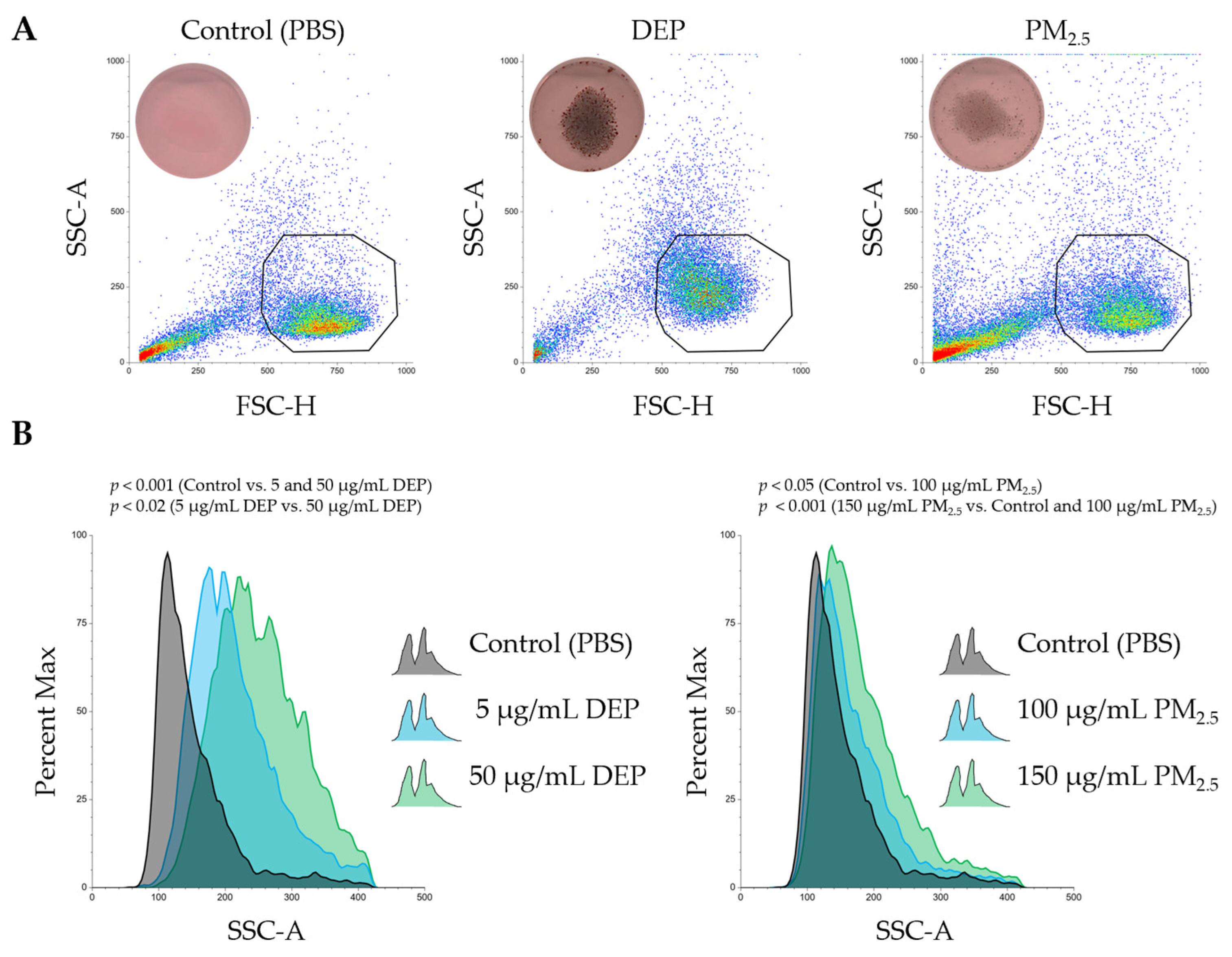
3.2. Dendritic Cell Model PMDC05 Visibly Uptakes DEPs and PM2.5 Particles Without Signs of Cytotoxicity
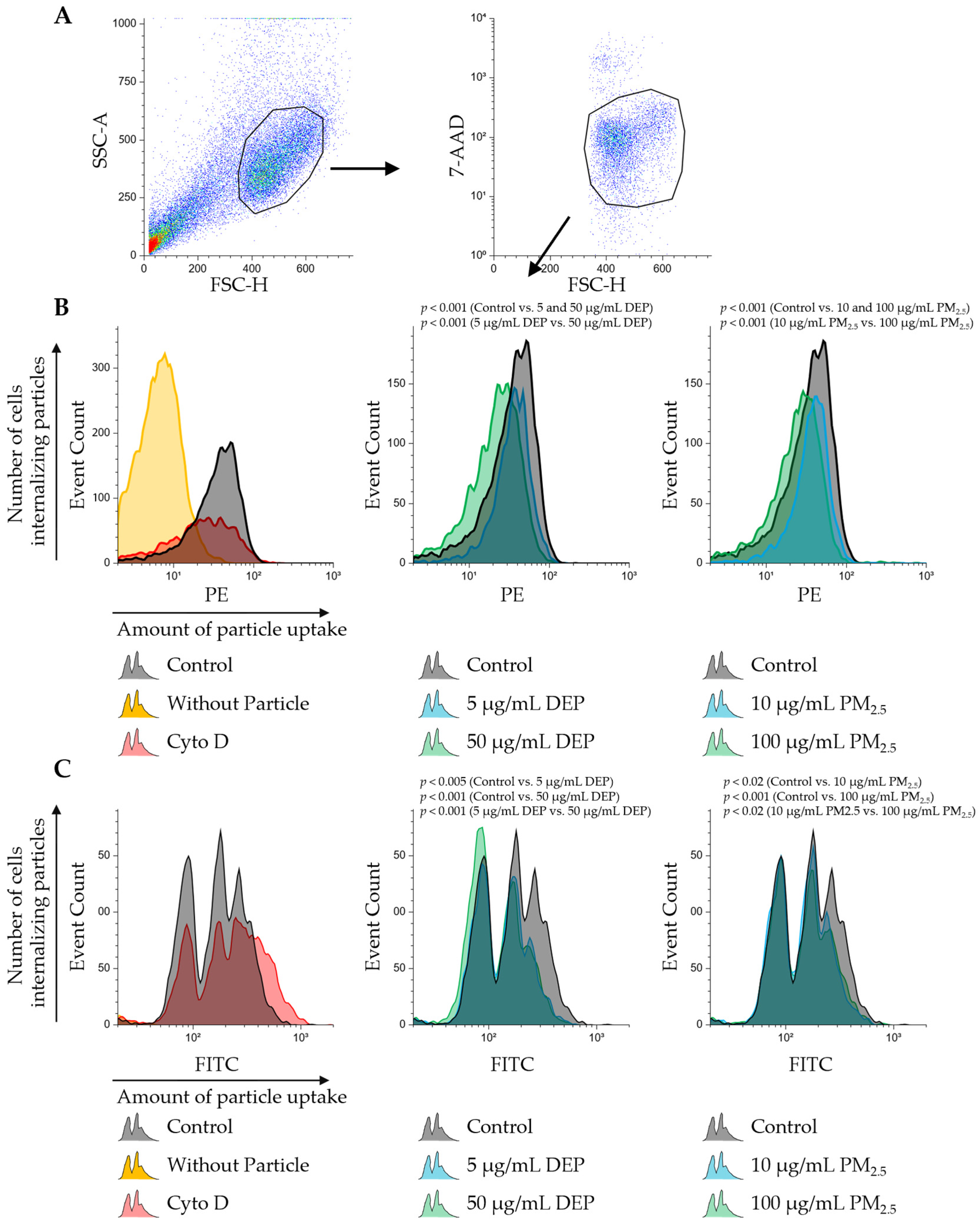
3.3. Exposure to DEPs and PM2.5 Suppresses Both Endocytosis and Phagocytosis in PMDC05 Cells
3.4. DEPs and PM2.5 Selectively Inhibit Interferon Gene Expression Induced by Virus-like Stimulation Through TLR7
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farahani, V.J.; Pirhadi, M.; Sioutas, C. Are standardized diesel exhaust particles (DEP) representative of ambient particles in air pollution toxicological studies? Sci. Total Environ. 2021, 788, e147854. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.-C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2021, 19, 7511. [Google Scholar] [CrossRef]
- Li, X.; Liu, X. Effects of PM2.5 on Chronic Airway Diseases: A Review of Research Progress. Atmosphere 2021, 12, 1068. [Google Scholar] [CrossRef]
- Jiang, Y.; Si, J.; Wang, Y.; Zhang, H.; Zhou, F.; Lu, X.; Li, X.; Sun, D.; Wang, Z. The Relationship Between PM2.5 and Eight Common Lung Diseases: A Two-Sample Mendelian Randomization Analysis. Toxics 2024, 12, 851. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C., Jr.; et al. Air Pollution and Cardiovascular Disease: A Statement for Healthcare Professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Ma, Y.; Cui, F.; Wang, J.; Tang, L.; Zheng, L.; Xing, M.; Zhao, X.; Zhang, J.; Yang, J.; et al. Long-term PM2.5 components exposure, genetic susceptibility, and myocardial infarction risk. Eur. J. Prev. Cardiol. 2025, 30, zwaf476. [Google Scholar] [CrossRef] [PubMed]
- Psoter, K.J.; Roos, A.J.D.; Wakefield, J.; Mayer, J.D.; Rosenfeld, M. Air pollution exposure is associated with MRSA acquisition in young U.S. children with cystic fibrosis. BMC Pulm. Med. 2017, 17, e106. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, W.; Shi, F.; Li, Y.; Li, Y.; Zhang, Q.; Shang, Y.; Chen, N.; Xu, W.; Cheng, Q. Critical exposure windows of PM2.5-Meteorological interactions triggering pediatric mycoplasma pneumoniae epidemics: An age-stratified distributed lag nonlinear modeling approach. J. Environ. Manag. 2025, 391, e126533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, W.; Gao, Y.; Li, J.; Wang, H. Exposure to particular matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells. Toxicology 2014, 325, 180–188. [Google Scholar] [CrossRef]
- Luo, L.; Jiang, M.; Xiong, Y.; Xiong, A.; Zhang, L.; Wu, D.; Liu, Y.; Ran, Q.; Liu, J.; Zhang, Y.; et al. Fine particulate matter 2.5 induces susceptibility to Pseudomonas aeruginosa infection via expansion of PD-L1high neutrophils in mice. Respir. Res. 2024, 25, e90. [Google Scholar] [CrossRef]
- Mahla, R.S.; Reddy, M.C.; Prasad, D.V.R.; Kumar, H. Sweeten PAMPs: Role of Sugar Complexed PAMPs in Innate Immunity and Vaccine Biology. Front. Immunol. 2013, 4, e248. [Google Scholar] [CrossRef] [PubMed]
- Zanna, M.Y.; Yasmin, A.R.; Omar, A.R.; Arshad, S.S.; Mariatulqabtiah, A.R.; Nur-Fazila, S.H.; Mahiza, M.I. Review of Dendritic Cells, Their Role in Clinical Immunology, and Distribution in Various Animal Species. Int. J. Mol. Sci. 2024, 22, 8044. [Google Scholar] [CrossRef]
- Kurilin, V.; Alshevskaya, A.; Sennikov, S. Development of Cell Technologies Based on Dendritic Cells for Immunotherapy of Oncological Diseases. Biomedicines 2024, 12, 699. [Google Scholar] [CrossRef]
- Matthews, N.C.; Pfeffer, P.E.; Mann, E.H.; Kelly, F.J.; Corrigan, C.J.; Hawrylowicz, C.M.; Lee, T.H. Urban Particulate Matter–Activated Human Dendritic Cells Induce the Expansion of Potent Inflammatory Th1, Th2, and Th17 Effector Cells. Am. J. Respir. Cell Mol. Biol. 2016, 54, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Stec, M.; Węglarczyk, K.; Kluczewska, A.; Siedlar, M.; Baran, J. Transition Metal Containing Particulate Matter Promotes Th1 and Th17 Inflammatory Response by Monocyte Activation in Organic and Inorganic Compounds Dependent Manner. Int. J. Environ. Res. Public Health 2020, 17, 1227. [Google Scholar] [CrossRef]
- Chan, R.C.F.; Wang, M.; Li, N.; Yanagawa, Y.; Onoé, K.; Lee, J.J.; Nel, A.E. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J. Allergy Clin. Immunol. 2006, 118, 455–465. [Google Scholar] [CrossRef]
- De Homdedeu, M.; Cruz, M.J.; Sánchez-Díez, S.; Gómez-Ollés, S.; Ojanguren, I.; Ma, D.; Muñoz, X. Role of diesel exhaust particles in the induction of allergic asthma to low doses of soybean. Environ. Res. 2021, 196, e110337. [Google Scholar] [CrossRef]
- Seydoux, E.; Rothen-Rutishauser, B.; Nita, I.; Balog, S.; Gazdhar, A.; Stumbles, P.; Petri-Fink, A.; Blank, F.; von Garnier, C. Size-dependent accumulation of particles in lysosomes modulates dendritic cell function through impaired antigen degradation. Int. J. Nanomed. 2014, 9, 3885–3902. [Google Scholar] [CrossRef]
- Alobaid, M.A.; Richards, S.-J.; Alexander, M.R.; Gibson, M.I.; Ghaemmaghami, A.M. Monosaccharide coating modulate the intracellular trafficking of gold nanoparticles in dendritic cells. Mater. Today Bio 2024, 29, e101371. [Google Scholar] [CrossRef]
- Kamath, A.T.; Henri, S.; Battye, F.; Tough, D.F.; Shortman, K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 2002, 100, 1731–1741. [Google Scholar] [CrossRef]
- He, W.; Peng, H.; Ma, J.; Wang, Q.; Li, A.; Zhang, J.; Kong, H.; Li, Q.; Sun, Y.; Zhu, Y. Autophagy changes in lung tissues of mice at 30 days after carbon black-metal ion co-exposure. Cell Prolif. 2020, 53, e12813. [Google Scholar] [CrossRef] [PubMed]
- Onodera, A.; Shimomura, T.; Ochi, H.; Sunada, R.; Fukutomi, E.; Hidaka, K.; Kawai, Y. The Cellular Accumulation of Vehicle Exhaust Particulates Changes the Acidic pH Environment of Lysosomes in BEAS-2B Airway Epithelial Cells. J. Xenobiotics 2023, 13, 653–661. [Google Scholar] [CrossRef]
- Narita, M.; Watanabe, N.; Yamahira, A.; Hashimoto, S.; Tochiki, N.; Saitoh, A.; Kaji, M.; Nakamura, T.; Furukawa, T.; Toba, K.; et al. A leukemic plasmacytoid dendritic cell line, PMDC05, with the ability to secrete. Leuk. Res. 2009, 33, 1224–1232. [Google Scholar] [CrossRef]
- Colombi, C.; Dacarro, G.; Diaz Fernandez, Y.A.; Taglietti, A.; Pallavicini, P.; Doveri, L. Human Serum Albumin Protein Corona in Prussian Blue Nanoparticles. Nanomaterials 2024, 14, 1336. [Google Scholar] [CrossRef]
- Baimanov, D.; Wang, J.; Zhang, J.; Liu, K.; Cong, Y.; Shi, X.; Zhang, X.; Li, Y.; Li, X.; Qiao, R.; et al. In situ analysis of nanoparticle soft corona and dynamic evolution. Nat. Commun. 2022, 13, e5389. [Google Scholar] [CrossRef]
- Alimov, Z.B.; Youn, H.; Iwata, A.; Nakano, K.; Okamoto, T.; Sasaki, A.; Katori, T.; Okuda, T. Comparison of the Chemical Characteristics and Toxicity of PM2.5 Collected Using Different Sizes of Cyclones. Asian J. Atmos. Environ. 2022, 16, e2022062. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; Bogaart, G.V.D. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2021, 11, e607945. [Google Scholar] [CrossRef]
- Tong, C.; Liang, Y.; Han, X.; Zhang, Z.; Zhen, X.; Wang, S.; Song, B. Research Progress of Dendritic Cell Surface Receptors and Targeting. Biomedicines 2023, 11, 1673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, C.; Zhao, G.; Li, M.; Ma, D.; Meng, Q.; Tang, W.; Huang, Q.; Shi, P.; Li, Y.; et al. PM2.5 Synergizes with Pseudomonas aeruginosa to Suppress Alveolar Macrophage Function in Mice Through the mTOR Pathway. Front. Pharmacol. 2022, 13, e924242. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.-J.; Cao, W.-J.; Li, M.-H.; Yang, L.; Zhang, Y.; Zhang, Y.-Y.; Li, X.-Y.; Li, Y.-X.; Zhang, Y.; Li, M.-Y. PM2.5 compromises antiviral immunity in influenza infection by inhibiting activation of NLRP3 inflammasome and expression of interferon-β. Mol. Immunol. 2020, 125, 178–186. [Google Scholar] [CrossRef]
- Porter, M.; Karp, M.; Killedar, S.; Bauer, S.M.; Guo, J.; Williams, D.; Breysse, P.; Georas, S.N.; Williams, M.A. Diesel-Enriched Particulate Matter Functionally Activates Human Dendritic Cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 706–719. [Google Scholar] [CrossRef]
- O’Driscoll, C.A.; Owens, L.A.; Gallo, M.E.; Hoffmann, E.J.; Afrazi, A.; Han, M.; Fechner, J.H.; Schauer, J.J.; Bradfield, C.A.; Mezrich, J.D. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part. Fibre Toxicol. 2018, 15, e35. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Korge, P.; Weiss, J.N.; Li, N.; Venkatesen, M.I.; Sioutas, C.; Nel, A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: Implications for ultrafine particle toxicity. Environ. Health Perspect. 2004, 112, 1347–1358. [Google Scholar] [CrossRef]
- Honda, A.; Inoue, K.-I.; Higashihara, M.; Ueda, K.; Takano, H. Differential Pattern of Cell Death and ROS Production in Human Airway Epithelial Cells Exposed to Quinones Combined with Heated-PM2.5 and/or Asian Sand Dust. Int. J. Mol. Sci. 2023, 24, 10544. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Xu, Y.; Yu, X.; Xiong, T.; Du, M.; Sun, J.; Liu, L.; Tang, Y.; Yao, P. Iron-Mediated Lysosomal Membrane Permeabilization in Ethanol-Induced Hepatic Oxidative Damage and Apoptosis: Protective Effects of Quercetin. Oxid. Med. Cell. Longev. 2016, 2016, e4147610. [Google Scholar] [CrossRef]
- Ziglari, T.; Wang, Z.; Holian, A. Contribution of Particle-Induced Lysosomal Membrane Hyperpolarization to Lysosomal Membrane Permeabilization. Int. J. Mol. Sci. 2021, 22, 2277. [Google Scholar] [CrossRef]
- Hwang, J.J.; Lee, S.-J.; Kim, T.-Y.; Cho, J.-H.; Koh, J.-Y. Zinc and 4-Hydroxy-2-Nonenal Mediate Lysosomal Membrane Permeabilization Induced by H2O2 in Cultured Hippocampal Neurons. J. Neurosci. 2008, 28, 3114–3122. [Google Scholar] [CrossRef]
- Liu, Z.; Roche, P.A. Macropinocytosis in phagocytes: Regulation of MHC class-II-restricted antigen presentation in dendritic cells. Front. Physiol. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chaiwut, R.; Kasinrer, W. Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC Res. Notes 2022, 15, e42. [Google Scholar] [CrossRef] [PubMed]
- Petes, C.; Odoardi, N.; Gee, K. The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome. Front. Immunol. 2017, 8, e1075. [Google Scholar] [CrossRef]
- Blasius, A.L.; Beutler, B. Intracellular toll-like receptors. Immunity 2010, 32, 305–306. [Google Scholar] [CrossRef]
- Hipp, M.M.; Shepherd, D.; Gileadi, U.; Aichinger, M.C.; Kessler, B.M.; Edelmann, M.J.; Essalmani, R.; Seidah, N.G.; Reis e Sousa, C.; Cerundolo, V. Processing of human Toll-like receptor 7 by furin-like proprotein convertases is required for its accumulation and activity in endosomes. Immunity 2013, 39, 711–721. [Google Scholar] [CrossRef]
- Elshikha, A.S.; Abboud, G.; Avdiaj, R.; Morel, L.; Song, S. The Inhibitory Effects of Alpha 1 Antitrypsin on Endosomal TLR Signaling Pathways. Biomolecules 2025, 15, 43. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of Carbon Nanotubes, Graphene, and Their Derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Mu, Q.; Wang, H.; Lin, G.; Zhang, M. Enzymatic and Cellular Degradation of Carbon-Based Biconcave Nanodisks. Micromachines 2022, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A. Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 1987, 105, 1473–1478. [Google Scholar] [CrossRef]
- Jing, W.; Saito, K.; Okamoto, T.; Saito, H.; Sugimoto, K.; Nishita-Hara, C.; Hara, K.; Hayashi, M.; Hasegawa, S.; Okuda, T. Characterization of Elemental Composition and Valence State of Cyclone-collected Aerosol Particles Using EDXRF and XAFS at Three Sites in Japan. Asian J. Atmos. Environ. 2022, 16, e2021137. [Google Scholar] [CrossRef]
- Okuda, T. Measurement of the specific surface area and particle size distribution. Atmos. Environ. 2013, 75, 1–5. [Google Scholar] [CrossRef]


| Component | DEP | PM2.5 |
|---|---|---|
| Organic carbon (Total) | ~20.25 µg/mL | 7.07 µg/mL |
| Elemental carbon (Total) | ~19.85 µg/mL | 2.27 µg/mL |
| Fe | ~0.063 µg/mL | 1.93 µg/mL |
| Zn | ~0.052 µg/mL | 0.24 µg/mL |
| Endotoxin | ~0.5 EU/mL | 0.41 EU/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakahira, Y.; Otomo, D.; Okuda, T.; Onodera, A. Sub-Toxic Exposure to DEPs and PM2.5 Impairs Dendritic Cell Function Through Intracellular Particle Accumulation. J. Xenobiot. 2025, 15, 142. https://doi.org/10.3390/jox15050142
Nakahira Y, Otomo D, Okuda T, Onodera A. Sub-Toxic Exposure to DEPs and PM2.5 Impairs Dendritic Cell Function Through Intracellular Particle Accumulation. Journal of Xenobiotics. 2025; 15(5):142. https://doi.org/10.3390/jox15050142
Chicago/Turabian StyleNakahira, Yuki, Daisuke Otomo, Tomoaki Okuda, and Akira Onodera. 2025. "Sub-Toxic Exposure to DEPs and PM2.5 Impairs Dendritic Cell Function Through Intracellular Particle Accumulation" Journal of Xenobiotics 15, no. 5: 142. https://doi.org/10.3390/jox15050142
APA StyleNakahira, Y., Otomo, D., Okuda, T., & Onodera, A. (2025). Sub-Toxic Exposure to DEPs and PM2.5 Impairs Dendritic Cell Function Through Intracellular Particle Accumulation. Journal of Xenobiotics, 15(5), 142. https://doi.org/10.3390/jox15050142







