Our study aimed to compare the effect of MSCs and a combination of MSCs+NPs with antimicrobial properties on skin wound healing in mice. The experiments consisted of two parts: (1) Whole genome mRNA expression and epigenetic regulation (miRNA expression, DNA methylation) changes were evaluated for Ag and ZnO NPs. (2) Based on the results, Ag NPs were used for further analyses of immune-response- and regeneration-related molecules. NPs for the skin injury experiments were selected based on a previous study in which the effects of metal NPs on the characteristics and functional properties of MSCs were tested in vitro [
12]. For our animal experiments, we selected the highest and the second-to-highest dose of the respective NP that was not toxic in the in vitro tests. ZnO administered alone had generally minimal biological effects when compared with Ag NPs, which might be related to the non-optimal dose selection or, rather, to the different physico-chemical properties of the tested NPs, as discussed further. To the best of our knowledge, this is the only study that has used such a comprehensive scenario to assess the mechanisms induced by a combination of MSCs+NPs in the healing of skin injury.
4.1. mRNA Expression and Epigenetic Modulation
As expected, the skin injury itself, without any treatment, was the factor with the most pronounced effects on mRNA expression. This observation is in agreement with substantial biological changes known to be initiated by wound infliction [
1]. In our study, the samples were collected seven days after injury, i.e., in the phases of inflammation and proliferation. As a result, neutrophils, macrophages, fibroblasts, and endothelial cells were expected to be present in the affected tissue, and collagen was expected to be produced [
2]. Considering the biological properties of MSCs, it is rather unexpected that the administration of MSCs alone affected the expression of only seven mRNAs when compared with wounded, untreated animals. Of these molecules, three were unique to MSC administration:
Acot11 (encoding acyl-CoA thioesterase 11, a protein involved in fatty acids metabolism; its role in wound healing might be related to its ability to affect the invasive potential of the cells),
Lair1 (encoding leukocyte-associated immunoglobulin-like receptor 1, a molecule expressed on immune cells that is crucial for maintaining immune homeostasis and for promoting tolerogenic immune response that helps to suppress excessive inflammation and promote tissue repair and regeneration), and
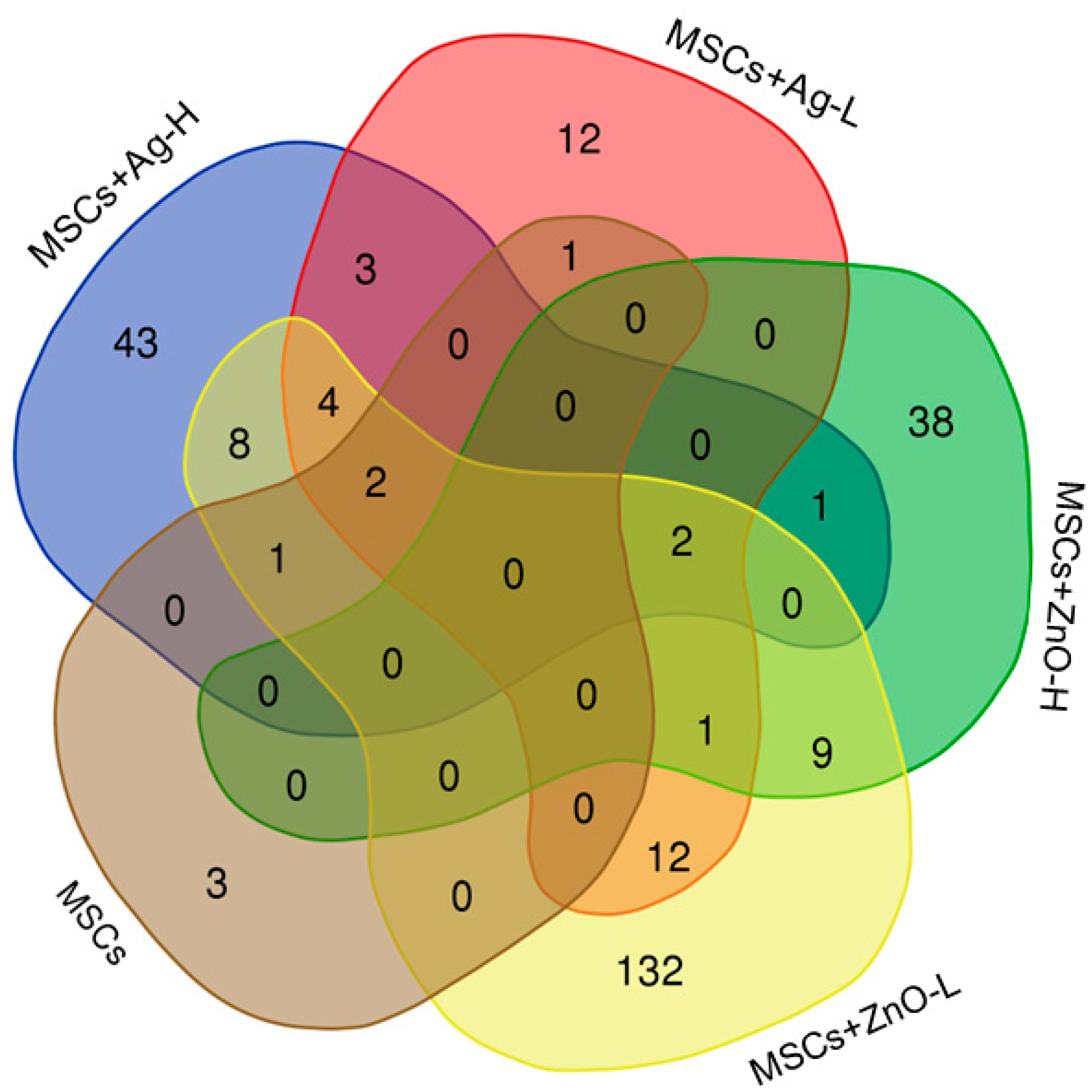
Pyhin1 (encoding pyrin and HIN domain family member 1, a molecule responsible for DNA recognition and regulation of the inflammatory response; its role in wound healing, however, is not clear yet). Adding NPs to the treatment scenarios clearly showed the importance of MSC-NP interactions for achieving a substantial molecular response; while treatments with NPs alone had mostly no effect on the number of deregulated mRNAs (Ag-L being a notable exception), simultaneous administration of MSCs and NPs affected the expression of up to 171 mRNAs (MSCs+ZnO-L). Genes discussed further were ones that were commonly deregulated by at least three treatment conditions.
Sprr2b and
Slc9b2 were both commonly induced by all the tested MSC+NP combinations (MSCs+Ag-L, MSCs+Ag-H, MSCs+ZnO-L, and MSCs+ZnO-H).
Sprr2b, encoding small proline-rich protein 2B, is involved in keratinocyte differentiation in response to tissue injury and inflammation. These roles were also recently reported in human keratinocytes [
16]. The encoded protein is crucial for maintaining epidermal homeostasis and for providing protection against bacterial infection. Our observation shows the involvement of the tested combinations of MSCs+NPs in the induction of healing processes. It should be noted that MSCs or NPs applied separately had no effect on the expression of this gene, indicating the importance of the combined treatment on the wound healing.
Slc9b2 encodes a member of the solute carrier (SLC) family of ion transporters. This protein contributes to cellular pH regulation and may influence cellular signaling pathways, both being generic mechanisms not directly linked to wound healing.
Combined treatments with MSCs+Ag-H, MSCs+Ag-L, and MSCs+ZnO-L all induced the expression of Sprr2a3, Alox8, Chil4, Rnase2a, Ocstamp, Atp10b. Apart from Ocstamp, which encodes a transmembrane protein involved in osteoclast differentiation, thus making its role in the healing of the wounded skin unclear, functions of all these genes are related to the expected processes at the injury site: Sprr2a3 encodes small proline-rich protein 2A3 with a function similar to that of Sprr2b; Alox8 encodes arachidonate 8-lipoxygenase, which is involved in the production of mediators that play a role in inflammation, immune response, and tissue repair; Chil4 encodes chitinase-like protein 4, which is expressed during inflammation and tissue remodeling; Rnase2a encodes ribonuclease A2, an enzyme important for antimicrobial activity and which also modulates the immune response and inflammation. Atp10b, which encodes ATPase phospholipid transporting 10B, the enzyme essential for membrane function and stability, was the only gene whose expression was downregulated by the treatment. Thus, its biological role in our experimental setting is not clear.
Aldh6A1 (induced in common by MSCs+Ag-L, MSCs+ZnO-L, and MSCs+ZnO-H treatment) encodes the aldehyde dehydrogenase 6 family member A1 that plays a role in the metabolism of fatty acids and some amino acids. Similarly to Atp10b, its expression was downregulated. As this enzyme contributes to the energy production of cells, which might be expected during the healing process, the reason for the common downregulation of the gene is not clear. However, the functions in which the enzymes are involved are rather generic and not specific to the mechanisms linked with MSC activities in damaged tissues. Thus, the biological significance of this observation is not clear, and it might be a spurious finding.
The pathway analysis showed similarities in the impact of MSCs+NPs and NPs alone on the processes involved in wound healing. “Neutrophil degranulation” along with “innate immune system” were the pathways affected by Ag-L and MSCs+Ag-H/MSCs+ZnO-H. Neutrophil degranulation is an antimicrobial mechanism in neutrophils that is based on the release of proteases from intracellular vesicles [
17]. The treatment with MSCs+Ag-L/MSCs+ZnO-L modulated the “formation of the cornified envelope” and “keratinization” pathways. Both processes refer to the formation of filaments that help to maintain the mechanical stability of cells and the epithelial tissue during healing [
18]. Overall, these results suggest comparable effects of the tested combinations of MSCs+NPs that depend on the dose of NPs: for higher doses, an inflammatory response is preferred (the earlier phase of the healing process), while the lower NP dose is, instead, linked with collagen formation, i.e., phases of proliferation and remodeling. For MSCs+ZnO-L, deregulation of the “fatty acid metabolism” process was further detected. This observation is in agreement with the metabolic reprogramming initiated in skin wounds that also includes changes in fatty acid synthesis. Lipids are the source of energy and support the angiogenic activity of MSCs [
19].
We further aimed to address the question of how epigenetic regulation is involved in the response to skin injury and the subsequent healing processes. Our data show that the role of miRNA is non-significant. In contrast, DNA methylation was affected by both types of NPs, although the response differs. While ZnO NPs need to be administered with MSCs to induce changes in the CpG site methylation profiles, for Ag NPs, both treatment scenarios (with and without simultaneous MSC application) affect these types of epigenetic changes, although the response differed for Ag-H and Ag-L doses. While for Ag-H, a combination with MSCs was needed to induce DNA methylation changes, Ag-L was effective only when administered alone. The explanation of such a result is not clear; however, it can be speculated that interactions between NPs and MSCs that induce biological response require a certain “optimal” ratio between the numbers of both factors.
Interestingly, the presence of Ag was linked with the modulation of the “desmosome organization” pathway. Desmosomes are intracellular junctions that participate in the formation of the cytoskeletal network by mechanical integration of the adjacent cells [
20]. Another affected pathway, “phosphatidylinositol 3-kinase/protein kinase B signal transduction (PI3K/AKT)”, is also known to participate in the healing process, particularly in cell proliferation and angiogenesis [
21,
22]. Although the impact of MSCs/NPs on DNA methylation changes in the healing wound is clear, it should be noted that the analyses indicate changes in epigenetic regulation but cannot specifically identify the modifications leading to the upregulation and downregulation of gene expression.
Overall, the genomic and epigenetic experiments showed the importance of the presence of NPs in the healing process in which MSCs were included. While there are common molecular impacts for both types of NPs, we selected Ag NPs for further investigation. The reason for this is the wider use of this type of NPs in clinical practice, as well as the unique impacts of Ag NPs on the epigenetic processes involved in cytoskeleton-remodeling PI3K/AKT signaling. In addition, our in vitro data suggest a more pronounced effect of Ag NPs on MSC differentiation and growth factor production [
12].
4.2. The Impacts of Treatment on Immunomodulatory and Regeneration-Related Molecules
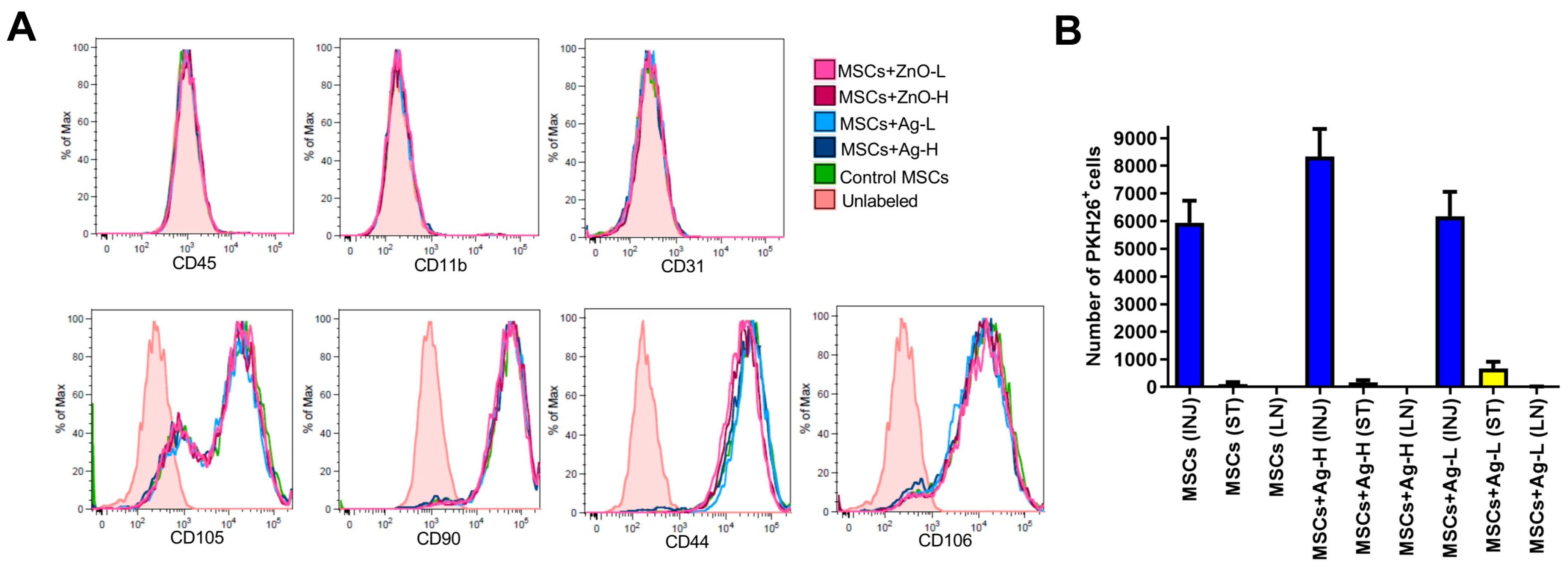
The following experiments were designed to make sure that the therapy with MSCs/NPs induces the cellular processes that regulate the immune response and accelerate healing. First, we checked the in vitro impact of NPs (both Ag and ZnO) on the phenotypic characteristics of MSCs and found no significant effects for either type at any tested dose. This was an important observation, confirming that the biological properties of MSCs would be preserved in the wound even in the presence of NPs. Although such experiments have also been conducted for other NP types (e.g., [
23]), to the best of our knowledge, our report is the only one focusing on Ag and ZnO NPs.
The presence of MSCs at the injury site is another important prerequisite for effective wound treatment. We confirmed that at the time of tissue collection (i.e., seven days after application), there was approximately 1% of the initially applied MSCs present in the wound; very few had migrated to the surrounding tissue and virtually none were detectable in the axillary lymph nodes. A comparable result was obtained for the samples treated with Ag NPs, confirming no negative effects of NPs on the presence of MSCs at the injury site.
Macrophages play a key role in the healing process [
24]. First, during the inflammatory phase, they exert pro-inflammatory functions, including the production of cytokines and growth factors. Subsequently, in the proliferative phase of healing, they stimulate fibroblasts, keratinocytes, and endothelial cells to induce extracellular matrix formation and angiogenesis. We thus checked for the presence of macrophages (F4/80
+ cells) at the injury site after individual treatment combinations. No significant impacts on the percentage of macrophages for MSCs, Ag, or MSCs+Ag treatment was detected when compared with injured, untreated tissue. This observation could be related to the fact that on day seven, the healing process should have progressed to the proliferative phase, when the activity of macrophages decreases. We further checked for the percentage of activated regulatory macrophages (CD80
+CD206
+ cells) among all macrophages. This subset of macrophages is anti-inflammatory and is responsible for the attenuation of the immune response, which is expected in the proliferative phase of healing. The treatment with MSCs alone and Ag-L alone significantly increased the percentage of these macrophages, suggesting their beneficial effects on the healing process.
Further experiments were performed to identify the potential reduction in the inflammatory response mediated by macrophages in the wound after the application of various treatments. The production of inflammatory markers (NO, TNF-α, and IL-6) was investigated in the skin lesion samples stimulated by IFN-γ and LPS [
25]. The most pronounced response was found for NO production, which is considered the gold standard when measuring the induction of inflammation [
26]. Namely, applications of MSCs alone, Ag-H alone, and a combination of MSCs+Ag-L significantly reduced the production of NO, suggesting anti-inflammatory effects of these treatment approaches. The production of TNF-α and IL-6, cytokines produced during the inflammation phase [
27], was only significantly decreased by Ag-H administration to the wound. Overall, the results indicate that at the time of sample collection (day seven), treatment is associated with a reduction in the inflammatory response, thus supporting the presence of a proliferative phase in the healing process.
To confirm the induction of proliferative/regenerative processes in the wound at the time of sample collection, we analyzed the mRNA expression of selected genes, including those encoding galectin 3 (implicated in the regulation of inflammation, angiogenesis, and re-epithelization [
28]); CCL-2 (an anti-inflammatory molecule involved in wound healing [
29]; HGF (a molecule supporting migration of keratinocytes [
30]); MMP-2 (a protease responsible for the regulation of inflammation and degradation of the extracellular matrix [
31]), collagen-1 (a key structural molecule of the extracellular matrix [
32]); and VEGF (a factor that plays a role in angiogenesis and wound closure [
33]). The data mostly did not show any significant response; however, the expression of
Col1, encoding collagen-1, was significantly induced by Ag-L treatment, indicating support for the healing process by the application of Ag NPs.