Arginine-Derived Cationic Surfactants Containing Phenylalanine and Tryptophan: Evaluation of Antifungal Activity, Biofilm Eradication, Cytotoxicity, and Ecotoxicity
Abstract
1. Introduction
2. Methods
2.1. Synthesis
2.2. Surface Tension Measurements
2.3. Antimicrobial Activity
- (a)
- Microorganisms and Culture Conditions
- (b)
- Minimum Inhibitory Concentration (MIC)
2.4. Antibiofilm Activity
2.5. Hemolytic Activity Assay
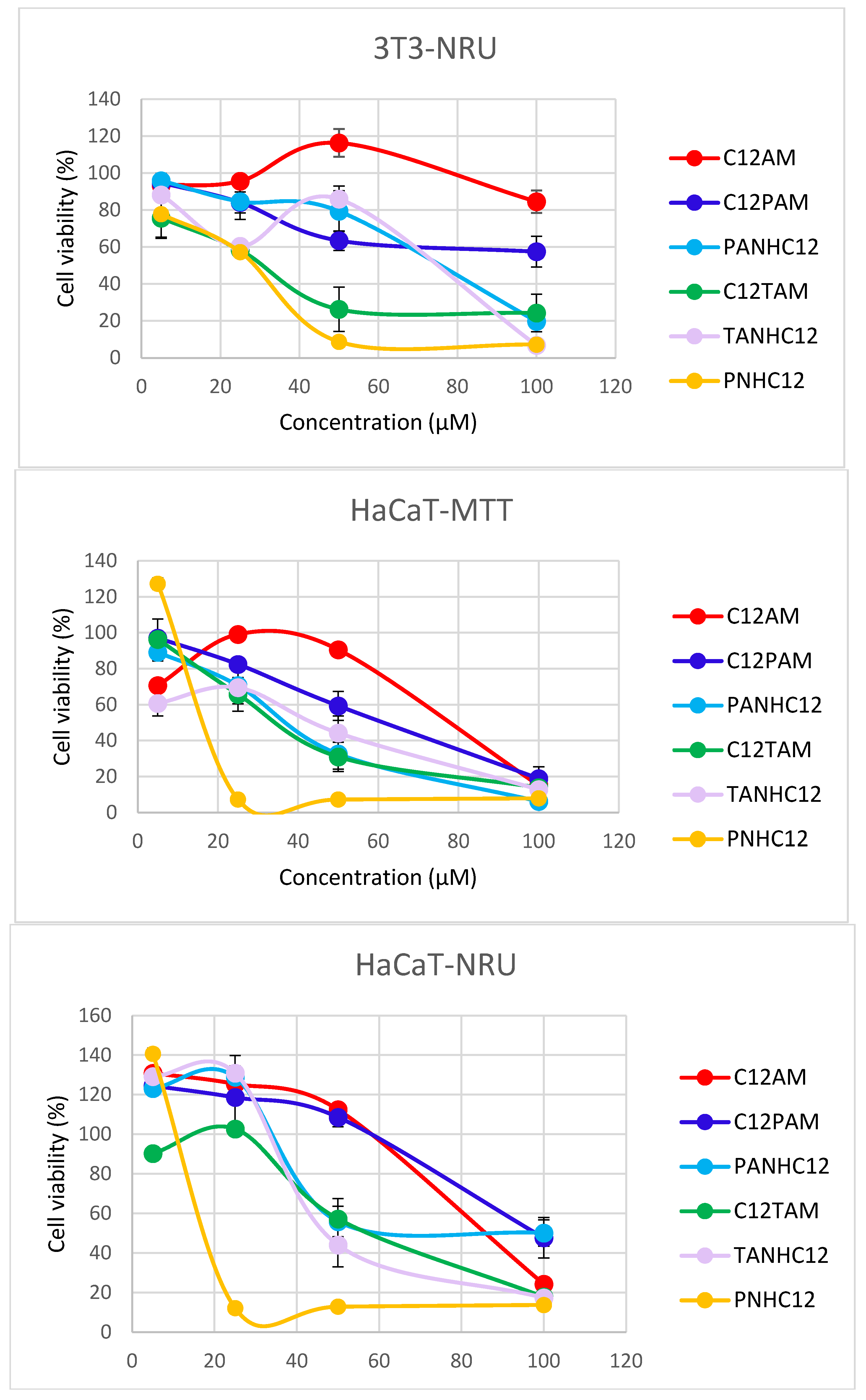
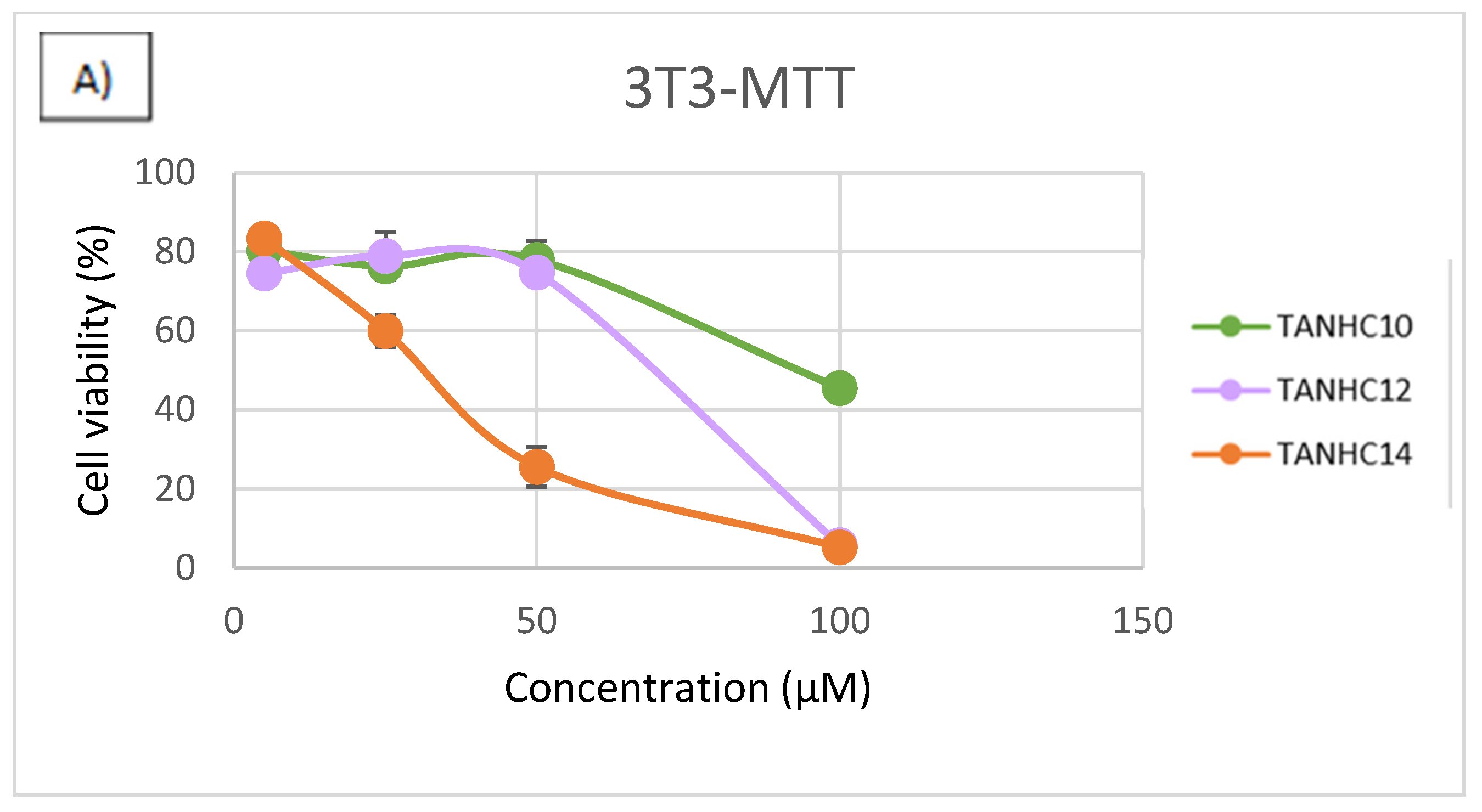
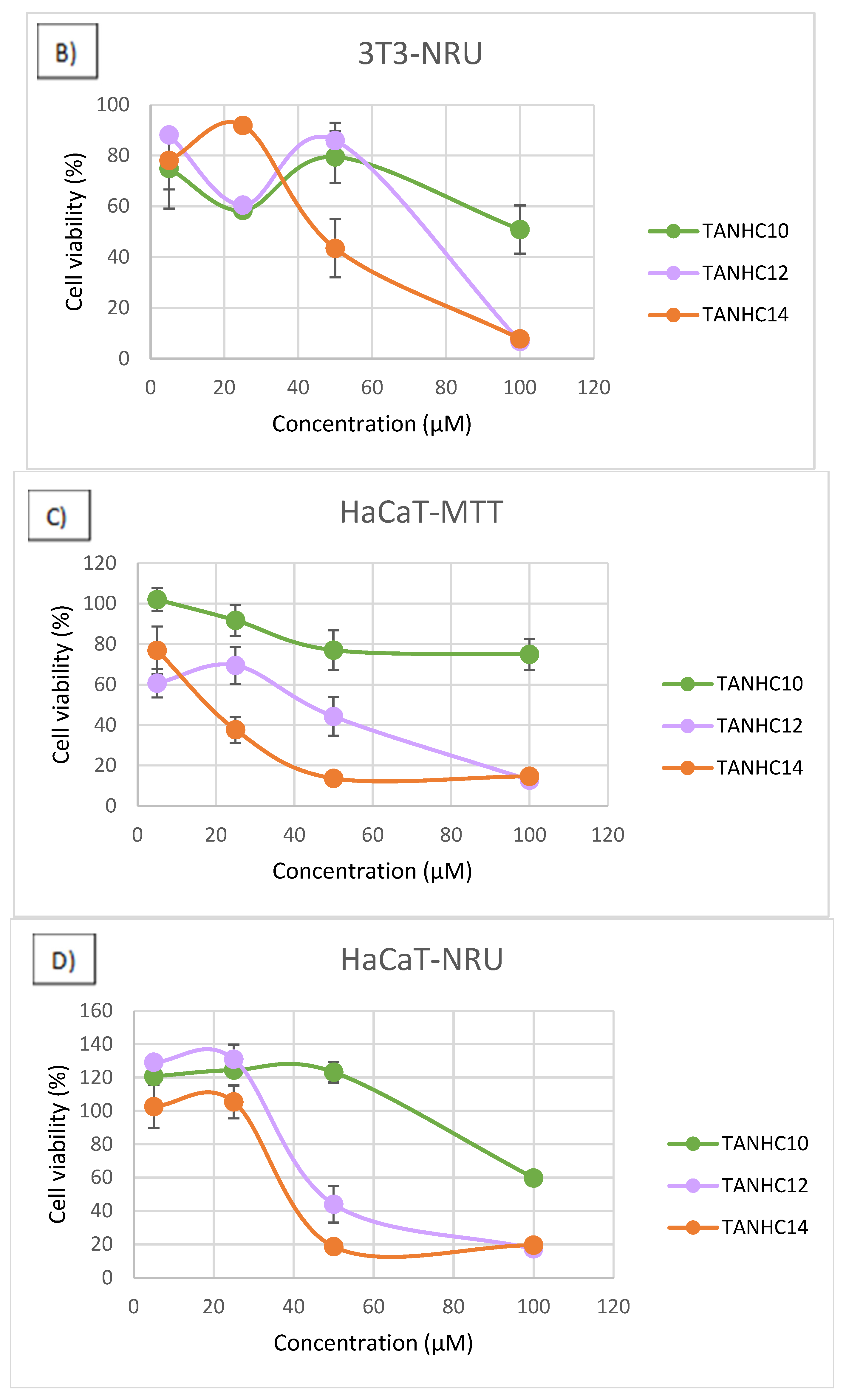
2.6. Cytotoxicity Assays
- Cell Viability Assays
- (a)
- Neutral Red Uptake (NRU Assay)
- (b)
- MTT Assay
2.7. Ecotoxicity Assays
- Acute Toxicity Assessment Using Daphnia magna
- Vibrio fischeri Luminescence Reduction Test
3. Results and Discussion
- (a)
- Six monocatenary surfactants in which, while keeping a constant C12 alkyl chain, we systematically varied the polar head in terms of the amino acid type, the number of cationic charges, and the attachment position of the alkyl chain: C12AM (Nα-dodecyl arginine methyl ester hydrochloride), with a C12 hydrophobic chain connected to the α-amine group of arginine and a positive charge on the protonated guanidine group; PNHC12 (phenylalanine dodecyl amide hydrochloride), with the cationic charge on the protonated amine group of the amino acid, and the C12 chain is linked to the carboxylic group of the phenylalanine; C12PAM (Nα-dodecyl phenylalanine-arginine methyl ester hydrochloride) and C12TAM (Nα-dodecyl-tryptophan-arginine methyl ester hydrochloride), with two aromatic amino acids on the hydrophilic moiety (Phe/Arg or Trp/Arg), one positive charge on the protonated guanidine group, and the C12 chain connected through an amide linkage to the α-amino group of the aromatic amino acid; PANHC12 (phenylalanine-arginine dodecyl amide dihydrochloride) and TANHC12 (tryptophan-arginine octyl amide dihydrochloride) compounds that also contain two amino acids on the polar head (Phe/Arg or Trp/Arg) but with two cationic charges (one on the protonated amine group of the aromatic amino acid and the other in the protonated guanidine group), and the C12 alkyl chain is linked to the carboxylic group of the arginine (Figure 1).
- (b)
- Seven monocatenary surfactants in which, keeping constant the polar head with two amino (Phe/Arg or Trp/Arg) and two cationic charges, we varied the alkyl chain from C8 to C14 for the Phe/Arg and C10 to C14 for the Trp/Arg homologs: PANHCn (phenylalanine-arginine alkyl amide) and TANHCn, (tryptophan-arginine alkyl amide).
3.1. Surface Tension
3.2. Antifungal Activity
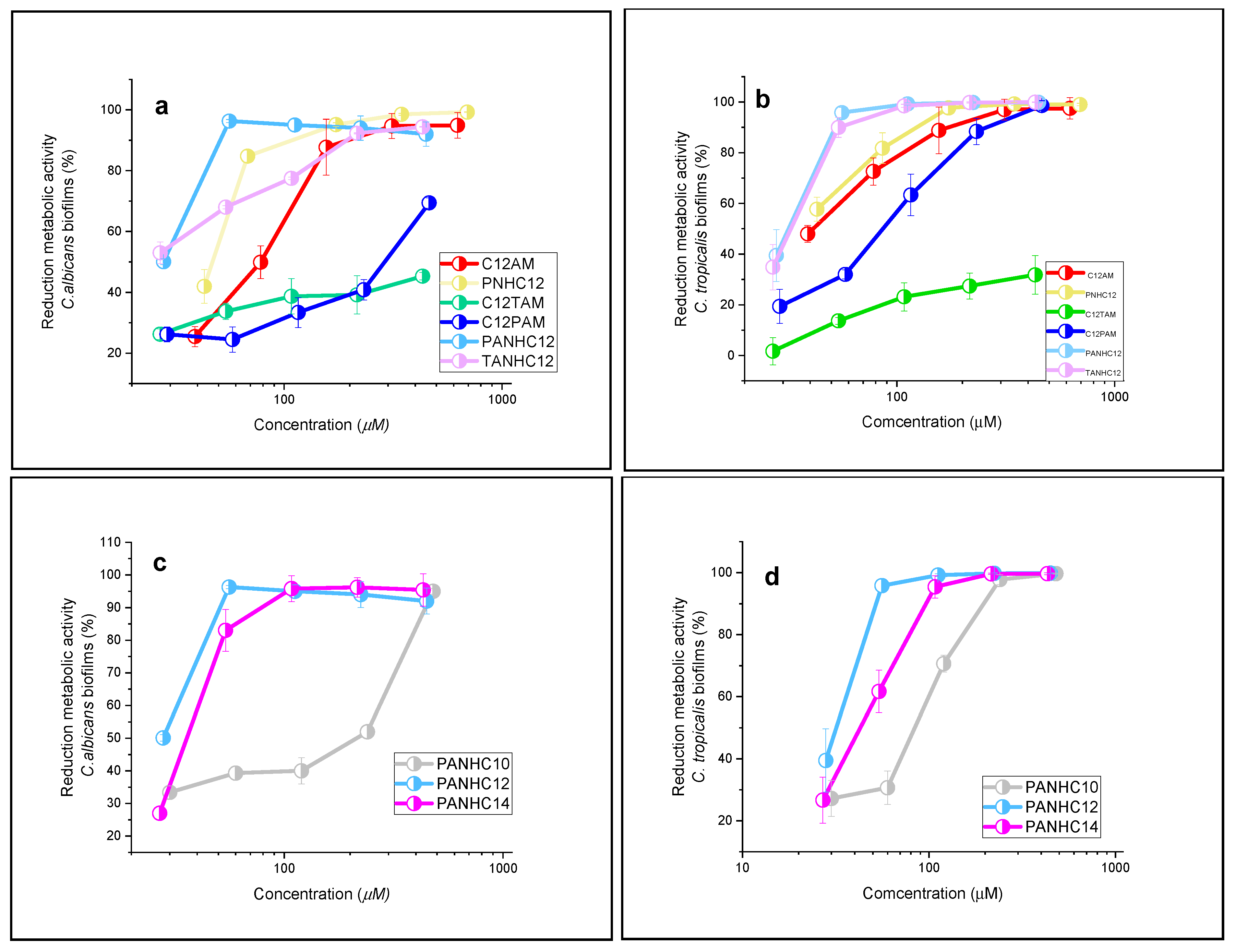
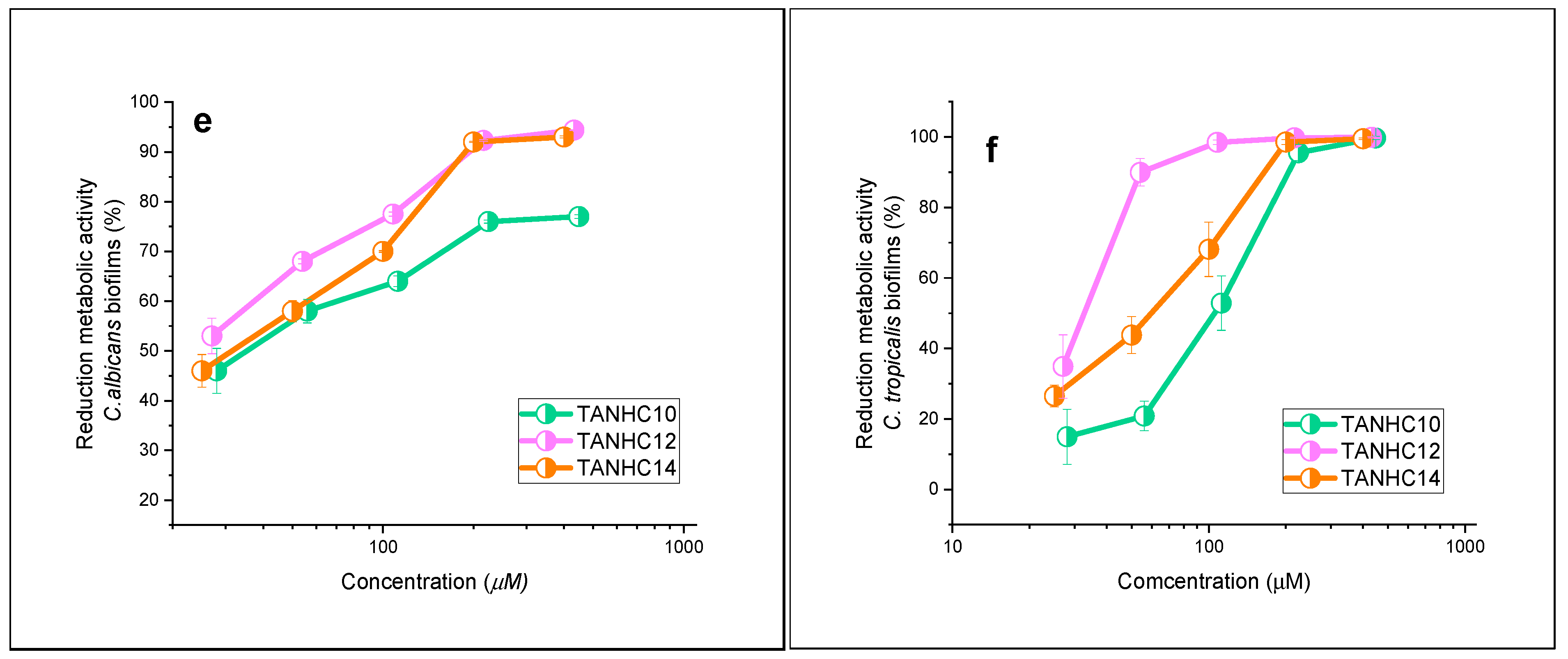
3.3. Antibiofilm Activity
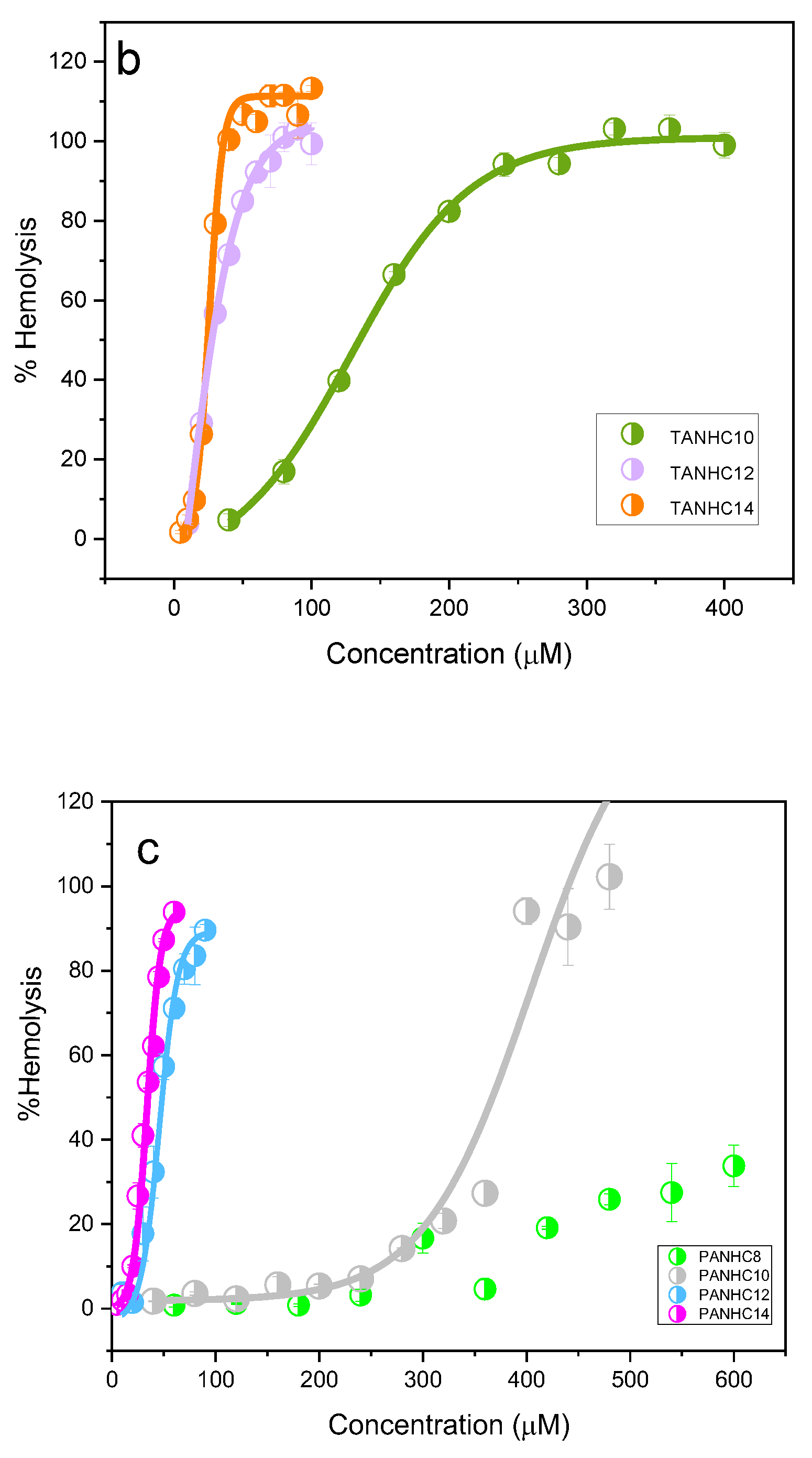
3.4. Cytotoxicity
3.5. Ecotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T. Hidden Killers: Human, Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The Changing Epidemiology of Invasive Fungal, Infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar] [CrossRef]
- Lockhart, S.R. Candida auris and multidrug resistance: Defining the New Normal. Fungal. Genet. Biol. 2019, 131, 103243. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The Global Problem of Antifungal Resistance: Prevalence, Mechanisms, and Management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Nobile, C.J.; Alexander Johnson, D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- García, M.T.; Ribosa, I.; Guindulain, T.; Sánchez-Leal, J.; Vives-Rego, J. Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment. Environ. Pollut. 2001, 111, 169–175. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Saczewski, J. Advances in the Synthesis of Biologically Active Quaternary Ammonium Compounds. J. Mol. Sci. 2024, 25, 4649. [Google Scholar] [CrossRef]
- Forman, M.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P. Building a Better Quaternary Ammonium Compound (QAC): Bran.ched Tetracationic Antiseptic Amphiphiles. ChemMedChem. 2016, 11, 1401–1405. [Google Scholar] [CrossRef]
- Asante, J.Y.D.; Casey, C.M.; Bezold, E.L.; Fernando, A.; McDonough, D.; Wuest, W.M.; Minbiole, K.P.C. Resorcinol-based Bolaamphiphilic Quaternary Ammonium Compounds. ChemMedChem 2025, 20, e202400932. [Google Scholar] [CrossRef]
- Paluch, E.; Szperlik, J.; Lamch, Ł.; Wilk, K.A.; Obłąk, E. Biofilm eradication and antifungal mechanism of action against Candida albicans of cationic dicephalic surfactants with a labile linker. Sci. Rep. 2021, 11, 8896. [Google Scholar] [CrossRef]
- Pérez, L.; García, M.T.; Pinazo, A.; Pérez-Matas, E.; Hafidi, Z.; Bautista, E. Cationic surfactants based on arginine-phenylalanine and arginine-tryptophan: Synthesis, aggregation behavior, antimicrobial activity, and biodegradation. Pharmaceutics 2022, 14, 2602. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, A.; Illa, O.; Ortuño, R.M.; Pons, R. Chiral Cyclobutane β-Amino Acid-Based Amphiphiles: Influence of Cis/Trans Stereochemistry on Condensed Phase and Monolayer Structure. Langmuir 2016, 32, 6977–6984. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadis, S.; Chen, J.K.; Koberstein, J.T.; Siegel, A.F.; Sohn, J.E.; Emerson, J. The determination of interfacial tension by video image processing of pendant fluid drops. J. Colloid Interface Sci. 1987, 119, 55–66. [Google Scholar] [CrossRef]
- Li, P.X.; Thomas, R.K.; Penfold, J. Limitations in the use of surface tension and the Gibbs equation to determine surface excesses of cationic surfactants. Langmuir 2014, 30, 6739–6747. [Google Scholar] [CrossRef]
- Goulden, C.E.; Comotto, R.M.; Hendrickson, J.A.; Hornig, L.L.; Johnson, K.L. On Procedures and recommendations for the culture and use of Daphnia in bioassay studies. In Aquatic Toxicology and Hazard Assessment; ASTM International: West Conshohocken, PA, USA, 1982; pp. 139–160, ISBN-10:0-8031-0796-X. [Google Scholar] [CrossRef]
- ISO 11348-3:2007; Water Quality_ Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fisheri (Luminiscent Bacteria Test). Part 3: Method Using Freeze-Dried Bacteria; ISO: Geneva, Switzerland, 2007.
- Hafidi, Z.; García, M.T.; Pons, R.; de Sousa Oliveira, F.F.; Bautista, M.E.; Vazquez, S.; Perez, L.J. Green cationic phenylalanine and tryptophan-based surfactants: Influence of the polar head amino acids and hydrophobic character on the self-aggregation, antimicrobial activity, and environmental behavior. Mol. Liq. 2025, 429, 127620. [Google Scholar] [CrossRef]
- Paluch, E.; Piecuch, A.; Obłąk, E.; Wilk, K. Antifungal activity of newly synthesized chemodegradable dicephalic-type cationic surfactants. Colloids Surf. B Biointerfaces 2018, 164, 34–41. [Google Scholar] [CrossRef]
- Piętka-Ottlik, M.; Frąckowiak, R.; Maliszewska, I.; Kołwzan, B.; Wilk, K.A. Ecotoxicity and biodegradability of antielectrostatic dicephalic cationic surfactants. Chemosphere 2012, 89, 1103–1111. [Google Scholar] [CrossRef]
- Serpa Sampaio Moreno, L.; Nobre Junior, H.V.; Ramos da Silva, A.; Aires do Nascimento, F.B.S.; Rocha da Silva, C.; de Andrade Neto, J.B.; Cavalcanti, B.C.; Odorico de Moraes, M.; Pinazo, A.; Pérez, L. Arginine-phenylalanine and arginine-tryptophan-based surfactants as new biocompatible antifungal agents and their synergistic effect with Amphotericin B against fluconazole-resistant Candida strains. Colloids Surf. B Biointerfaces 2021, 207, 112017. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Manjunath, G.B.; Akkapeddi, P.; Yarlagadda, V.; Hoque, J.; Uppu, D.; Konai, M.M.; Haldar, J. Small Molecular Antibacterial Peptoid Mimics: The Simpler the better. J. Med. Chem. 2014, 57, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Bravo Ruiz, G.; Lorenz, A. What do we know about the biology of the emerging fungal pathogen of humans Candida. auris. Microbiol. Res. 2021, 242, 126621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar] [CrossRef]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N. Cationic surfactants as antifungal agents. Appl. Microbiol. Biotechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef]
- Kula, N.; Lamch, Ł.; Futoma-Kołoch, B.; Wilk, K.A.; Obłąk, E. The effectiveness of newly synthesized quaternary ammonium salts differing in chain length and type of counterion against priority human pathogens. Sci. Rep. 2022, 12, 21799. [Google Scholar] [CrossRef] [PubMed]
- Obłak, E.; Piecuch, A.; Krasowska, A.; Łuczyński, J. Antifungal activity of Gemini quaternary ammonium salts. Microbiol. Res. 2013, 168, 630–638. [Google Scholar] [CrossRef]
- Piecuch, A.; Obłąk, E.; Guz-Regner, K. Antibacterial activity of alanine-derived Gemini quaternary ammonium compounds. J. Surfactants Deterg. 2016, 19, 275–282. [Google Scholar] [CrossRef]
- Pérez, L.; Pons, R.; Oliveira de Sousa, F.F.; Morán, M.C.; Ramos da Silva, A.; Pinazo, A. Green cationic arginine surfactants: Influence of the polar head cationic character on the self-aggregation and biological properties. J. Mol. Liq. 2021, 339, 116819. [Google Scholar] [CrossRef]
- Ke, F.; Huayang, L.; Haoning, G.; Zhang, L.; Liao, M.; Hu, X.; Ciumac, D.; Li, P.; Webster, J.; Petkov, J.; et al. In-Membrane Nanostructuring of Cationic Amphiphiles Affects Their Antimicrobial Efficacy and Cytotoxicity: A Comparison Study between a De Novo Antimicrobial Lipopeptide and Traditional Biocides. Langmuir 2022, 38, 6623–6637. [Google Scholar] [CrossRef]
- Da Silva, A.; Nobre, H., Jr.; Sampaio, L.; Nascimento, B.D.; da Silva, C.; de Andrade Neto, J.B.; Manresa, Á.; Pinazo, A.; Cavalcanti, B.; de Moraes, M.O.; et al. Antifungal and antiprotozoal green amino acid-based rhamnolipids: Mode of action, antibiofilm efficiency and selective activity against resistant Candida spp. strains and Acanthamoeba castellanii. Colloids Surf. B Biointerfaces 2020, 193, 111148. [Google Scholar] [CrossRef] [PubMed]
- Minbiole, K.P.C.; Jennings, M.C.; Ator, L.E.; Black, J.W.; Grenier, M.C.; LaDow, J.E.; Caran, K.L.; Seifert, K.; Wuest, W.M. From antimicrobial activity to mechanism of resistance: The multifaceted role of simple quaternary ammonium compounds in bacterial eradication. Tetrahedron 2016, 72, 3559–3566. [Google Scholar] [CrossRef]
- Jennings, M.C.; Buttaro, B.A.; Minbiole, K.P.; Wuest, W.M. Bioorganic Investigation of Multicationic Antimicrobials to Combat QAC-Resistant Staphylococcus aureus. ACS Infect. Dis. 2015, 1, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; McLaughlin, M.; Shaw, C.; Gorman, S.P.; Gilmore, B.F. Gilmore Antimicrobial Activity of Short, Synthetic Cationic Lipopeptides. Chem. Biol. Drug. Des. 2010, 75, 563–569. [Google Scholar] [CrossRef]
- Dawgul, M.A.; Greber, K.E.; Bartoszewska, S.; Baranska-Rybak, W.; Sawicki, W.; Kamysz, W. In Vitro Evaluation of Cytotoxicity and Permeation Study on Lysine- and Arginine-Based Lipopeptides with Proven Antimicrobial Activity. Molecules 2017, 22, 2173. [Google Scholar] [CrossRef]
- Neubauer, D.; Jaśkiewicz, M.; Bauer, M.; Gołacki, K.; Kamysz, W. Ultrashort Cationic Lipopeptides-Effect of N-Terminal Amino Acid and Fatty Acid Type on Antimicrobial Activity and Hemolysis. Molecules 2020, 25, 257. [Google Scholar] [CrossRef]
- Makovitzki, A.; Avrahami, D.; Shai, Y. Ultrashort Antibacterial and Antifungal Lipopeptides. Proc. Natl. Acad. Sci. USA 2006, 103, 15997–16002. [Google Scholar] [CrossRef]
- Mitra, R.N.; Shome, A.; Pauk, P.; Das, P.K. Antimicrobial activity, biocompatibility and hydrogelation ability of dipeptide-based amphiphiles. Org. Biomol. Chem. 2009, 7, 94–102. [Google Scholar] [CrossRef]
- Kothavade, R.J.; Kura, M.M.; Valand, A.G.; Panthaki, M.H. Candida tropicalis: Its prevalence, pathogenicity and increasing resistance to fluconazole. J. Med. Microbiol. 2010, 59, 873–880. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Vega-González, A.; Mendoza-Novelo, B.; López-Romero, E.; Ruiz-Baca, E.; Quintanar-Escorza, M.A.; Villagómez-Castro, J.C. The effect of biomaterials and antifungals on biofilm formation by Candida species: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2513–2527. [Google Scholar] [CrossRef] [PubMed]
- Seferyan, M.A.; Saverina, E.A.; Frolov, N.A.; Detusheva, E.V.; Kamanina, O.A.; Arlyapov, V.A.; Ostashevskaya, I.I.; Ananikov, V.P.; Vereshchagin, A.N. Multicationic Quaternary Ammonium Compounds: A Framework for Combating Bacterial Resistance. ACS Infect. Dis. 2023, 9, 1206–1220. [Google Scholar] [CrossRef]
- Ooi, N.; Miller, K.; Randall, C.; Rhys-Williams, W.; Love, W.; Chopra, I. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. J. Antimicrob. Chemother. 2009, 65, 72–78. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilm: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- García, M.T.; Bautista, E.; de la Fuente, A.; Pérez, L. Cholinium-Based Ionic Liquids as Promising Antimicrobial Agents in Pharmaceutical Applications: Surface Activity, Antibacterial Activity and Ecotoxicological Profile. Pharmaceutics 2023, 15, 1806. [Google Scholar] [CrossRef]
- Sousa, F.F.O.d.; Pinazo, A.; Hafidi, Z.; García, M.T.; Bautista, E.; Moran, M.d.C.; Pérez, L. Arginine Gemini-Based Surfactants for Antimicrobial and Antibiofilm Applications: Molecular Interactions, Skin-Related Anti-Enzymatic Activity and Cytotoxicity. Molecules 2023, 28, 6570. [Google Scholar] [CrossRef]
- Mazurkiewicz, E.; Lamch, L.G.; Wilk, K.A.; Obląk, E. Anti-adhesive, anti-biofilm and fungicidal action of newly synthesized gemini quaternary ammonium salts. Sci. Rep. 2024, 14, 14110. [Google Scholar] [CrossRef]
- Frolov, N.A.; Seferyan, M.A.; Valeev, A.B.; Saverina, E.A.; Detusheva, E.V.; Vereshchagin, A.N. The Antimicrobial and Antibiofilm Potential of New Water-Soluble Tris-Quaternary Ammonium Compounds. Int. J. Mol. Sci. 2023, 24, 10512. [Google Scholar] [CrossRef] [PubMed]
- Fait, M.E.; Grillo, P.D.; Garrote, G.L.; Prieto, E.D.; Vázquez, R.F.; Saparrat, M.C.N.; Morcelle, S.R. Biocidal and antibiofilm activities of arginine-based surfactants against Candida isolates. Amino Acids 2023, 55, 1083–1102. [Google Scholar] [CrossRef] [PubMed]
- Sikora, K.; Bauer, M.; Bartoszewska, S.; Neubauer, D.; Kamysz, W. Glycosylated Lipopeptides-Synthesis and Evaluation of Antimicrobial Activity and Cytotoxicity. Biomolecules 2023, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Fait, M.E.; Garrote, G.L.; Clapés, P.; Tanco, S.; Lorenzo, J.; Morcelle, S.R. Biocatalytic synthesis, antimicrobial properties and toxicity studies of arginine derivative surfactants. Amino Acids 2015, 47, 1465–1477. [Google Scholar] [CrossRef]
- Joondan, N.; Caumul, P.; Jackson, G.; Laulloo, S.J. Novel quaternary ammonium compounds derived from aromatic and cyclic amino acids: Synthesis, physicochemical studies and biological evaluation. Chem. Phys. Lipids 2021, 235, 105051. [Google Scholar] [CrossRef]
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic surfactants derived from lysine: Effects of their structure and charge type on antimicrobial and hemolytic activities. J. Med. Chem. 2011, 54, 989–1002. [Google Scholar] [CrossRef]
- Vlachy, N.; Touraud, D.; Heilmann, J.; Kunz, W. Determining the cytotoxicity of catanionic surfactant mixtures on HeLa cells. Colloids Surf. B Biointerfaces 2009, 70, 278–280. [Google Scholar] [CrossRef]
- Brahmachari, S.; Debnath, S.; Dutta, S.; Das, P.K. Pyridinium based amphiphilic hydrogelators as potential antibacterial agents. Beilstein J. Org. Chem. 2010, 6, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Petrelli, D.; Vitali, L.A.; Bonacucina, G.; Cespi, M.; Vllasaliu, D.; Giorgioni, G.; Palmieri, G.F. Quaternary Ammonium Leucine-Based Surfactants: The Effect of a Benzyl Group on Physicochemical Properties and Antimicrobial Activity. Pharmaceutics 2019, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhang, F.; Zhu, N.; Zhu, Y.; Yao, J.; Gou, S.; Xie, J.; Ni, J. Ultra-short lipopeptides against gram-positive bacteria while alleviating antimicrobial resistance. Eur. J. Med. Chem. 2021, 212, 113138. [Google Scholar] [CrossRef] [PubMed]
- Op den Kamp, J.A. Lipid asymmetry in membranes. Ann. Rev. Biochem. 1979, 48, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Crowston, J.G.; Wang, X.Y.; Khaw, P.T.; Zoellner, H.; Healey, P.R. Human serum reduces mitomycin-C cytotoxicity in human tenon’s fibroblasts. Invest. Ophthalmol. Vis. Sci. 2006, 47, 946–952. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends. Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- García, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and aquatic toxicity of new cleavable betainate cationic oligomeric surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef]
- García, M.T.; Bautista, E.; Pérez, L.; Vázquez, S. Self-Assembly, Antimicrobial Properties and Biodegradability of Ester-Functionalized Choline-Based Surface-Active Ionic Liquids. Molecules 2025, 30, 1280. [Google Scholar] [CrossRef]

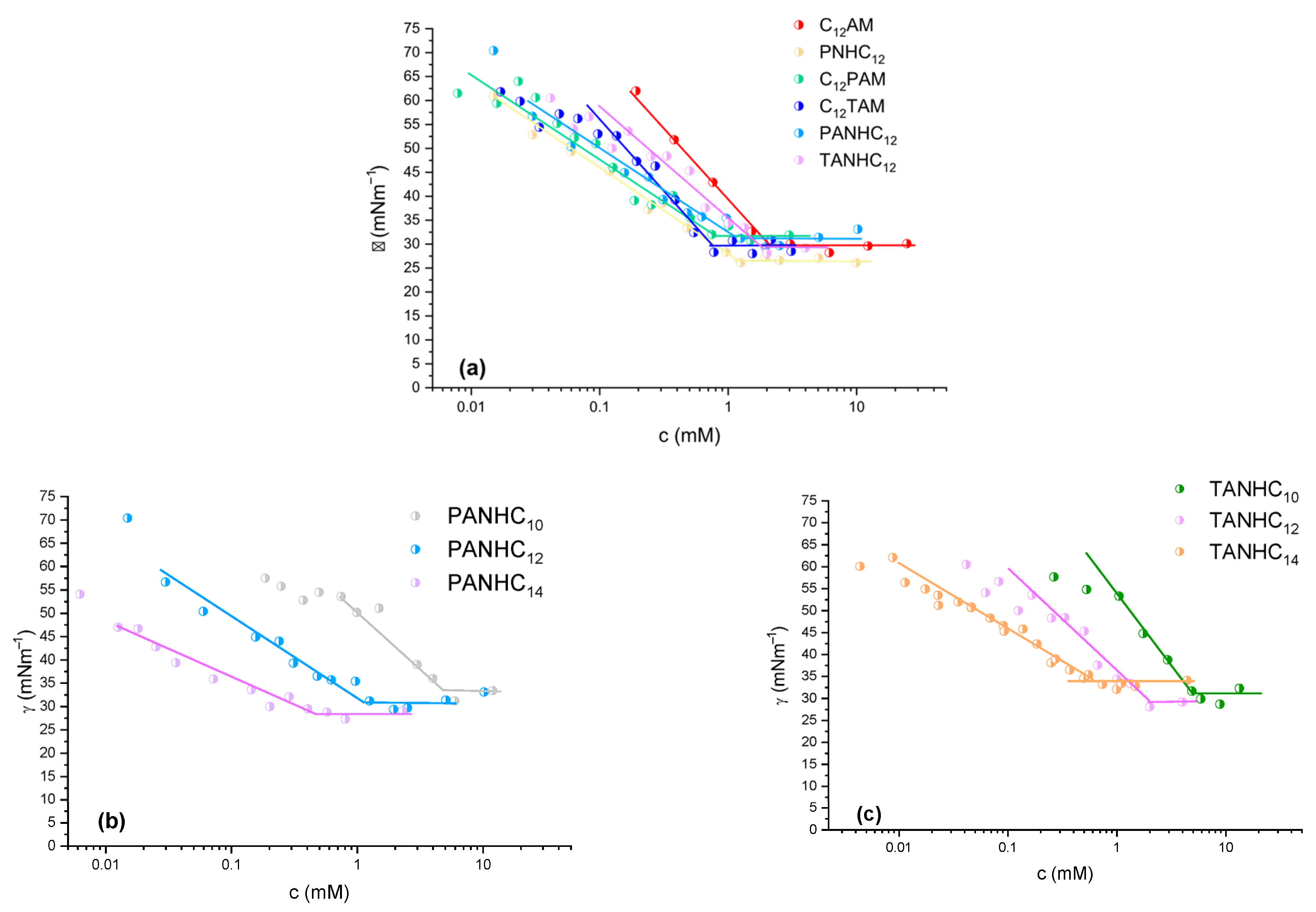


| logP | cmc (mM) | cmc Range * (mM) | cmc Cond (mM) ** | γc (mNm−1) | Rt (min) | |
|---|---|---|---|---|---|---|
| C12AM | 1.14 | 1.7 | [1.1, 2.8] | 6 | 29.5 ± 1.5 | 12.4 |
| PNHC12 | 3.15 | 1.2 | [0.7, 2.1] | 1.2 | 26.6 ± 0.8 | 14.8 |
| LPAM | 2.03 | 0.7 | [0.3, 1.8] | 1.2 | 29.5 ± 2.7 | 15.4 |
| LTAM | 2.50 | 0.65 | [0.3, 1.5] | 0.5 | 32.1 ± 2.3 | 15.9 |
| PANHC12 | 1.71 | 1.5 | [0.7, 3.2] | 2.2 | 30.4 ± 2.6 | 13.5 |
| TANHC12 | 2.17 | 2.5 | [1.0, 6.5] | 0.6 | 28.7 ± 3.3 | 15.1 |
| PANHC10 | 0.69 | 6.7 | [1.9, 23] | 7.2 | 30.6 ± 9.7 | 11.8 |
| PANHC12 | 1.71 | 1.5 | [0.7, 3.2] | 2.2 | 30.4 ± 2.6 | 13.5 |
| PANHC14 | 2.72 | 0.35 | [0.13, 0.95] | 0.7 | 28.7 ± 1.5 | 15.0 |
| TANHC10 | 1.16 | 5.4 | [3.8, 7.6] | 1.7 | 30.6 ± 2.0 | 13.5 |
| TANHC12 | 2.17 | 2.5 | [1.0, 6.5] | 0.6 | 28.7 ± 3.3 | 15.1 |
| TANHC14 | 3.18 | 0.7 | [0.4, 1.3] | 0.3 | 32.8 ± 1.2 | 16.2 |
| BAC | C12AM | PNHC12 | PANHC12 | TANHC12 | C12PAM | C12TAM | |
|---|---|---|---|---|---|---|---|
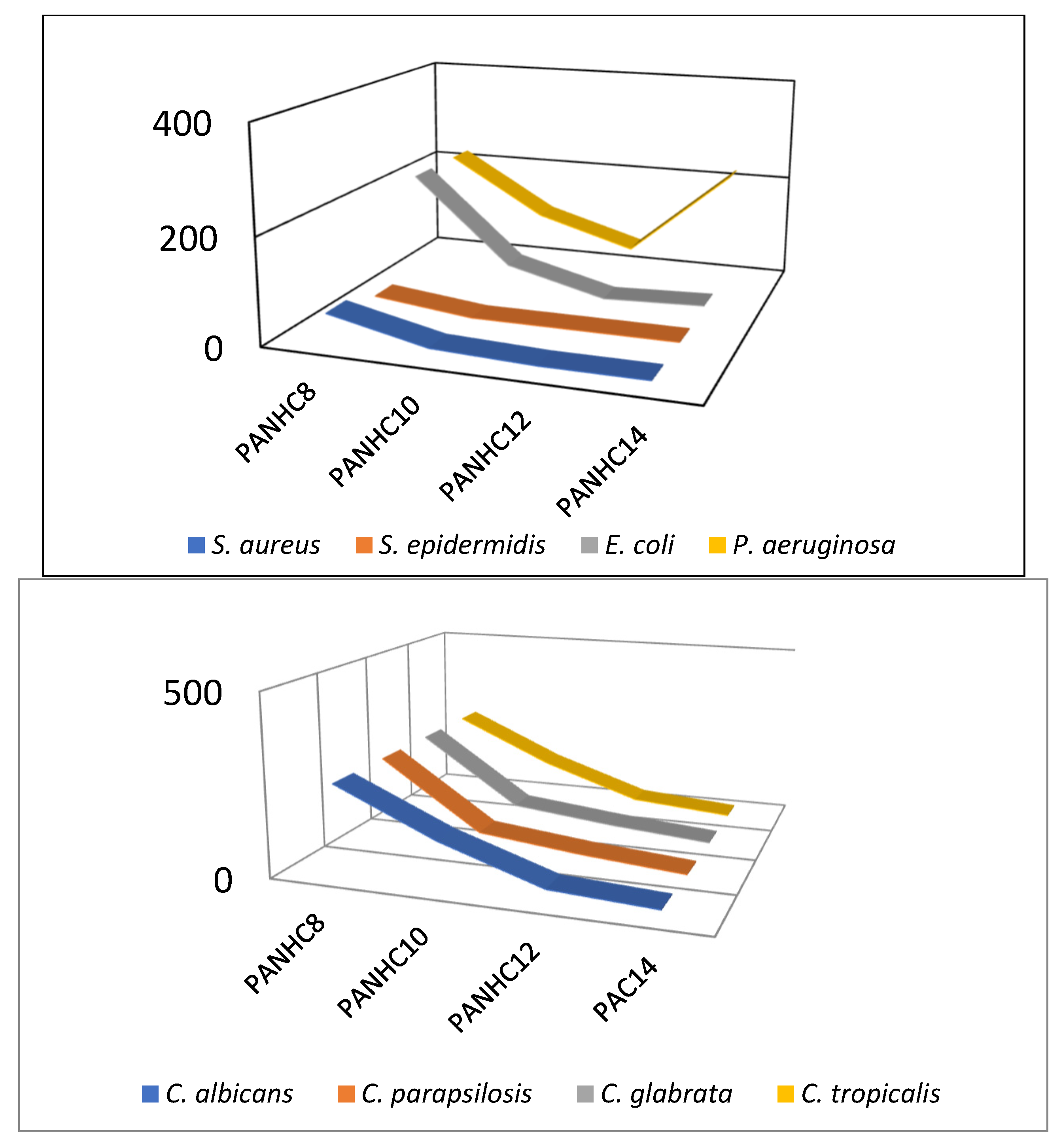
| C. albicans | 8 (24) | 32 (78) | 8 (22) | 32 (56) | 64 (108) | 16 (29) | 32 (54) |
| C. tropicalis | 4 (12) | 8 (19) | 32 (88) | 32 (56) | 32 (54) | 16 (29) | 16 (27) |
| C. parapsilosis | 4 (12) | 16 (39) | 32 (88) | 32 (56) | 32 (54) | 16 (29) | 16 (27) |
| C. glabrata | 8 (24) | 16 (39) | 32 (88) | 32 (56) | 128 (216) | 16 (29) | 16 (27) |
| C. rugosa | 8 (24) | 64 (156) | 32 (88) | 64 (112) | 4 (7) | 4 (7) | 8 (13) |
| C. auris | 32 (98) | 128 (312) | 32 (88) | 64 (112) | 128 (216) | 32 (58) | 16 (27) |
| C. jardinii | 16 (48) | 64 (156) | 32 (88) | 32 (88) | 64 (108) | 64 (116) | 32 (54) |
| PANHC8 | PANHC10 | PANHC12 | PANHC14 | TANHC10 | TANHC12 | TANHC14 | |
|---|---|---|---|---|---|---|---|
| C. albicans | >256 (>512) | 128 (240) | 32 (56) | 16 (27) | 64 (112) | 64 (108) | 16 (25) |
| C. tropicalis | 256 (512) | 128 (240) | 32 (56) | 8 (14) | 64 (112) | 32 (54) | 4 (7) |
| C. parapsilosis | 256 (512) | 64 (120) | 32 (56) | 8 (14) | 64 (112) | 32 (54) | 16 (25) |
| C. glabrata | 256 (512) | 64 (120) | 32 (56) | 8 (14) | 64 (112) | 64 (108) | 8 (14) |
| C. rugosa | >256 (>512) | 128 (240) | 64 (112) | 16 (27) | 4 (7) | 4 (7) | 4 (7) |
| C. auris | >256 (>512) | 256 (480) | 64 (112) | 16 (27) | 128 (224) | 128 (216) | 16 (25) |
| C. jardinii | >256 (>512) | 128 (240) | 32 (56) | 16 (27) | 64 (112) | 64 (108) | 8 (14) |
| HC50 μg/mL (μM) | |
|---|---|
| C12AM | 109 ± 0.6 (270 ± 1.7) |
| PNHC12 | 20 ± 0.6 (55 ± 1.7) |
| LPAM | 23 ± 0.8 (42 ± 1.5) |
| LTAM | 24 ± 0.8 (40 ± 1.6) |
| PANHC12 | 26 ± 1.2 (46 ± 2.2) |
| TANHC12 | 16 ± 0.3 (27 ± 0.6) |
| BAC | 31 ± 0.3 (92 ± 0.8) |
| PANHC10 | 215 ± 22 (404 ± 42) |
| PANHC12 | 26 ± 1.2 (46 ± 2.2) |
| PANHC14 | 20 ± 0.5 (34 ± 0.8) |
| TANHC10 | 74 ± 2.8 (129 ± 5) |
| TANHC12 | 16 ± 0.3 (27 ± 0.6) |
| TANHC14 | 16 ± 0.18 (26 ± 0.3) |
| Alkyl Chain Length (n) | PANHCn | TANHCn | C12AM | BAC a | DDAB b |
|---|---|---|---|---|---|
| 10 | 22 (20–24) | 9.7 (8.6–11) | - | - | 0.15 (0.14–0.17) |
| 12 | 3.4 (2.3–5.3) | 3.0 (2.7–3.4) | 4.4 (3.5–5.4) | 0.039 (0.023–0.05) | - |
| 14 | 0.71 (0.60–1.1) | 0.85 (0.75–1.1) | - | - | - |
| Alkyl Chain Length (n) | PANHCn | TANHCn | C12AM |
|---|---|---|---|
| 10 | 5.6 (5.3–5.9) | 2.2 (1.8–2.8) | |
| 12 | 3.0 (2.6–3.5) | 1.9 (1.3–2.7) | 1.9 (1.8–2.1) |
| 14 | 1.8 (1.3–2.3) | 2.6 (2.1–3.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, M.T.; Morán, M.C.; Pons, R.; Hafidi, Z.; Bautista, E.; Vazquez, S.; Pérez, L. Arginine-Derived Cationic Surfactants Containing Phenylalanine and Tryptophan: Evaluation of Antifungal Activity, Biofilm Eradication, Cytotoxicity, and Ecotoxicity. J. Xenobiot. 2025, 15, 140. https://doi.org/10.3390/jox15050140
García MT, Morán MC, Pons R, Hafidi Z, Bautista E, Vazquez S, Pérez L. Arginine-Derived Cationic Surfactants Containing Phenylalanine and Tryptophan: Evaluation of Antifungal Activity, Biofilm Eradication, Cytotoxicity, and Ecotoxicity. Journal of Xenobiotics. 2025; 15(5):140. https://doi.org/10.3390/jox15050140
Chicago/Turabian StyleGarcía, M. Teresa, M. Carmen Morán, Ramon Pons, Zakaria Hafidi, Elena Bautista, Sergio Vazquez, and Lourdes Pérez. 2025. "Arginine-Derived Cationic Surfactants Containing Phenylalanine and Tryptophan: Evaluation of Antifungal Activity, Biofilm Eradication, Cytotoxicity, and Ecotoxicity" Journal of Xenobiotics 15, no. 5: 140. https://doi.org/10.3390/jox15050140
APA StyleGarcía, M. T., Morán, M. C., Pons, R., Hafidi, Z., Bautista, E., Vazquez, S., & Pérez, L. (2025). Arginine-Derived Cationic Surfactants Containing Phenylalanine and Tryptophan: Evaluation of Antifungal Activity, Biofilm Eradication, Cytotoxicity, and Ecotoxicity. Journal of Xenobiotics, 15(5), 140. https://doi.org/10.3390/jox15050140









