Abstract
Curcumin, the principal active component of turmeric, is a polyphenol that has been used in various countries for the treatment of numerous conditions due to its wide range of health benefits. Curcumin exhibits bifunctional antioxidant properties: the first is attributed to its chemical structure, which enables it to directly neutralize reactive oxygen species (ROS); the second is related to its ability to induce the expression of antioxidant enzymes via the transcription factor nuclear factor erythroid 2–related factor 2 (Nrf2). Both ROS and Nrf2 are closely associated with mitochondrial function and metabolism, and their dysregulation may lead to mitochondrial dysfunction, potentially contributing to the development of various pathological conditions. Therefore, curcumin treatment appears highly promising and is strongly associated with the preservation of mitochondrial function. The aim of this review is to summarize the current literature on the impact of curcumin’s antioxidant properties on mitochondrial function. Specifically, studies conducted in different biological models are included, with emphasis on aspects such as mitochondrial respiration, antioxidant enzyme activity, interactions with mitochondrial membranes, and the role of curcumin in the regulation of intrinsic apoptosis.
1. Introduction
The turmeric (Curcuma longa), also known as Indian saffron, is a plant that has been used for many years in traditional Asian medicine, in addition to its use as a spice and coloring agent. This plant belongs to the same family as ginger, is native to India and Southeast Asia, and is currently cultivated in tropical and subtropical regions [1].
Historically, the use of the plant has been limited primarily to the rhizomes, with the leaves often discarded during processing. However, recent studies have demonstrated that the leaves of Curcuma longa are a valuable source of protein, fiber, and bioactive compounds, and contain significant concentrations of trace elements such as sodium (Na), magnesium (Mg), calcium (Ca), and manganese (Mn) [2].
The underground stem of the plant consists mainly of water (approximately 80%), followed by carbohydrates (13%), proteins (2%), minerals (2%), lipids (1%), and curcuminoids (3–5%). Curcuminoids are a group of phenolic phytochemicals, including curcumin, that share a diarylheptanoid structure, comprising two aromatic rings connected by a seven-carbon linker, with various potential substituents [3]. These compounds are known for a broad spectrum of biological activities, including anti-inflammatory [4], antioxidant [5], antimicrobial [6], and antitumor effects [7].
The three major curcuminoids are curcumin (approximately 70%), demethoxycurcumin (17%), and bisdemethoxycurcumin (3–6%) [8]. Although curcuminoids are found in other species of the Zingiberaceae family, such as Curcuma zedoaria and Curcuma aromatica, Curcuma longa remains the most prominent and studied source [9].
Due to its high natural abundance, curcumin is more easily isolated compared to other curcuminoids, which has facilitated its widespread use in experimental studies. These investigations have revealed a wide range of biological effects beyond those already mentioned, including immunomodulatory [10], hepatoprotective [11], and neuroprotective [12] activities. Curcumin has been shown to act on various cell types and, in some cases, its molecular targets have been identified, including growth factors, transcription factors, redox-regulating enzymes, and cell surface receptors.
Curcumin is recognized and used in multiple countries in diverse forms due to its extensive range of health benefits. A clinical trial has demonstrated that curcumin is well-tolerated and safe in pancreatic cancer patients, even at doses up to 8000 mg/day [13]. However, to date, the United States Food and Drug Administration (FDA) has not approved curcumin as a therapeutic agent for any condition; it remains classified as a safe food additive.
This review aims to provide a comprehensive overview of curcumin, focusing on its chemical structure, metabolism, and antioxidant mechanisms of action. Furthermore, it summarizes the current literature on the impact of curcumin’s antioxidant properties on mitochondrial function, highlighting findings from studies conducted in various biological models. Particular attention is given to aspects such as mitochondrial respiration, antioxidant enzyme activity, interactions with mitochondrial membranes, and curcumin’s role in regulating intrinsic apoptotic pathways.
2. Curcumin
Curcumin is a yellow-orange solid compound widely used as a food additive and coloring agent. Also known as diferuloylmethane, curcumin is a symmetrical molecule belonging to the diarylheptanoid group. Chemically, it is a polyphenol composed of two aromatic rings, each bearing a hydroxyl and a methoxy group as substituents. These rings are connected by a seven-carbon chain that includes two carbonyl groups located at positions 3 and 5 (commonly referred to as the β-diketone moiety), which can undergo keto-enol tautomerism [14] (Figure 1). Its chemical formula is C21H20O6, with a molecular weight of 368 g/moL and a melting point of 183 °C [15]. Curcumin is practically insoluble in water but is readily soluble in organic solvents such as DMSO, methanol, ethanol, and chloroform [16].
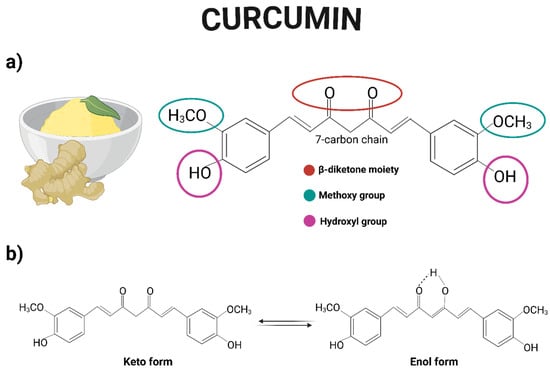
Figure 1.
(a) Chemical structure of curcumin, highlighting the hydroxyl and methoxy functional groups, as well as the β-diketone moiety. (b) Representation of the keto-enol tautomerism of curcumin. Created with BioRender.com.
Curcumin is a photosensitive molecule, and exposure to light is the main route of its degradation. Although the photodegradation mechanism is not yet fully understood, it has been proposed that it primarily involves the cleavage of the β-diketone moiety, leading to the formation of smaller phenolic compounds. Among the colorless degradation products identified in this process are vanillin and ferulic acid [17].
The liver is considered the primary site of curcumin metabolism, along with the intestine. Curcumin undergoes both phase I and phase II biotransformation. Phase I metabolism involves the action of reductases, which reduce the double bonds in the molecule, converting curcumin into dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin, and octahydrocurcumin. In phase II metabolism, both curcumin and its reduced metabolites can be conjugated with glucuronic acid and sulfate at the phenolic site [18,19]. Additionally, curcumin may be sulfated in the cytosol, primarily by phenol sulfotransferase isoenzymes SULT1A1 and SULT1A3 [20]; meanwhile, uridine 5’-diphospho-glucuronosyltransferases (UGTs), mainly UGT1A1, UGT1A8, and UGT1A10, catalyze glucuronidation in intestinal and hepatic microsomes [21].
Moreover, it has been proposed that curcumin can be alternatively metabolized by the gut microbiota. In 2011, Hassanniaasab and colleagues [22] screened human fecal microorganisms for curcumin-converting activity and successfully isolated Escherichia coli, which exhibited the highest activity among the Enterobacteriaceae tested. The responsible enzyme was later isolated, purified, and characterized from E. coli strain K-12, which showed the highest curcumin-converting capacity. The researchers identified the enzyme as an NADPH-dependent reductase that catalyzes a two-step reduction pathway, beginning with the conversion of curcumin to dihydrocurcumin and subsequently to tetrahydrocurcumin as the final product [22].
3. Strategies to Enhance the Bioavailability of Curcumin
Despite its beneficial effects, the most frequent criticism of curcumin consumption is its poor systemic bioavailability. Numerous studies have reported low or even undetectable blood concentrations following oral administration of curcumin. The main reasons for this appear to be the chemical instability of the molecule, poor absorption, and rapid systemic elimination.
To address these limitations, various strategies have been developed to enhance curcumin’s bioavailability. One of the most well-known approaches involves co-administration with compounds that inhibit or delay curcumin metabolism. Piperine, the major active component of black pepper, is a well-established inhibitor of hepatic and intestinal glucuronidation. A pioneering study conducted in 1998 by Shoba et al. [23] demonstrated that co-administration of piperine (20 mg/kg) with curcumin (2 mg/kg) significantly enhanced the bioavailability of the polyphenol. In animal models (rats), bioavailability increased by 154%, while in humans, it rose by as much as 2000%, with no reported adverse effects associated with the combination [23]. Twelve years later, Suresh and Srinivasan [24] confirmed these findings in rats, showing significantly improved bioavailability of curcumin when administered orally alongside piperine. This enhancement was attributed to improved intestinal absorption and prolonged tissue retention of curcumin, with intact curcumin detected in the brain up to 96 h after administration.
Other strategies to improve curcumin bioavailability include the use of nanoparticles. Various formulations, such as those based on high molecular weight poly (lactic-co-glycolic acid) (PLGA) and coatings with saponins, have shown enhanced absorption and stability of curcumin in animal models [25,26].
In addition to the aforementioned approaches, alternative routes of administration have been explored, including subcutaneous, intraperitoneal, and intravenous injection. In general, these methods have yielded promising results for increasing the bioavailability of curcumin, thereby enhancing its therapeutic effects across a wide range of diseases. For instance, a single subcutaneous dose of PLGA microparticles in mice was able to maintain detectable curcumin levels in blood and various tissues for nearly a month. Furthermore, this formulation exhibited strong anticancer activity in immunodeficient mice bearing MDA-MB-231 human breast cancer xenografts and significantly reduced tumor angiogenesis [27]. Likewise, intravenous injection of curcumin formulated in human serum albumin nanoparticles enhanced the anticancer efficacy of curcumin in xenografts of human HT116 colon cancer cells [28].
Interestingly, a marked difference in curcumin’s effectiveness was observed in the same study depending on the route of administration used to attenuate bleomycin-induced lung injury. Intraperitoneal administration was significantly more effective than oral delivery. The authors hypothesized that curcumin bioavailability varied significantly with the route of administration. To test this hypothesis, they measured plasma curcumin levels in mice using high-performance liquid chromatography (HPLC) following oral or intraperitoneal administration of curcumin (300 mg/kg body weight). Plasma collected two hours after oral dosing showed a concentration of 15.7 ± 3.8 ng/mL (approximately 43 nM), whereas intraperitoneal administration resulted in a much higher plasma concentration of 181 ± 23.1 ng/mL (506 nM). Therefore, intraperitoneal delivery of curcumin led to higher plasma concentrations and a greater effective systemic dose than oral administration [29].
Despite these advances, intraperitoneal, intravenous, and subcutaneous delivery of curcumin have been more commonly studied in animals. Further research is needed to validate their efficacy and safety in humans. Oral administration remains the preferred option due to its convenience, high compliance, and strong patient acceptance.
4. Antioxidant Activity of Curcumin
Curcumin is a bifunctional antioxidant, as it has the ability to directly react with reactive oxygen species (ROS), and additionally, it can induce the expression of cytoprotective and antioxidant proteins through the nuclear factor erythroid 2–related factor 2 (Nrf2) pathway.
The chemical structure of curcumin determines its antioxidant activity, as the phenolic hydroxyl (OH) groups are involved in the scavenging or neutralization of free radicals [30,31]. These OH groups donate a hydrogen atom to the radicals, thereby reducing their reactivity. As a result, a relatively stable oxidized product known as the phenoxyl ion/radical is formed [32].
Moreover, the β-diketone moiety of curcumin enables it to react with metals to form complexes. These complexes not only alter the physicochemical properties of curcumin but also affect the biological reactivity of the metals involved [33,34]. Several studies have demonstrated that curcumin-metal complexes possess greater antioxidant capacity than curcumin alone [35,36,37]. For instance, in a study conducted by Thakam and Saewan [35], curcumin was complexed with bivalent metal ions (Zn2+, Se2+, Cu2+, Fe2+, Mg2+, and Mn2+), and the resulting complexes were characterized and evaluated for their radical-scavenging activity. The researchers reported that the complexes showed superior antioxidant performance compared to curcumin alone, with zinc being the most effective in enhancing antioxidant capacity.
On the other hand, Nrf2 is the master transcriptional regulator of cellular responses to oxidative stress. It is well established that various chemical and natural inducers (including curcumin) enhance endogenous antioxidant defenses through the activation of Nrf2. Multiple cellular factors are involved in the regulation of the stability and activation (i.e., nuclear translocation) of this protein [38]. The most well-known regulatory mechanism is described below:
Under normal homeostatic conditions, the Kelch-like ECH-associated protein 1 (Keap1) anchors Nrf2 in the cytosol and facilitates its degradation. Keap1 acts as an adaptor for the Cullin 3 (Cul3)-based E3 ubiquitin ligase complex, promoting Nrf2 ubiquitination and subsequent proteasomal degradation, thereby preventing its activation. However, curcumin induces the modification of cysteine residue 151 in Keap1, leading to conformational changes that hinder Keap1’s ability to mediate Nrf2 ubiquitination by the Cul3 complex. As a result, Nrf2 accumulates, stabilizes, and translocates into the nucleus, where it dimerizes with small proteins (such as Maf or Jun). This dimerization allows Nrf2 to bind to antioxidant response elements (AREs), activating the transcription of a variety of genes encoding cytoprotective, antioxidant, and phase II detoxification proteins [39,40,41,42]. Figure 2 summarizes the antioxidant mechanisms of action of curcumin, including both direct and indirect pathways.
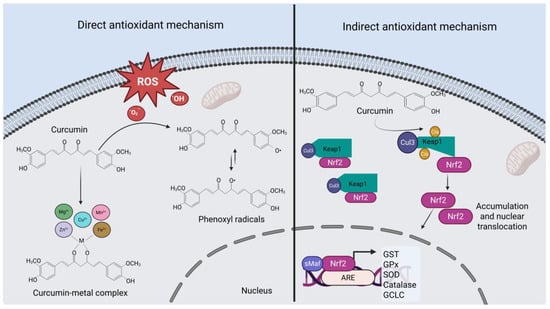
Figure 2.
Antioxidant mechanism of curcumin. The direct mechanism (left panel) relies on the phenolic OH groups of curcumin, which act as electron donors to neutralize free radicals. This leads to the formation of phenoxyl radicals, which are relatively stable. The β-diketone moiety enables curcumin to interact with metals, thereby modifying their biological reactivity. On the other hand, the indirect antioxidant mechanism (right panel) is mediated by the activation of the transcription factor Nrf2, promoting the expression of endogenous antioxidant enzymes. ARE, Antioxidant Response Elements; Cis, Cysteines; Cu2+, Copper; CUL3, Cullin E3 Ubiquitin Ligase; ROS, Reactive Oxygen Species; Fe2+, Iron; GCLC, Glutamate–Cysteine Ligase; GST, Glutathione S-Transferase; GPX, Glutathione Peroxidase; H2O2, Hydrogen Peroxide; Keap1, Kelch-like ECH-associated Protein 1; Mg2+, Magnesium; Mn2+, Manganese; Nrf2, Nuclear Factor Erythroid 2–Related Factor 2; O2−, Superoxide Radical; SOD, Superoxide Dismutase. Created with BioRender.com.
5. Curcumin and the Mitochondrion
Mitochondria play a central role in cellular metabolism by generating the majority of adenosine triphosphate (ATP) through the catabolism of nutrients. This is accomplished via mitochondrial respiration, a complex process that includes the tricarboxylic acid (TCA) cycle (also known as the Krebs cycle). The TCA cycle takes place in the mitochondrial matrix and its primary functions include the production of guanosine triphosphate (GTP), which is energetically equivalent to ATP, as well as the generation of the electron carrier’s nicotinamide adenine dinucleotide in its reduced form (NADH) and flavin adenine dinucleotide in its reduced form (FADH2). These carriers shuttle electrons to the electron-transport chain, a series of protein complexes embedded in the inner mitochondrial membrane. As electrons traverse the chain, protons are pumped into the intermembrane space, creating an electrochemical gradient that ultimately drives ATP synthesis [43].
Given its pivotal role in energy production, mitochondrial integrity is essential to cellular homeostasis. The following section examines the involvement of mitochondria in the etiology of various diseases, highlighting the impact of mitochondrial genome mutations, the generation of and defense against ROS, and mitochondrial contributions to biosynthetic and signaling pathways, as well as the modulatory effects of curcumin on these functions.
5.1. Role of Mitochondria in the Etiology of Diseases
ATP synthesis is an intricate process; even minor disruptions can precipitate a variety of diseases, particularly in high-energy-demand tissues such as brain and skeletal muscle. Some mitochondrial dysfunctions arise from inherited or acquired mutations in the mitochondrial genome. Such mutations may disrupt overall mitochondrial protein synthesis, via large-scale deletions of mitochondrial DNA or point mutations in specific genes. Notably, pathogenic mutations in mitochondrial tRNA and rRNA genes often impair the translation of the thirteen polypeptides encoded by mitochondrial DNA, leading to defects in oxidative phosphorylation. For example, mutations in the mitochondrial tRNA^Lys gene are frequently associated with epilepsy, elongated muscle fibers, and myoclonus (involuntary, jerky muscle contractions) [44].
Oxygen serves as the terminal electron acceptor in the respiratory chain, combining with protons and electrons to form water according to the reaction:
O2 + 4H+ + 4e− ⟶ 2H2O
However, mitochondria are also a major source of intracellular ROS, generated when electrons “leak” from the transport chain and partially reduce oxygen. Under physiological conditions, an estimated 0.2–2% of electrons escape the normal transfer pathway, directly reacting with oxygen to produce superoxide (O2−), a highly reactive species [45]. The O2− is subsequently converted into hydrogen peroxide (H2O2) and other ROS. At low concentrations, ROS function as signaling molecules that regulate processes such as cell differentiation; but uncontrolled ROS production can inflict both mitochondrial and widespread cellular damage [46].
To mitigate ROS, mitochondria possess antioxidant defense systems, most prominently superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which neutralize ROS before they cause harm [47]. When these defenses are overwhelmed, excessive ROS provoke oxidative damage to mitochondrial proteins, DNA, and lipids, impairing respiratory-chain enzyme activities, interrupting ATP production, and compromising other essential functions. Such mitochondrial dysfunction contributes to a range of pathological conditions, including aging and metabolic diseases [48].
Beyond energy generation, mitochondria supply key precursors for macromolecular biosynthesis, participating in the synthesis of nucleotides, fatty acids, cholesterol, amino acids, and heme. A central example is citrate, a TCA-cycle intermediate that not only regulates energy production but also modulates anabolic pathways. Following its synthesis from acetyl-CoA and oxaloacetate by citrate synthase, citrate may either continue through the TCA cycle or be exported to the cytosol via a specific transporter, depending on the cell’s energetic and biosynthetic state. High cytosolic citrate levels inhibit glycolysis, via allosteric suppression of phosphofructokinase-1, and stimulate ATP-consuming pathways such as lipid synthesis. In the cytosol, ATP-citrate lyase cleaves citrate into oxaloacetate and acetyl-CoA, the latter being the key precursor for fatty-acid and sterol biosynthesis. Moreover, certain metabolites and their concentrations act as signals to reshape gene expression; in this context, newly generated acetyl-CoA plays a significant role in epigenetic modifications, such as histone acetylation [49,50]. These biosynthetic pathways are integral to stress responses and are often dysregulated in disease.
Finally, mitochondria serve as environmental sensors that communicate with other subcellular compartments. A principal mode of communication is through physical contacts at mitochondrial-associated membranes (MAMs), specialized contact sites between mitochondria and the endoplasmic reticulum. MAMs form a crucial platform for interorganelle molecule exchange and cross-talk, governing calcium homeostasis, lipid metabolism, and apoptosis regulation. Disruption of MAM composition or aberrant MAM formation is linked to various pathologies, notably neurological and cardiovascular diseases [51,52,53]
Together, these pleiotropic mitochondrial functions underscore how subtle perturbations in organelle structure or activity can disrupt cellular homeostasis and contribute to the pathogenesis of numerous diseases.
5.2. Effects of Curcumin on Mitochondria
5.2.1. Mitochondrial Respiration, ROS, and Antioxidant Enzymes
Curcumin attenuates oxidative stress in both animal models and cell-culture systems in which ROS generation has been induced by various stimuli, including chemical agents or pathological conditions (e.g., hyperglycemia, neurotoxicity, and aging) (Table 1). Administration of curcumin reduces oxidative damage, as evidenced by decreases in biomarkers such as malondialdehyde (MDA), a lipid-peroxidation product, and protein carbonyl content. These antioxidant effects are intimately linked to curcumin’s ability to protect against mitochondrial dysfunction elicited by these stressors, since it enhances the efficiency of mitochondrial respiration and preserves mitochondrial membrane potential. Moreover, curcumin induces both the expression and activity of SOD, CAT, GPx, and glutathione reductase (GR), thereby reinforcing the endogenous antioxidant defense system.

Table 1.
In vivo and in vitro studies on the effects of curcumin in diseases associated with mitochondrial dysfunction.
A representative study by Moselhy et al. [54], evaluated cellular toxicity and mitochondrial dysfunction during cellular senescence in rat brain tissue exposed to γ-radiation, and assessed the therapeutic potential of curcumin nanoparticles. The researchers found that cranial irradiation induced ROS production and oxidative stress in rat brain, as indicated by a significant increase in MDA concentrations and a significant decrease in antioxidant biomarkers, namely SOD activity, reduced glutathione (GSH) content, and total antioxidant capacity, compared with controls. Furthermore, activities of mitochondrial Complexes I and II and ATP production were diminished. In contrast, rats receiving oral curcumin (10 mg/kg) for eight weeks post-irradiation exhibited significantly lower MDA levels and significantly higher SOD activity and GSH content. These animals also demonstrated significantly increased activities of mitochondrial complexes and ATP production relative to irradiated controls; notably, Complex II activity was fully restored to control levels following curcumin treatment [54].
However, other investigations indicate that direct addition of curcumin to isolated mitochondria or to cells in the absence of a stressor may exert the opposite effect on redox balance. Curcumin appears to possess both antioxidant and pro-oxidant properties, a duality that depends primarily on its concentration and the cell type. For example, Yu et al. [64] reported that, in mouse C2C12 myoblasts under basal conditions, low curcumin concentrations (1–5 µM) did not alter cell morphology or viability but did elicit a mild increase in ROS levels associated with elevated mitochondrial mass and membrane potential. Conversely, cells incubated with higher curcumin concentrations (10, 20, and 50 µM) underwent marked morphological changes, rounding and detachment from the culture substrate, indicative of cell death. These high-dose treatments decreased viability and raised ROS levels. Specifically, exposure to 20 µM curcumin induced mitochondrial permeability transition pore opening, cytochrome c release, caspase 9 and 3 activation, and ultimately apoptotic cell death [64].
Similarly, Atsumi et al. [67] demonstrated that, in normal human gingival fibroblasts, curcumin can also act as a pro-oxidant: incubation with 10–50 µM curcumin provoked significant ROS generation, which was accompanied by loss of mitochondrial membrane potential, an early indicator of mitochondrial dysfunction, and externalization of phosphatidylserine, a hallmark of apoptosis. All induced damage was mitigated by the addition of exogenous antioxidants, such as GSH and N-acetyl-L-cysteine (NAC), suggesting that ROS mediated curcumin’s cytotoxicity [67].
Although definitive conclusions cannot be drawn from current evidence, findings from animal models suggest that high doses of curcumin (up to 400 mg/kg body weight) may exert mitoprotective effects in the presence of a stressor [62], likely due to the physiological complexity of these organisms. In contrast, in vitro studies have shown a biphasic response that depends both on curcumin concentration and the presence of a stress-inducing stimulus. When such a stimulus is present, concentrations between 10 and 20 µM appear sufficient to trigger a mitoprotective effect [65,66]. However, in the absence of a stressor, cytotoxic effects have been observed at concentrations exceeding 10 µM [64,67]. These findings highlight the need for further studies to clarify the direct effects of curcumin on cells and mitochondria under basal (non-stressed) conditions in order to better define the threshold between its cytoprotective and cytotoxic actions.
5.2.2. Curcumin’s Interaction with Mitochondrial Components and Its Modulation of Membrane Fluidity
The lipophilic nature of curcumin and its poor solubility in water allow it to interact with cellular membranes. However, there is still no consensus regarding curcumin’s preferential location within the lipid bilayer, nor about its precise effects on membrane properties. Among the most relevant findings is that curcumin affects membrane fluidity; both an increase in acyl chain order across various membrane models [68,69,70] and a bilayer fluidization after curcumin administration have been reported [71].
In a recent study, the interaction of curcumin with membranes and its ability to modify membrane barrier properties, such as water permeability, was investigated using protein-free model membranes composed of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC). The findings revealed a concentration-dependent biphasic effect: water permeability decreased at low concentrations of curcumin (up to 2 mol%), while at higher concentrations (above 3 mol%), permeability increased. Based on these results and complementary biophysical techniques, the authors proposed that at low concentrations, curcumin interacts at the lipid–water interface through hydrogen bonding with phosphate head groups, which reduces membrane fluidity and sterically hinders the passage of water molecules, ultimately leading to decreased water permeability. In contrast, at higher concentrations, curcumin may penetrate the bilayer and interact with acyl chains, increasing membrane fluidity and, consequently, water permeability [72].
Moreover, curcumin has shown significant interactions with cardiolipin, a phospholipid found in the mitochondrial membrane of eukaryotic cells and in bacterial membranes. A study investigating curcumin’s interaction with model membrane systems, specifically fully hydrated bilayers and Langmuir monolayers composed of dimyristoylphosphatidylcholine (DMPC) or a mixture of DMPC with 4 mol% tetramyristoylcardiolipin (TMCL), used biophysical techniques such as spectrophotometry and fluorescence to assess the compound’s behavior. The authors demonstrated that curcumin associates with the polar/apolar interfaces of lipid bilayers, and that this association is enhanced by the presence of cardiolipin. Additionally, atomic force microscopy revealed that at high curcumin concentrations (10 mol%), aggregates form within DMPC monolayers, suggesting oversaturation of the system and potential disruption of membrane stability. However, in the presence of TMCL, aggregate formation was reduced, contributing to membrane stability [73].
In biomimetic models mimicking the inner mitochondrial membrane, it was observed that curcumin readily incorporates into the bilayer in the presence of cardiolipin. This behavior appears to be related to cardiolipin’s tendency to adopt a non-lamellar hexagonal phase, which may facilitate more effective curcumin association and insertion. Furthermore, curcumin insertion into the bilayers reduces lipid packing order and increases membrane fluidity. These findings are particularly relevant given that several mitochondrial membrane proteins, including cytochrome c and respiratory complexes, are closely associated with cardiolipin. Therefore, changes in membrane fluidity may affect the activity of these enzymes and, consequently, mitochondrial function. Indeed, the authors suggest that curcumin’s interactions with mitochondrial membranes, mediated by cardiolipin, could underlie its high efficacy against a broad range of metabolic diseases [71].
In addition to its effects mediated by alterations in mitochondrial membrane fluidity, curcumin has also been shown to influence mitochondrial proteins involved in the regulation of mitochondrial dynamics. For instance, in a recent study using an animal model of sepsis, curcumin was reported to inhibit the mitochondrial translocation of dynamin-related protein 1 (DRP1), a key GTPase involved in mitochondrial fission. This inhibition was associated with activation of the SIRT1 pathway, which not only reduced mitochondrial fragmentation but also promoted mitochondrial biogenesis [63].
Complementarily, in an in vitro model of heat stress using mouse C2C12 myoblasts, curcumin induced a moderate increase in non-mitochondrial ROS levels, generated via NADPH oxidase. This increase functioned as a signal that directly regulated the expression of proteins involved in mitochondrial dynamics. Specifically, an upregulation of mitofusin 2 (MFN2) and dynamin-like 120 kDa mitochondrial protein OPA1 (OPA1) was observed—proteins responsible for the fusion of the outer and inner mitochondrial membranes, respectively. Additionally, DRP1 expression was downregulated, preventing mitochondrial fragmentation. Taken together, these findings indicate that curcumin promotes mitochondrial fusion, thereby increasing the number of elongated and tubular mitochondria. This morphology has been proposed as a mechanism that enhances ATP production efficiency, potentially representing a pro-survival adaptation to thermal stress [74].
Furthermore, in a model of oxidative stress-induced damage using H2O2 in R28 retinal neuronal cells, pretreatment with 5 µM curcumin was shown to attenuate the H2O2-induced overexpression of DRP1, while simultaneously preventing the reduction in expression of the mitochondrial fusion protein MFN2. Curcumin pretreatment also preserved mitochondrial morphology and led to a reduction in intracellular ROS production. Additionally, curcumin attenuated caspase-3 cleavage and decreased cellular apoptosis. These findings suggest that curcumin may exert a neuroprotective effect against oxidative damage through modulation of mitochondrial dynamics [75]. These findings suggest that some of curcumin’s mitochondrial actions are due to both specific effects on key proteins and changes in the lipid microenvironment.
6. Intrinsic Apoptosis
Curcumin exhibits broad therapeutic potential, including notable antiproliferative properties. Multiple studies have demonstrated its efficacy against various cancer types, such as lung [76], kidney [77], breast [78], pancreatic [79], and colon tumors [80]. This effect is partly attributed to its preferential uptake by tumor cells over normal cells [81,82], which enhances its ability to induce cell cycle arrest, autophagy, and apoptosis [83].
Apoptosis, a tightly regulated form of programmed cell death essential for tissue homeostasis, occurs via two main pathways: the intrinsic (mitochondrial) and the extrinsic (death receptor) pathways [84]. The intrinsic pathway is triggered by internal stress signals such as elevated ROS levels and DNA damage, leading to mitochondrial outer membrane permeabilization (MOMP), cytochrome c release, apoptosome formation, caspase activation, and ultimately cell death. This pathway is critically regulated by the Bcl-2 protein family, comprising pro-apoptotic (Bax, Bad, Bak) and anti-apoptotic (Bcl-2, Bcl-xL) members that govern MOMP [85].
Treatment of various tumor cell lines with curcumin consistently alters ROS levels, decreases mitochondrial membrane potential, and upregulates pro-apoptotic markers such as Bax and cytochrome c, while downregulating anti-apoptotic proteins (see Table 2). These findings highlight mitochondrial membrane depolarization as a key event in ROS-mediated apoptotic signaling, positioning mitochondria as central players in curcumin’s anticancer action.

Table 2.
Main effects of curcumin on mitochondria in different tumor cell lines.
This mechanism is exemplified by a study conducted by Bao et al. [86], in which human ovarian cancer cell lines HO9810 and OVCAR3 were treated with 5–30 µM curcumin. Treated cells detached and floated, with the number of floating cells increasing in a concentration-dependent manner, indicating enhanced cell death. After 48 h of treatment, intracellular ROS levels rose significantly. Curcumin also elevated mitochondrial H2O2 and O2− levels, reduced mitochondrial membrane potential, and decreased ATP production. Notably, pre-treatment with the antioxidant N-acetylcysteine (NAC) prevented both oxidative stress and mitochondrial dysfunction, suggesting that curcumin-induced apoptosis in ovarian cancer cells is largely driven by oxidative stress [86].
While many studies have focused on evaluating the pro-apoptotic effects of curcumin in various tumor cell lines, it is also important to understand its impact on normal and/or stem/progenitor cells. At low concentrations (1–20 µM), curcumin has been shown to preserve mitochondrial integrity, prevent the loss of membrane potential, and reduce cytochrome c release across different cell types. For example, in bone marrow-derived stem cells (BMSCs), curcumin combined with hypoxic preconditioning enhanced mitochondrial quality by promoting both mitochondrial fusion and complex I enzymatic activity. It also supported cell survival and inhibited mitochondrial cytochrome c release, thereby suppressing the intrinsic apoptotic pathway, as evidenced by reduced caspase-3 activation. These effects were closely associated with activation of the PGC-1α/SIRT3/HIF-1α pathway [93].
Furthermore, curcumin pretreatment was reported to protect human periodontal ligament stem cells (hPDLSCs) from oxidative stress-induced injury by enhancing cell proliferation, reducing ROS levels, and significantly decreasing apoptosis [94]. Similarly, in mouse lung mesenchymal stem cells (LMSCs) exposed to H2O2, treatment with 10 µM curcumin restored mitochondrial membrane potential, decreased ROS production, and reduced caspase-3 activation. These effects were linked to positive modulation of the AKT/Nrf2/HO-1 signaling pathway [95].
Collectively, these findings support a dual role of curcumin: on one hand, it promotes mitochondrial apoptosis in cancer cells through ROS-induced mitochondrial dysfunction; on the other, it exerts a cytoprotective effect in progenitor cells under stress conditions by maintaining mitochondrial homeostasis and attenuating apoptotic signaling. This highlights the therapeutic potential of curcumin in both oncology and regenerative medicine.
7. Conclusions and Future Perspectives
Several studies have demonstrated that curcumin attenuates oxidative stress in models where the production of ROS has been induced by various factors. This antioxidant effect appears to be closely associated with its ability to preserve mitochondrial function, by improving respiratory efficiency and maintaining membrane potential.
However, in the absence of a stressor, curcumin can exert pro-oxidant effects when directly applied to cells or mitochondria, leading to apoptosis. These effects depend primarily on curcumin concentration and cell type and have gained attention due to their potential application in cancer therapies, given that curcumin tends to accumulate at higher concentrations in tumor tissues.
In this context, increased ROS levels, upregulation of pro-apoptotic markers, and a reduction in mitochondrial membrane potential, an essential event in oxidative stress-mediated apoptotic signaling, have been observed.
Recently, interest has grown in understanding how curcumin interacts with mitochondria, particularly at the membrane level. Studies using lipid bilayer models suggest that curcumin insertion into the membrane is facilitated by cardiolipin, resulting in reduced lipid ordering and increased bilayer fluidity. These changes may affect the activity of mitochondrial enzymes, such as respiratory complexes, by altering the lipid microenvironment.
Together, these findings highlight the central role of mitochondria not only as targets of curcumin’s effects but also as mediators of its cytoprotective or cytotoxic actions, depending on the experimental context. However, the potential clinical application of these effects still faces significant challenges, mainly due to curcumin’s low systemic bioavailability, which limits its therapeutic efficacy. Although various strategies have been proposed to improve its pharmacokinetic profile (such as co-administration with piperine, the use of nanoparticles, and alternative routes of administration), its efficacy and safety in humans still require validation.
Therefore, it is essential to address these limitations by developing well-structured and context-specific protocols that allow for rigorous evaluation and reliable establishment of curcumin’s therapeutic potential as a modulator of mitochondrial function.
Author Contributions
Conceptualization, K.A.A.-B.; formal analysis, K.A.A.-B.; investigation, K.A.A.-B. and E.Y.H.-C.; resources, J.P.-C.; writing—original draft preparation, K.A.A.-B.; writing—review and editing, E.Y.H.-C., J.E.-M., and J.P.-C.; visualization, K.A.A.-B., J.E.-M., and E.Y.H.-C.; supervision, E.Y.H.-C. and J.P.-C.; funding acquisition, J.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The funding for this article was supported by Secretaría de Ciencia, Humanidades, Tecnología e Innovación (Secihti; CBF2023-2024-190), Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT IN202725), and Programa de Apoyo a la Investigación y al Posgrado (PAIP, 5000-9105).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
All the authors are thankful to their respective affiliated institutions for providing research facilities to carry out this work. Karla Alejandra Avendaño Briseño and Jorge Escutia- Martínez are doctoral students from Programa de Doctorado en Ciencias Biológicas from Universidad Nacional Autónoma de México (UNAM). They received a fellowship (No. CVU: 1035130 and 1035117) from Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| ARE | Antioxidant response elements |
| ATP | Adenosine triphosphate |
| ATPase | Adenosine triphosphatase |
| Bcl-2 | Apoptosis regulator Bcl-2 |
| BMSCs | Bone marrow-derived stem cells |
| CAT | Catalase |
| DMPC | Dimyristoylphosphatidylcholine |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DRP1 | Dynamin-related protein 1 |
| FADH2 | Flavin adenine dinucleotide in its reduced form |
| FDA | Food and Drug Administration |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GTP | Guanosine triphosphate |
| H2O2 | Hydrogen peroxide |
| hPDLSCs | Human periodontal ligament stem cells |
| HL-1 | Mouse atrial cardiomyocyte cell line |
| HPLC | High-performance liquid chromatography |
| i.p. | Intraperitoneal (injection) |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LPS | Lipopolysaccharide |
| MAMs | Mitochondrial-associated membranes |
| MFN2 | Mitofusin 2 |
| MDA | Malondialdehyde |
| MOMP | Mitochondrial outer membrane permeabilization |
| mPTP | Mitochondrial permeability transition pore |
| MSC | Mesenchymal stem cells |
| MSCs | Mouse lung mesenchymal stem cells |
| NAC | N-acetylcysteine |
| NADH | Nicotinamide adenine dinucleotide in its reduced form |
| NO | Nitric oxide |
| NRF1 | Nuclear respiratory factor 1 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| O2 | Oxygen |
| O2− | Superoxide radical |
| OH | Hydroxyl groups |
| OPA1 | Dynamin-like 120 kDa mitochondrial protein OPA1 |
| PLGA | Poly(lactic-co-glycolic acid) |
| ROS | Reactive oxygen species |
| rRNA | Ribosomal RNA |
| SOD | Superoxide dismutase |
| SULT | Sulfotransferase isoenzymes |
| TBARS | Thiobarbituric acid reactive substances |
| TCA | Tricarboxylic acid |
| TFAM | Transcription factor A, mitochondrial. |
| TMCL | Tetramyristoylcardiolipin |
| tRNA | Transfer RNA |
| UGTs | 5’-diphospho-glucuronosyltransferases |
References
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological Activities of Curcuminoids, Other Biomolecules from Turmeric and Their Derivatives—A Review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.C.; Vieira, E.C.S.; de Oliveira, T.F. Curcuma Longa L. Leaves: Characterization (Bioactive and Antinutritional Compounds) for Use in Human Food in Brazil. Food Chem. 2018, 265, 308–315. [Google Scholar] [CrossRef]
- Ganapathy, G.; Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Diarylheptanoids as Nutraceutical: A Review. Biocatal. Agric. Biotechnol. 2019, 19, 101109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, Z.; Chen, G.; Wang, D.; Tang, S.; Deng, H.; Wang, J.; Li, S.; Lan, J.; Tong, J.; et al. Curcumin Attenuates Asthmatic Airway Inflammation and Mucus Hypersecretion Involving a PPAR γ -Dependent NF-κ B Signaling Pathway In Vivo and In Vitro. Mediat. Inflamm. 2019, 2019, 4927430. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant Effects of Curcumin in Models of Neurodegeneration, Aging, Oxidative and Nitrosative Stress: A Review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Negi, P.S.; Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Antibacterial Activity of Turmeric Oil: A Byproduct from Curcumin Manufacture. J. Agric. Food Chem. 1999, 47, 4297–4300. [Google Scholar] [CrossRef]
- Doello, K.; Ortiz, R.; Alvarez, P.J.; Melguizo, C.; Cabeza, L.; Prados, J. Latest in Vitro and in Vivo Assay, Clinical Trials and Patents in Cancer Treatment Using Curcumin: A Literature Review. Nutr. Cancer 2018, 70, 569–578. [Google Scholar] [CrossRef]
- Zhang, H.A.; Kitts, D.D. Turmeric and Its Bioactive Constituents Trigger Cell Signaling Mechanisms That Protect against Diabetes and Cardiovascular Diseases. Mol. Cell. Biochem. 2021, 476, 3785–3814. [Google Scholar] [CrossRef]
- Zhang, J.; Jinnai, S.; Ikeda, R.; Wada, M.; Hayashidac, S.; Nakashima, K. A Simple HPLC-Fluorescence Method for Quantitation of Curcuminoids and Its Application to Turmeric Products. Anal. Sci. 2009, 25, 385–388. [Google Scholar] [CrossRef]
- Allegra, A.; Mirabile, G.; Ettari, R.; Pioggia, G.; Gangemi, S. The Impact of Curcumin on Immune Response: An Immunomodulatory Strategy to Treat Sepsis. Int. J. Mol. Sci. 2022, 23, 14710. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective Effects of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the Active Substance of Turmeric: Its Effects on Health and Ways to Improve Its Bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of Curcumin through Reduction and Glucuronidation in Mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the Cancer Chemopreventive Agent Curcumin in Human and Rat Intestine. Cancer Epidemiol. Biomark. Prev. 2002, 11, 105–111. [Google Scholar]
- Hoehle, S.I.; Pfeiffer, E.; Metzler, M. Glucuronidation of Curcuminoids by Human Microsomal and Recombinant UDP-glucuronosyltransferases. Mol. Nutr. Food Res. 2007, 51, 932–938. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the Curcumin Metabolic Pathway Involving a Unique Enzyme in an Intestinal Microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivasan, K. Tissue Distribution & Elimination of Capsaicin, Piperine & Curcumin Following Oral Intake in Rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Improving Curcumin Solubility and Bioavailability by Encapsulation in Saponin-Coated Curcumin Nanoparticles Prepared Using a Simple PH-Driven Loading Method. Food Funct. 2018, 9, 1829–1839. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Chang-Liao, W.-L.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Effects of Polymer Molecular Weight on Relative Oral Bioavailability of Curcumin. Int. J. Nanomed. 2012, 7, 2957–2966. [Google Scholar] [CrossRef]
- Shahani, K.; Swaminathan, S.K.; Freeman, D.; Blum, A.; Ma, L.; Panyam, J. Injectable Sustained Release Microparticles of Curcumin: A New Concept for Cancer Chemoprevention. Cancer Res. 2010, 70, 4443–4452. [Google Scholar] [CrossRef]
- Kim, T.H.; Jiang, H.H.; Youn, Y.S.; Park, C.W.; Tak, K.K.; Lee, S.; Kim, H.; Jon, S.; Chen, X.; Lee, K.C. Preparation and Characterization of Water-Soluble Albumin-Bound Curcumin Nanoparticles with Improved Antitumor Activity. Int. J. Pharm. 2011, 403, 285–291. [Google Scholar] [CrossRef]
- Smith, M.R.; Gangireddy, S.R.; Narala, V.R.; Hogaboam, C.M.; Standiford, T.J.; Christensen, P.J.; Kondapi, A.K.; Reddy, R.C. Curcumin Inhibits Fibrosis-Related Effects in IPF Fibroblasts and in Mice Following Bleomycin-Induced Lung Injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 298, L616–L625. [Google Scholar] [CrossRef]
- Feng, J.-Y.; Liu, Z.-Q. Phenolic and Enolic Hydroxyl Groups in Curcumin: Which Plays the Major Role in Scavenging Radicals? J. Agric. Food Chem. 2009, 57, 11041–11046. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of Phenolic O-H and Methylene Hydrogen on the Free Radical Reactions and Antioxidant Activity of Curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Malik, P.; Mukherjee, T.K. Structure-Function Elucidation of Antioxidative and Prooxidative Activities of the Polyphenolic Compound Curcumin. Chin. J. Biol. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Indira Priyadarsini, K. Chemical and Structural Features Influencing the Biological Activity of Curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal–Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef] [PubMed]
- Thakam, A.; Saewan, N. Antioxidant Activities of Curcumin-Metal Complexes. Thai J. Agric. Sci. 2011, 44, 188–193. [Google Scholar]
- Mei, X.; Xu, D.; Xu, S.; Zheng, Y.; Xu, S. Gastroprotective and Antidepressant Effects of a New Zinc(II)–Curcumin Complex in Rodent Models of Gastric Ulcer and Depression Induced by Stresses. Pharmacol. Biochem. Behav. 2011, 99, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.Q.; Thao, D.T.T. Enhancing the Solubility of Curcumin Metal Complexes and Investigating Some of Their Biological Activities. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 Signaling. React. Oxyg. Species 2019, 8, 312–322. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase to Regulate Proteasomal Degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a Regulator of Cell Metabolism and Inflammation in Cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin Induces Stabilization of Nrf2 Protein through Keap1 Cysteine Modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef] [PubMed]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and Molecular Mechanisms of Mitochondrial Function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Schon, E.A. Mitochondrial Genetics and Disease. Trends Biochem. Sci. 2000, 25, 555–560. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial Dysfunction in Cell Senescence and Aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Iacobazzi, V.; Infantino, V. Citrate—New Functions for an Old Metabolite. Biol. Chem. 2014, 395, 387–399. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, J.; Zhang, M.; Wang, Y.; Shi, X. Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs): Possible Therapeutic Targets in Heart Failure. Front. Cardiovasc. Med. 2023, 10, 1083935. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, S.; Ou, Y.; Hu, F.; Long, D. Mitochondria-Associated Endoplasmic Reticulum Membranes: A Promising Toxicity Regulation Target. Acta Histochem. 2023, 125, 152000. [Google Scholar] [CrossRef]
- Schon, E.A.; Area-Gomez, E. Mitochondria-Associated ER Membranes in Alzheimer Disease. Mol. Cell. Neurosci. 2013, 55, 26–36. [Google Scholar] [CrossRef]
- Moselhy, O.A.; Abdel-Aziz, N.; El-bahkery, A.; Moselhy, S.S.; Ibrahim, E.A. Curcumin Nanoparticles Alleviate Brain Mitochondrial Dysfunction and Cellular Senescence in γ-Irradiated Rats. Sci. Rep. 2025, 15, 3857. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, J.; Li, X.; Zhu, H.; Sun, J.; Jiang, L.; Xue, C.; Zhang, L.; Xu, C.; Xing, S.; et al. Tetrahydrocurcumin Ameliorates Postinfarction Cardiac Dysfunction and Remodeling by Inhibiting Oxidative Stress and Preserving Mitochondrial Function via SIRT3 Signaling Pathway. Phytomedicine 2023, 121, 155127. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Singh, S.S.; Birla, H.; Zahra, W.; Keshri, P.K.; Dilnashin, H.; Singh, R.; Singh, S.; Singh, S.P. Curcumin Modulates P62-Keap1-Nrf2-Mediated Autophagy in Rotenone-Induced Parkinson’s Disease Mouse Models. ACS Chem. Neurosci. 2023, 14, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin Prevents Mitochondrial Dynamics Disturbances in Early 5/6 Nephrectomy: Relation to Oxidative Stress and Mitochondrial Bioenergetics. BioFactors 2017, 43, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Soto-Urquieta, M.G.; López-Briones, S.; Pérez-Vázquez, V.; Saavedra-Molina, A.; González-Hernández, G.A.; Ramírez-Emiliano, J. Curcumin Restores Mitochondrial Functions and Decreases Lipid Peroxidation in Liver and Kidneys of Diabetic Db/Db Mice. Biol. Res. 2014, 47, 74. [Google Scholar] [CrossRef]
- Rastogi, M.; Ojha, R.P.; Sagar, C.; Agrawal, A.; Dubey, G.P. Protective Effect of Curcuminoids on Age-Related Mitochondrial Impairment in Female Wistar Rat Brain. Biogerontology 2014, 15, 21–31. [Google Scholar] [CrossRef]
- Kuo, J.-J.; Chang, H.-H.; Tsai, T.-H.; Lee, T.-Y. Positive Effect of Curcumin on Inflammation and Mitochondrial Dysfunction in Obese Mice with Liver Steatosis. Int. J. Mol. Med. 2012, 30, 673–679. [Google Scholar] [CrossRef]
- Sood, P.K.; Nahar, U.; Nehru, B. Curcumin Attenuates Aluminum-Induced Oxidative Stress and Mitochondrial Dysfunction in Rat Brain. Neurotox. Res. 2011, 20, 351–361. [Google Scholar] [CrossRef]
- Molina-Jijón, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Medina-Campos, O.N.; Zarco-Márquez, G.; Torres, I.; Pedraza-Chaverri, J. Curcumin Prevents Cr(VI)-Induced Renal Oxidant Damage by a Mitochondrial Pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Liao, H.; Hao, S.; Liu, R.; Huang, H.; Duan, C. Curcumin Simultaneously Improves Mitochondrial Dynamics and Myocardial Cell Bioenergy after Sepsis via the SIRT1-DRP1/PGC-1α Pathway. Heliyon 2024, 10, e28501. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Dohl, J.; Elenberg, F.; Chen, Y.; Deuster, P. Curcumin Induces Concentration-dependent Alterations in Mitochondrial Function through ROS in C2C12 Mouse Myoblasts. J. Cell. Physiol. 2019, 234, 6371–6381. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, J.; Wang, Y.; Zhao, B.; Zhang, Y.; Han, F.; Zheng, Z.; Hu, D. Curcumin Pretreatment Prevents Hydrogen Peroxide-Induced Oxidative Stress through Enhanced Mitochondrial Function and Deactivation of Akt/Erk Signaling Pathways in Rat Bone Marrow Mesenchymal Stem Cells. Mol. Cell. Biochem. 2018, 443, 37–45. [Google Scholar] [CrossRef]
- Jat, D.; Parihar, P.; Kothari, S.C.; Parihar, M.S. Curcumin Reduces Oxidative Damage by Increasing Reduced Glutathione and Preventing Membrane Permeability Transition in Isolated Brain Mitochondria. Cell. Mol. Biol. 2013, 59, OL1899–OL1905. [Google Scholar]
- Atsumi, T.; Tonosaki, K.; Fujisawa, S. Induction of Early Apoptosis and ROS-Generation Activity in Human Gingival Fibroblasts (HGF) and Human Submandibular Gland Carcinoma (HSG) Cells Treated with Curcumin. Arch. Oral Biol. 2006, 51, 913–921. [Google Scholar] [CrossRef]
- Duda, M.; Cygan, K.; Wisniewska-Becker, A. Effects of Curcumin on Lipid Membranes: An EPR Spin-Label Study. Cell Biochem. Biophys. 2020, 78, 139–147. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.S.; Lee, D.-K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef]
- Starok, M.; Preira, P.; Vayssade, M.; Haupt, K.; Salomé, L.; Rossi, C. EGFR Inhibition by Curcumin in Cancer Cells: A Dual Mode of Action. Biomacromolecules 2015, 16, 1634–1642. [Google Scholar] [CrossRef]
- Ben-Zichri, S.; Kolusheva, S.; Danilenko, M.; Ossikbayeva, S.; Stabbert, W.J.; Poggio, J.L.; Stein, D.E.; Orynbayeva, Z.; Jelinek, R. Cardiolipin Mediates Curcumin Interactions with Mitochondrial Membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2019, 1861, 75–82. [Google Scholar] [CrossRef]
- Gudyka, J.; Ceja-Vega, J.; Ivanchenko, K.; Morocho, Z.; Panella, M.; Gamez Hernandez, A.; Clarke, C.; Perez, E.; Silverberg, S.; Lee, S. Concentration-Dependent Effects of Curcumin on Membrane Permeability and Structure. ACS Pharmacol. Transl. Sci. 2024, 7, 1546–1556. [Google Scholar] [CrossRef]
- Aloi, E.; Tone, C.M.; Barberi, R.C.; Ciuchi, F.; Bartucci, R. Effects of Curcumin in the Interaction with Cardiolipin-Containg Lipid Monolayers and Bilayers. Biophys. Chem. 2023, 301, 107082. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Dohl, J.; Wang, L.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Curcumin Ameliorates Heat-Induced Injury through NADPH Oxidase–Dependent Redox Signaling and Mitochondrial Preservation in C2C12 Myoblasts and Mouse Skeletal Muscle. J. Nutr. 2020, 150, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, K.; Qu, X.-H.; Wang, T.; Yang, P.; Yang, Y.; Jiang, L.-P.; Wan, Y.-Y.; Tou, F.-F.; Chen, Z.-P.; et al. Curcumin Protects Retinal Neuronal Cells against Oxidative Stress-Induced Damage by Regulating Mitochondrial Dynamics. Exp. Eye Res. 2022, 224, 109239. [Google Scholar] [CrossRef]
- Mehta, H.J.; Patel, V.; Sadikot, R.T. Curcumin and Lung Cancer—A Review. Target. Oncol. 2014, 9, 295–310. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, L.; Li, W.; Liang, Q.; Li, Z. Curcumin Induces Apoptosis and Autophagy Inhuman Renal Cell Carcinoma Cells via Akt/MTOR Suppression. Bioengineered 2021, 12, 5017–5027. [Google Scholar] [CrossRef]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin Inhibits Proliferation and Promotes Apoptosis of Breast Cancer Cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef]
- Guo, W.; Ding, Y.; Pu, C.; Wang, Z.; Deng, W.; Jin, X. Curcumin Inhibits Pancreatic Cancer Cell Proliferation by Regulating Beclin1 Expression and Inhibiting the Hypoxia-Inducible Factor-1α-Mediated Glycolytic Pathway. J. Gastrointest. Oncol. 2022, 13, 3254–3262. [Google Scholar] [CrossRef]
- Li, P.; Pu, S.; Lin, C.; He, L.; Zhao, H.; Yang, C.; Guo, Z.; Xu, S.; Zhou, Z. Curcumin Selectively Induces Colon Cancer Cell Apoptosis and S Cell Cycle Arrest by Regulates Rb/E2F/P53 Pathway. J. Mol. Struct. 2022, 1263, 133180. [Google Scholar] [CrossRef]
- Bolger, G.T.; Licollari, A.; Bagshaw, R.; Tan, A.; Greil, R.; Vcelar, B.; Majeed, M.; Sordillo, P. Intense Uptake of Liposomal Curcumin by Multiple Myeloma Cell Lines: Comparison to Normal Lymphocytes, Red Blood Cells and Chronic Lymphocytic Leukemia Cells. Anticancer Res. 2019, 39, 1161–1168. [Google Scholar] [CrossRef]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative Cellular Uptake, Localization and Cytotoxicity of Curcumin in Normal and Tumor Cells. Biochim. Biophys. Acta—Gen. Subj. 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.-C. Curcumin in Cancer Therapy: Exploring Molecular Mechanisms and Overcoming Clinical Challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.-A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 Family: From Apoptosis Mechanisms to New Advances in Targeted Therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Wang, Z.; Yang, T.; Su, X.; Chen, Y.; Liu, L.; Deng, Q.; Liu, Q.; Shao, C.; Zhu, W. Curcumin Induces Mitochondrial Dysfunction-Associated Oxidative DNA Damage in Ovarian Cancer Cells. PLoS ONE 2025, 20, e0319846. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, E.; Kotiya, A.; Bhuyan, R.; Raza, S.T.; Misra, A.; Ahmad, R.; Mahdi, A.A. Curcumin Chemo-Sensitizes Intrinsic Apoptosis through ROS-Mediated Mitochondrial Hyperpolarization and DNA Damage in Breast Cancer Cells. Cell. Signal. 2025, 128, 111637. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin Induces Apoptosis and Cell Cycle Arrest via the Activation of Reactive Oxygen Species–Independent Mitochondrial Apoptotic Pathway in Smad4 and P53 Mutated Colon Adenocarcinoma HT29 Cells. Nutr. Res. 2018, 51, 67–81. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M. Curcumin Induce DNA Damage and Apoptosis through Generation of Reactive Oxygen Species and Reducing Mitochondrial Membrane Potential in Melanoma Cancer Cells. Cell. Mol. Biol. 2017, 63, 97–105. [Google Scholar] [CrossRef]
- Jayakumar, S.; Patwardhan, R.S.; Pal, D.; Singh, B.; Sharma, D.; Kutala, V.K.; Sandur, S.K. Mitochondrial Targeted Curcumin Exhibits Anticancer Effects through Disruption of Mitochondrial Redox and Modulation of TrxR2 Activity. Free Radic. Biol. Med. 2017, 113, 530–538. [Google Scholar] [CrossRef]
- Moustapha, A.; Pérétout, P.; Rainey, N.; Sureau, F.; Geze, M.; Petit, J.-M.; Dewailly, E.; Slomianny, C.; Petit, P. Curcumin Induces Crosstalk between Autophagy and Apoptosis Mediated by Calcium Release from the Endoplasmic Reticulum, Lysosomal Destabilization and Mitochondrial Events. Cell Death Discov. 2015, 1, 15017. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Zheng, S.; Wu, T. Curcumin Induces Apoptosis through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar] [CrossRef]
- Wang, X.; Shen, K.; Wang, J.; Liu, K.; Wu, G.; Li, Y.; Luo, L.; Zheng, Z.; Hu, D. Hypoxic Preconditioning Combined with Curcumin Promotes Cell Survival and Mitochondrial Quality of Bone Marrow Mesenchymal Stem Cells, and Accelerates Cutaneous Wound Healing via PGC-1α/SIRT3/HIF-1α Signaling. Free Radic. Biol. Med. 2020, 159, 164–176. [Google Scholar] [CrossRef]
- Tan, L.; Cao, Z.; Chen, H.; Xie, Y.; Yu, L.; Fu, C.; Zhao, W.; Wang, Y. Curcumin Reduces Apoptosis and Promotes Osteogenesis of Human Periodontal Ligament Stem Cells under Oxidative Stress in Vitro and in Vivo. Life Sci. 2021, 270, 119125. [Google Scholar] [CrossRef]
- Ke, S.; Zhang, Y.; Lan, Z.; Li, S.; Zhu, W.; Liu, L. Curcumin Protects Murine Lung Mesenchymal Stem Cells from H2O2 by Modulating the Akt/Nrf2/HO-1 Pathway. J. Int. Med. Res. 2020, 48, 0300060520910665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).