An Integrative Approach to Assessing the Impact of Mercury (Hg) on Avian Behaviour: From Molecule to Movement
Abstract
1. Introduction
2. Mercury in the Environment and Avian Exposure
2.1. Sources and Concentrations of Mercury in the Environment
2.2. Birds as Bioindicators and Their Susceptibility
2.3. Routes of Exposure
3. Behavioural Effects of Mercury on Birds
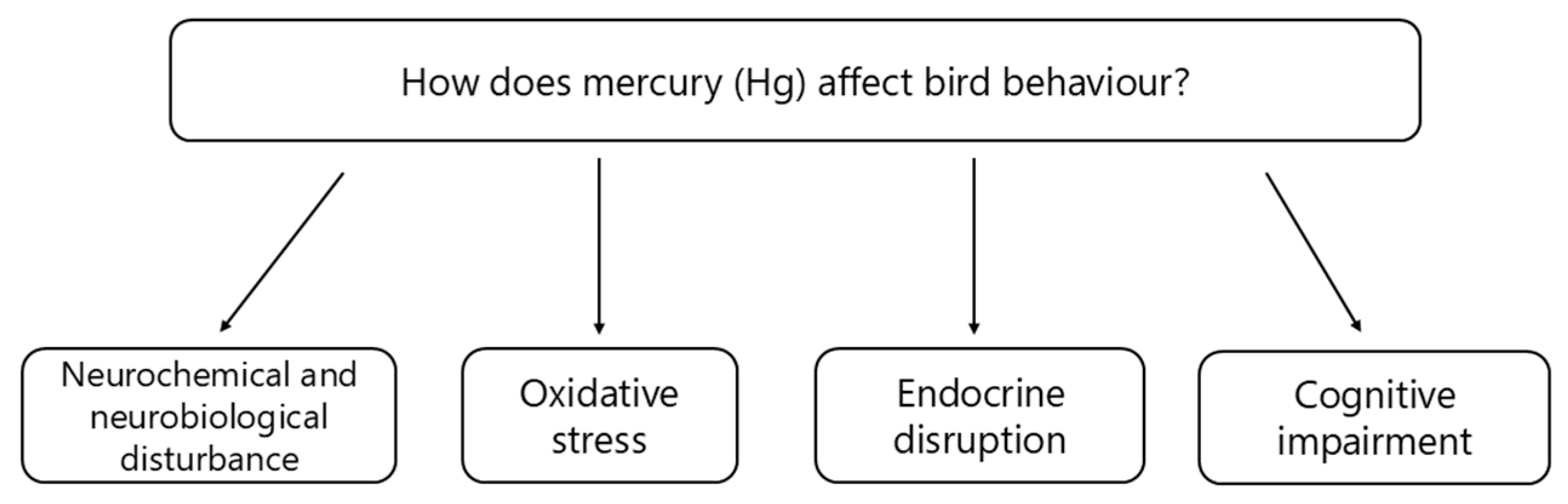
3.1. Mechanism (Causation): How Does Mercury Affect Bird Behaviour?
3.2. Ontogeny (Development): How Does Mercury Exposure Affect Bird Behaviour Across Life Stages?
3.3. Function (Adaptation): How Do Mercury-Induced Behavioural Changes Affect Birds’ Survival and Reproduction?
3.4. Evolution (Phylogeny): How Have Evolutionary Processes Shaped Species Differences in Responses to Mercury Exposure?
4. Empirical Insights into the Behavioural Impacts of Mercury Exposure
4.1. Species-Specific Sensitivity
4.2. Tissue Type and Interpretation of Exposure
4.3. Field vs. Laboratory Insights
5. Considerations and Challenges
Recommendations for Future Research
- Mechanistic studies at the molecular and neuroendocrine levels: there is a critical need for research elucidating the molecular, neurological, and endocrine pathways through which Hg disrupts avian behaviour. Understanding these mechanisms will improve our ability to link behavioural symptoms with specific biochemical effects and dose-response relationships.
- Long-term behavioural monitoring in natural populations: extended field-based studies are essential to capture subtle and cumulative behavioural effects that develop over time. Such studies should be designed to span multiple breeding seasons and account for natural variability in behaviour and environmental conditions.
- Species-specific toxicity thresholds: due to interspecific differences in sensitivity and exposure routes, future work must aim to define species-specific ecotoxicological thresholds for both physiological and behavioural endpoints. This includes establishing reliable reference values for tissue Hg concentrations and their behavioural consequences.
- Standardisation of behavioural endpoints in ecotoxicology: to integrate behavioural changes more effectively into ecotoxicological frameworks, standard protocols and validated metrics must be developed. Harmonising laboratory and field approaches will allow for stronger causal inferences and more ecologically meaningful assessments.
- Advancement of non-invasive biomonitoring techniques: refining the use of feathers, blood, and egg samples to assess Hg exposure requires improved calibration against known behavioural outcomes. Validated models converting Hg concentrations across tissue types (e.g., feather to blood, blood to egg) should be expanded across species and ecological contexts.
- Assessment of pollutant interactions: future studies should examine how Hg interacts with other pollutants, such as organochlorines and PFAS, in shaping behavioural outcomes. Synergistic, antagonistic, or amplification effects may obscure Hg toxicity, necessitating multi-pollutant risk assessments.
- Scalable detoxification and mitigation strategies: research into practical and ecologically sound detoxification strategies. Studies must assess their safety, effectiveness, scalability, and potential ecological consequences in real-world avian habitats.
- Population-level modelling and risk assessment: continued development of population models will help forecast demographic responses to changes in Hg exposure and evaluate the conservation benefits of mitigation efforts, including reductions in global Hg emissions.
- Citizen science and community engagement: incorporating trained volunteers and birdwatchers into long-term behavioural monitoring programs offers a cost-effective means of collecting large-scale data. Citizen science initiatives should be carefully structured to ensure data reliability and enhance public awareness of Hg ecological impacts.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayers, C.J.; Evers, D.C.; Ruiz-Gutierrez, V.; Adams, E.; Vega, C.M.; Pisconte, J.N.; Tejeda, V.; Regan, K.; Lane, O.P.; Ash, A.A.; et al. Mercury in Neotropical Birds: A Synthesis and Prospectus on 13 Years of Exposure Data. Ecotoxicology 2023, 32, 1096–1123. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.T.; Eagles-Smith, C.A.; Herzog, M.P.; Hartman, C.A.; Peterson, S.H.; Evers, D.C.; Jackson, A.K.; Elliott, J.E.; Vander Pol, S.S.; Bryan, C.E. Avian Mercury Exposure and Toxicological Risk across Western North America: A Synthesis. Sci. Total Environ. 2016, 568, 749–769. [Google Scholar] [CrossRef]
- Uddin, S.; Khanom, S.; Islam, M.R. Source and Distribution of Mercury in Environment—A Review. In Mercury Toxicity Mitigation: Sustainable Nexus Approach; Springer: Berlin/Heidelberg, Germany, 2024; pp. 3–43. [Google Scholar]
- Vince, G. An Epoch Debate. Science 2011, 334, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Esdaile, L.J.; Chalker, J.M. The Mercury Problem in Artisanal and Small-Scale Gold Mining. Chem. A Eur. J. 2018, 24, 6905–6916. [Google Scholar] [CrossRef]
- Pfeiffer, W.C.; de Lacerda, L.D. Mercury Inputs into the Amazon Region, Brazil. Environ. Technol. Lett. 1988, 9, 325–330. [Google Scholar] [CrossRef]
- Little, E.E. Behavioral Toxicology: Stimulating Challenges for a Growing Discipline. Environ. Toxicol. Chem. 1990, 9, 1–2. [Google Scholar] [CrossRef]
- Gerhardt, A. Aquatic Behavioral Ecotoxicology—Prospects and Limitations. Human. Ecol. Risk Assess. Int. J. 2007, 13, 481–491. [Google Scholar] [CrossRef]
- Simon, M.; Jönk, P.; Wühl-Couturier, G.; Halbach, S. Mercury, Mercury Alloys, and Mercury Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Bourdineaud, J.P.; Gonzalez-Rey, M.; Rovezzi, M.; Glatzel, P.; Nagy, K.L.; Manceau, A. Divalent Mercury in Dissolved Organic Matter Is Bioavailable to Fish and Accumulates as Dithiolate and Tetrathiolate Complexes. Environ. Sci. Technol. 2019, 53, 4880–4891. [Google Scholar] [CrossRef]
- Weber, L.P. Phenylmercuric Acetate. In Encyclopedia of Toxicology, Fourth Edition: Volume 1–9; Elsevier: Amsterdam, The Netherlands, 2024; Volume 7, pp. 549–552. [Google Scholar] [CrossRef]
- Dórea, J.G. Low-Dose Thimerosal in Pediatric Vaccines: Adverse Effects in Perspective. Environ. Res. 2017, 152, 280–293. [Google Scholar] [CrossRef]
- Nierenberg, D.W.; Nordgren, R.E.; Chang, M.B.; Siegler, R.W.; Blayney, M.B.; Hochberg, F.; Toribara, T.Y.; Cernichiari, E.; Clarkson, T. Delayed Cerebellar Disease and Death after Accidental Exposure to Dimethylmercury. N. Engl. J. Med. 1998, 338, 1672–1676. [Google Scholar] [CrossRef]
- Siegler, R.W.; Nierenberg, D.W.; Hickey, W.F. Fatal Poisoning from Liquid Dimethylmercury: A Neuropathologic Study. Hum. Pathol. 1999, 30, 720–723. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.M.; Cui, X.; Lamborg, C.H.; Schartup, A.T. Dimethylmercury as a Source of Monomethylmercury in a Highly Productive Upwelling System. Environ. Sci. Technol. 2024, 58, 10591–10600. [Google Scholar] [CrossRef] [PubMed]
- Boening, D.W. Ecological Effects, Transport, and Fate of Mercury: A General Review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of Environmental Methylmercury on the Health of Wild Birds, Mammals, and Fish. AMBIO A J. Hum. Environ. 2007, 36, 12–19. [Google Scholar] [CrossRef]
- Manceau, A.; Enescu, M.; Simionovici, A.; Lanson, M.; Gonzalez-Rey, M.; Rovezzi, M.; Tucoulou, R.; Glatzel, P.; Nagy, K.L.; Bourdineaud, J.P. Chemical Forms of Mercury in Human Hair Reveal Sources of Exposure. Environ. Sci. Technol. 2016, 50, 10721–10729. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Bourdineaud, J.P.; Oliveira, R.B.; Sarrazin, S.L.F.; Krabbenhoft, D.P.; Eagles-Smith, C.A.; Ackerman, J.T.; Stewart, A.R.; Ward-Deitrich, C.; Del Castillo Busto, M.E.; et al. Demethylation of Methylmercury in Bird, Fish, and Earthworm. Environ. Sci. Technol. 2021, 55, 1527–1534. [Google Scholar] [CrossRef]
- Amde, M.; Yin, Y.; Zhang, D.; Liu, J. Methods and Recent Advances in Speciation Analysis of Mercury Chemical Species in Environmental Samples: A Review. Chem. Speciat. Bioavailab. 2016, 28, 51–65. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, Toxicity, and Remediation of Mercury: An Essence Review. Environ. Monit. Assess. 2019, 191, 566. [Google Scholar] [CrossRef]
- Evers, D.C.; Savoy, L.J.; DeSorbo, C.R.; Yates, D.E.; Hanson, W.; Taylor, K.M.; Siegel, L.S.; Cooley, J.H.; Bank, M.S.; Major, A.; et al. Adverse Effects from Environmental Mercury Loads on Breeding Common Loons. Ecotoxicology 2008, 17, 69–81. [Google Scholar] [CrossRef]
- Fleming, E.J.; Mack, E.E.; Green, P.G.; Nelson, D.C. Mercury Methylation from Unexpected Sources: Molybdate-Inhibited Freshwater Sediments and an Iron-Reducing Bacterium. Appl. Environ. Microbiol. 2006, 72, 457–464. [Google Scholar] [CrossRef]
- Wiener, J.G.; Fitzgerald, W.F.; Watras, C.J.; Rada, R.G. Partitioning and Bioavailability of Mercury in an Experimentally Acidified Wisconsin Lake. Environ. Toxicol. Chem. 1990, 9, 909–918. [Google Scholar] [CrossRef]
- Cristol, D.A.; Brasso, R.L.; Condon, A.M.; Fovargue, R.E.; Friedman, S.L.; Hallinger, K.K.; Monroe, A.P.; White, A.E. The Movement of Aquatic Mercury through Terrestrial Food Webs. Science 2008, 320, 335. [Google Scholar] [CrossRef] [PubMed]
- Seewagen, C.L. Threats of Environmental Mercury to Birds: Knowledge Gaps and Priorities for Future Research. Bird. Conserv. Int. 2010, 20, 112–123. [Google Scholar] [CrossRef]
- Ackerman, J.T.; Eagles-Smith, C.A.; Herzog, M.P.; Yee, J.L.; Hartman, C.A. Egg-laying Sequence Influences Egg Mercury Concentrations and Egg Size in Three Bird Species: Implications for Contaminant Monitoring Programs. Environ. Toxicol. Chem. 2016, 35, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Whitney, M.C.; Cristol, D.A. Impacts of Sublethal Mercury Exposure on Birds: A Detailed Review. In Reviews of Environmental Contamination and Toxicology; Springer LLC: New York, NY, USA, 2017; Volume 244, pp. 113–163. [Google Scholar]
- Burger, J.; Gochfeld, M. Risk, Mercury Levels, and Birds: Relating Adverse Laboratory Effects to Field Biomonitoring. Environ. Res. 1997, 75, 160–172. [Google Scholar] [CrossRef]
- Bjedov, D.; Velki, M.; Toth, L.; Filipović Marijić, V.; Mikuška, T.; Jurinović, L.; Ečimović, S.; Turić, N.; Lončarić, Z.; Šariri, S.; et al. Heavy Metal(loid) Effect on Multi-Biomarker Responses in Apex Predator: Novel Assays in the Monitoring of White Stork Nestlings. Environ. Pollut. 2023, 324, 121398. [Google Scholar] [CrossRef]
- Bjedov, D.; Mikuška, A.; Gvozdić, V.; Glavaš, P.; Gradečak, D.; Sudarić Bogojević, M. White Stork Pellets: Non-Invasive Solution to Monitor Anthropogenic Particle Pollution. Toxics 2024, 12, 236. [Google Scholar] [CrossRef]
- Bjedov, D.; Bernal-Alviz, J.; Buelvas-Soto, J.A.; Jurman, L.A.; Marrugo-Negrete, J.L. Elevated Heavy Metal(loid) Blood and Feather Concentrations in Wetland Birds from Different Trophic Levels Indicate Exposure to Environmental Pollutants. Arch. Environ. Contam. Toxicol. 2024, 87, 127–143. [Google Scholar] [CrossRef]
- Bjedov, D.; Mikuška, A.; Velki, M. From Wetlands to Landfills: White Stork (Ciconia ciconia L., 1758) as a Reliable Bioindicator of Ecosystem Health. Arch. Ind. Hyg. Toxicol. 2025, 76, 1–15. [Google Scholar] [CrossRef]
- López-Berenguer, G.; Peñalver, J.; Martínez-López, E. A Critical Review about Neurotoxic Effects in Marine Mammals of Mercury and Other Trace Elements. Chemosphere 2020, 246, 125688. [Google Scholar] [CrossRef]
- Ji, X.; Hu, W.; Cheng, J.; Yuan, T.; Xu, F.; Qu, L.; Wang, W. Oxidative Stress on Domestic Ducks (Shaoxing duck) Chronically Exposed in a Mercury–Selenium Coexisting Mining Area in China. Ecotoxicol. Environ. Saf. 2006, 64, 171–177. [Google Scholar] [CrossRef]
- Kobiela, M.E.; Cristol, D.A.; Swaddle, J.P. Risk-Taking Behaviours in Zebra Finches Affected by Mercury Exposure. Anim. Behav. 2015, 103, 153–160. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Osborn, D. Kidney Lesions in Juvenile Starlings Sturnus Vulgaris Fed on a Mercury-Contaminated Synthetic Diet. Environ. Pollut. Ser. A Ecol. Biol. 1984, 33, 195–206. [Google Scholar] [CrossRef]
- Wada, H.; Cristol, D.A.; McNabb, F.M.A.; Hopkins, W.A. Suppressed Adrenocortical Responses and Thyroid Hormone Levels in Birds near a Mercury-Contaminated River. Environ. Sci. Technol. 2009, 43, 6031–6038. [Google Scholar] [CrossRef] [PubMed]
- Mallory, M.L.; Provencher, J.F.; Robertson, G.J.; Braune, B.M.; Holland, E.R.; Klapstein, S.; Stevens, K.; O’Driscoll, N.J. Mercury Concentrations in Blood, Brain and Muscle Tissues of Coastal and Pelagic Birds from Northeastern Canada. Ecotoxicol. Environ. Saf. 2018, 157, 424–430. [Google Scholar] [CrossRef]
- Burger, J.; Mizrahi, D.; Tsipoura, N.; Jeitner, C.; Gochfeld, M. Mercury, Lead, Cadmium, Cobalt, Arsenic and Selenium in the Blood of Semipalmated Sandpipers (Calidris pusilla) from Suriname, South America: Age-Related Differences in Wintering Site and Comparisons with a Stopover Site in New Jersey, USA. Toxics 2018, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, G.; Esmaili-Sari, A.; Ghasempouri, S.M.; Kiabi, B.H. Examination of Mercury Concentration in the Feathers of 18 Species of Birds in Southwest Iran. Environ. Res. 2007, 104, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, D.; Mikuska, A.; Begović, L.; Bollinger, E.; Bustnes, J.O.; Deme, T.; Mikuška, T.; Morocz, A.; Schulz, R.; Søndergaard, J.; et al. Effects of White-Tailed Eagle (Haliaeetus albicilla) Nestling Diet on Mercury Exposure Dynamics in Kopački Rit Nature Park, Croatia. Environ. Pollut. 2023, 336, 122377. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, D.; Mikuška, A.; Velki, M.; Lončarić, Z.; Mikuška, T. The First Analysis of Heavy Metals in the Grey Heron Ardea Cinerea Feathers from the Croatian Colonies. Larus Godišnjak Zavoda Ornitol. Hrvat. Akad. Znan. I Umjet. 2020, 55, 7–25. [Google Scholar] [CrossRef]
- Aarif, K.M.; Rubeena, K.A.; Nefla, A.; Musilova, Z.; Musil, P.; Bin Muzaffar, S. Bio-Concentration of Hazardous Metals in Migrant Shorebirds in a Key Conservation Reserve and Adjoining Areas on the West Coast of India. Ecotoxicol. Environ. Saf. 2025, 289, 117690. [Google Scholar] [CrossRef]
- Eeva, T.; Raivikko, N.; Espín, S.; Sánchez-Virosta, P.; Ruuskanen, S.; Sorvari, J.; Rainio, M. Bird Feces as Indicators of Metal Pollution: Pitfalls and Solutions. Toxics 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Bean, T.G.; Beasley, V.R.; Berny, P.; Eisenreich, K.M.; Elliott, J.E.; Eng, M.L.; Fuchsman, P.C.; Johnson, M.S.; King, M.D.; Mateo, R.; et al. Toxicological Effects Assessment for Wildlife in the 21st Century: Review of Current Methods and Recommendations for a Path Forward. Integr. Environ. Assess. Manag. 2024, 20, 699–724. [Google Scholar] [CrossRef]
- Bertram, M.G.; Martin, J.M.; McCallum, E.S.; Alton, L.A.; Brand, J.A.; Brooks, B.W.; Cerveny, D.; Fick, J.; Ford, A.T.; Hellström, G.; et al. Frontiers in Quantifying Wildlife Behavioural Responses to Chemical Pollution. Biol. Rev. 2022, 97, 1346–1364. [Google Scholar] [CrossRef]
- Peterson, E.K.; Buchwalter, D.B.; Kerby, J.L.; LeFauve, M.K.; Varian-Ramos, C.W.; Swaddle, J.P. Integrative Behavioral Ecotoxicology: Bringing Together Fields to Establish New Insight to Behavioral Ecology, Toxicology, and Conservation. Curr. Zool. 2017, 63, 185–194. [Google Scholar] [CrossRef]
- Abbey-Lee, R.N.; Mathot, K.J.; Dingemanse, N.J. Behavioral and Morphological Responses to Perceived Predation Risk: A Field Experiment in Passerines. Behav. Ecol. 2016, 27, 857–864. [Google Scholar] [CrossRef]
- Frederick, P.; Jayasena, N. Altered Pairing Behaviour and Reproductive Success in White Ibises Exposed to Environmentally Relevant Concentrations of Methylmercury. Proc. R. Soc. B Biol. Sci. 2011, 278, 1851–1857. [Google Scholar] [CrossRef]
- Tartu, S.; Angelier, F.; Wingfield, J.C.; Bustamante, P.; Labadie, P.; Budzinski, H.; Weimerskirch, H.; Bustnes, J.O.; Chastel, O. Corticosterone, Prolactin and Egg Neglect Behavior in Relation to Mercury and Legacy POPs in a Long-Lived Antarctic Bird. Sci. Total Environ. 2015, 505, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.T.; Ågerstrand, M.; Brooks, B.W.; Allen, J.; Bertram, M.G.; Brodin, T.; Dang, Z.; Duquesne, S.; Sahm, R.; Hoffmann, F.; et al. The Role of Behavioral Ecotoxicology in Environmental Protection. Environ. Sci. Technol. 2021, 55, 5620. [Google Scholar] [CrossRef]
- Lutscher, F.; Nisbet, R.M.; Pachepsky, E. Population Persistence in the Face of Advection. Theor. Ecol. 2010, 3, 271–284. [Google Scholar] [CrossRef]
- Carravieri, A.; Vincze, O.; Bustamante, P.; Ackerman, J.T.; Adams, E.M.; Angelier, F.; Chastel, O.; Cherel, Y.; Gilg, O.; Golubova, E.; et al. Quantitative Meta-analysis Reveals No Association between Mercury Contamination and Body Condition in Birds. Biol. Rev. 2022, 97, 1253–1271. [Google Scholar] [CrossRef]
- Tinbergen, N. On Aims and Methods of Ethology. Z. Tierpsychol. 1963, 20, 410–433. [Google Scholar] [CrossRef]
- Fitzgerald, W.; Lamborg, C. Geochemistry of Mercury. In Environmental Geochemistry; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Selin, N.E. Global Biogeochemical Cycling of Mercury: A Review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Nriagu, J.O. Legacy of Mercury Pollution. Nature 1993, 363, 589. [Google Scholar] [CrossRef]
- UN Environment. Global Mercury Assessment; UN Environment: Nairobi, Kenya, 2019. [Google Scholar]
- Global Mercury Assessment|UNEP—UN Environment Programme. Available online: https://www.unep.org/topics/chemicals-and-pollution-action/pollution-and-health/heavy-metals/mercury/global-mercury-2 (accessed on 3 July 2025).
- Landers, D.H.; Gubala, C.; Verta, M.; Lucotte, M.; Johansson, K.; Vlasova, T.; Lockhart, W.L. Using Lake Sediment Mercury Flux Ratios to Evaluate the Regional and Continental Dimensions of Mercury Deposition in Arctic and Boreal Ecosystems. Atmos. Environ. 1998, 32, 919–928. [Google Scholar] [CrossRef]
- Muir, D.; Braune, B.; DeMarch, B.; Norstrom, R.; Wagemann, R.; Lockhart, L.; Hargrave, B.; Bright, D.; Addison, R.; Payne, J.; et al. Spatial and Temporal Trends and Effects of Contaminants in the Canadian Arctic Marine Ecosystem: A Review. Sci. Total Environ. 1999, 230, 83–144. [Google Scholar] [CrossRef] [PubMed]
- Cooke, C.A.; Martínez-Cortizas, A.; Bindler, R.; Sexauer Gustin, M. Environmental Archives of Atmospheric Hg Deposition—A Review. Sci. Total Environ. 2020, 709, 134800. [Google Scholar] [CrossRef]
- Engstrom, D.R.; Balogh, S.J.; Swain, E.B. History of Mercury Inputs to Minnesota Lakes: Influences of Watershed Disturbance and Localized Atmospheric Deposition. Limnol. Oceanogr. 2007, 52, 2467–2483. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A Review of Global Environmental Mercury Processes in Response to Human and Natural Perturbations: Changes of Emissions, Climate, and Land Use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef]
- Sonke, J.E.; Teisserenc, R.; Heimbürger-Boavida, L.E.; Petrova, M.V.; Marusczak, N.; Le Dantec, T.; Chupakov, A.V.; Li, C.; Thackray, C.P.; Sunderland, E.M.; et al. Eurasian River Spring Flood Observations Support Net Arctic Ocean Mercury Export to the Atmosphere and Atlantic Ocean. Proc. Natl. Acad. Sci. USA 2018, 115, E11586–E11594. [Google Scholar] [CrossRef]
- Schartup, A.T.; Heimbuerger-Boavida, L.-E.; Soerensen, A.; Sonke, J.; Sunderland, E.M.; Schartup, A.T.; Heimbuerger-Boavida, L.-E.; Soerensen, A.; Sonke, J.; Sunderland, E.M. Biogeochemical Controls on the Mercury Cycle in the Arctic Ocean. In Proceedings of the American Geophysical Union, Ocean Sciences Meeting, San Diego, CA, USA, 16–21 February 2020; p. CT33A-08. [Google Scholar]
- Kirk, J.L.; St Louis, V.L.; Sharp, M.J. Rapid Reduction and Reemission of Mercury Deposited into Snowpacks during Atmospheric Mercury Depletion Events at Churchill, Manitoba, Canada. Environ. Sci. Technol. 2006, 40, 7590–7596. [Google Scholar] [CrossRef]
- Cohen, M.J.; Lamsal, S.; Osborne, T.Z.; Bonzongo, J.C.J.; Newman, S.; Reddy, K.R. Soil Total Mercury Concentrations across the Greater Everglades. Soil. Sci. Soc. Am. J. 2009, 73, 675–685. [Google Scholar] [CrossRef]
- Qiu, G.; Feng, X.; Li, P.; Wang, S.; Li, G.; Shang, L.; Fu, X. Methylmercury Accumulation in Rice (Oryza sativa L.) Grown at Abandoned Mercury Mines in Guizhou, China. J. Agric. Food Chem. 2008, 56, 2465–2468. [Google Scholar] [CrossRef]
- Meng, M.; Li, B.; Shao, J.J.; Wang, T.; He, B.; Shi, J.B.; Ye, Z.H.; Jiang, G. Bin Accumulation of Total Mercury and Methylmercury in Rice Plants Collected from Different Mining Areas in China. Environ. Pollut. 2014, 184, 179–186. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Bakshi, P.; Parihar, R.D.; Radziemska, M.; Kumar, R. Mercury in the Natural Environment: Biogeochemical Cycles and Associated Health Risks. J. Geochem. Explor. 2024, 267, 107594. [Google Scholar] [CrossRef]
- Gregory, R.D.; van Strien, A.; Vorisek, P.; Gmelig Meyling, A.W.; Noble, D.G.; Foppen, R.P.B.; Gibbons, D.W. Developing Indicators for European Birds. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Egwumah, F.A.; Egwumah, P.; Edet, D. Paramount Roles of Wild Birds as Bioindicators of Contamination. Int. J. Avian Wildl. Biol. 2017, 2, 194–199. [Google Scholar] [CrossRef]
- Chambers, S. Birds as Environmental Indicators Review of Literature; Parks Victoria: Melbourne, Australia, 2008.
- Seewagen, C.L. The Threat of Global Mercury Pollution to Bird Migration: Potential Mechanisms and Current Evidence. Ecotoxicology 2020, 29, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, C.C.; Mcfarland, K.P.; Evers, D.C.; Miller, E.K.; Aubry, Y.; Busby, D.; Taylor, R.J. Mercury Concentrations in Bicknell’s Thrush and Other Insectivorous Passerines in Montane Forests of Northeastern North America. Ecotoxicology 2005, 14, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, C.C.; Miller, E.K.; McFarland, K.P.; Taylor, R.J.; Faccio, S.D. Mercury Bioaccumulation and Trophic Transfer in the Terrestrial Food Web of a Montane Forest. Ecotoxicology 2010, 19, 697–709. [Google Scholar] [CrossRef]
- Espin, S.; García-Fernández, A.; Herzke, D.; Shore, R.; van Hattum, B.; Martínez-López, E.; Coeurdassier, M.; Eulaers, I.; Fritsch, C.; Gómez-Ramírez, P.; et al. Sampling and Contaminant Monitoring Protocol for Raptors; Research Networking Programme-EURAPMON (Research and Monitoring for and with Raptors in Europe): Brussels, Belgium, 2016. [Google Scholar] [CrossRef]
- Hernández, L.M.; González, M.J.; Rico, M.C.; Fernández, M.A.; Aranda, A. Organochlorine and Heavy Metal Residues in Falconiforme and Ciconiforme Eggs (Spain). Bull. Environ. Contam. Toxicol. 1988, 40, 86–93. [Google Scholar] [CrossRef]
- Bjedov, D.; Mikuška, A.; Lackmann, C.; Begović, L.; Mikuška, T.; Velki, M. Application of Non-Destructive Methods: Biomarker Assays in Blood of White Stork (Ciconia ciconia) Nestlings. Animals 2021, 11, 2341. [Google Scholar] [CrossRef]
- Bjedov, D.; Velki, M.; Lackmann, C.; Begović, L.; Mikuška, T.; Jurinović, L.; Mikuška, A. Blood Biomarkers in White Stork (Ciconia ciconia) Nestlings Show Different Responses in Several Areas of Croatia. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, D.; Velki, M.; Kovačić, L.S.; Begović, L.; Lešić, I.; Jurinović, L.; Mikuska, T.; Sudarić Bogojević, M.; Ečimović, S.; Mikuška, A. White Stork (Ciconia ciconia) Nestlings Affected by Agricultural Practices? Assessment of Integrated Biomarker Responses. Agriculture 2023, 13, 1045. [Google Scholar] [CrossRef]
- Goutner, V.; Becker, P.H.; Liordos, V.; Tsachalidis, E.P. Mercury in White Stork (Ciconia ciconia) Chick Feathers from Northeastern Mediterranean Areas in Relation to Age, Brood Size, and Hatching Order. Arch. Environ. Contam. Toxicol. 2011, 61, 327–336. [Google Scholar] [CrossRef]
- Piedra, J.L.V.; Zienkiewicz, A.; Khudhur, H.B.; Szymańczyk, S.E.; Rumińska, E. Influence of the Administration of Propolis and Bee Pollen Preparation on the Concentration of Mercury in the Muscles, Kidneys and Liver of White Storks. Med. Weter. 2018, 74, 171–174. [Google Scholar] [CrossRef]
- Townsend, J.M.; Driscoll, C.T.; Rimmer, C.C.; McFarland, K.P. Avian, salamander, and forest floor mercury concentrations increase with elevation in a terrestrial ecosystem. Environ. Toxicol. Chem. 2014, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Eagles-Smith, C.A.; Wiener, J.G.; Eckley, C.S.; Willacker, J.J.; Evers, D.C.; Marvin-DiPasquale, M.; Obrist, D.; Fleck, J.A.; Aiken, G.R.; Lepak, J.M.; et al. Mercury in Western North America: A Synthesis of Environmental Contamination, Fluxes, Bioaccumulation, and Risk to Fish and Wildlife. Sci. Total Environ. 2016, 568, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.C.; Burgess, N.M.; Champoux, L.; Hoskins, B.; Major, A.; Goodale, W.M.; Taylor, R.J.; Poppenga, R.; Daigle, T. Patterns and Interpretation of Mercury Exposure in Freshwater Avian Communities in Northeastern North America. Ecotoxicology 2005, 14, 193–221. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Hylland, K.; Julshamn, K.; Lundebye, A.K.; Waagbø, R. Maximum Limits of Organic and Inorganic Mercury in Fish Feed. Aquac. Nutr. 2004, 10, 83–97. [Google Scholar] [CrossRef]
- Muccillo–Baisch, A.L.; Mirlean, N.; Carrazzoni, D.; Soares, M.C.F.; Goulart, G.P.; Baisch, P. Health Effects of Ingestion of Mercury-Polluted Urban Soil: An Animal Experiment. Environ. Geochem. Health 2012, 34, 43–53. [Google Scholar] [CrossRef]
- Biser, J.A.; Vogel, L.A.; Berger, J.; Hjelle, B.; Loew, S.S. Effects of Heavy Metals on Immunocompetence of White-Footed Mice (Peromyscus leucopus). J. Wildl. Dis. 2004, 40, 173–184. [Google Scholar] [CrossRef]
- Rebolloso-Hernández, C.A.; Vallejo-Pérez, M.R.; Carrizales-Yáñez, L.; Garrigos-Lomelí, G.J.; Razo-Soto, I.; Diaz-Barriga, F. Arsenic and Mercury Exposure in Different Insect Trophic Guilds from Mercury Mining Areas in Mexico. Environ. Monit. Assess. 2024, 196, 422. [Google Scholar] [CrossRef]
- Ssenku, J.E.; Naziriwo, B.; Kutesakwe, J.; Mustafa, A.S.; Kayeera, D.; Tebandeke, E. Mercury Accumulation in Food Crops and Phytoremediation Potential of Wild Plants Thriving in Artisanal and Small-Scale Gold Mining Areas in Uganda. Pollutants 2023, 3, 181–196. [Google Scholar] [CrossRef]
- Li, R.; Wu, H.; DIng, J.; Fu, W.; Gan, L.; Li, Y. Mercury Pollution in Vegetables, Grains and Soils from Areas Surrounding Coal-Fired Power Plants. Sci. Rep. 2017, 7, 46545. [Google Scholar] [CrossRef]
- Dell’Omo, G. Behavioural Ecotoxicology; John Wiley & Sons: Hoboken, NJ, USA, 2002; p. 463. [Google Scholar]
- Mcwilliams, S.R.; Guglielmo, C.; Pierce, B.; Klaassen, M.; Mcwilliams, S.R.; Pierce, B.; Guglielmo, C. Flying, Fasting, and Feeding in Birds during Migration: A Nutritional and Physiological Ecology Perspective. J. Avian Biol. 2004, 35, 377–393. [Google Scholar] [CrossRef]
- Weber, J.M. Metabolic Fuels: Regulating Fluxes to Select Mix. J. Exp. Biol. 2011, 214, 286–294. [Google Scholar] [CrossRef]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From Molecular Action to Physiological Outputs: Peroxisome Proliferator-Activated Receptors Are Nuclear Receptors at the Crossroads of Key Cellular Functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Heinz, G.H. Effects of Mercury and Selenium on Glutathione Metabolism and Oxidative Stress in Mallard Ducks. Environ. Toxicol. Chem. 1998, 17, 161–166. [Google Scholar] [CrossRef]
- Spalding, M.G.; Frederick, P.C.; McGill, H.C.; Bouton, S.N.; Richey, L.J.; Schumacher, I.M.; Blackmore, C.G.; Harrison, J. Histologic, Neurologic, and Immunologic Effects of Methylmercury in Captive Great Egrets. J. Wildl. Dis. 2000, 36, 423–435. [Google Scholar] [CrossRef]
- Lindstrom, A.; Alerstam, T. Optimal Fat Loads in Migrating Birds: A Test of the Time-Minimization Hypothesis. Am. Nat. 1992, 140, 477–491. [Google Scholar] [CrossRef]
- Klaassen, M.; Hoye, B.J.; Nolet, B.A.; Buttemer, W.A. Ecophysiology of Avian Migration in the Face of Current Global Hazards. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1719–1732. [Google Scholar] [CrossRef]
- Ramenofsky, M. Hormones in Migration and Reproductive Cycles of Birds. In Hormones and Reproduction of Vertebrates; Academic Press: Cambridge, MA, USA, 2011; pp. 205–237. [Google Scholar] [CrossRef]
- Zhu, X.; Kusaka, Y.; Sato, K.; Zhang, Q. The endocrine disruptive effects of mercury. Environ. Health Prev. Med. 2000, 4, 174–183. [Google Scholar] [CrossRef]
- Ackerman, J.T.; Eagles-Smith, C.A.; Heinz, G.; La Cruz, S.E.W.D.; Takekawa, J.Y.; Miles, A.K.; Adelsbach, T.L.; Herzog, M.P.; Bluso-Demers, J.D.; Demers, S.A.; et al. Mercury in Birds of San Francisco Bay-Delta, California: Trophic Pathways, Bioaccumulation, and Ecotoxicological Risk to Avian Reproduction; US Geological Survey: Reston, VA, USA, 2014.
- Basu, N.; Stamler, C.J.; Loua, K.M.; Chan, H.M. An Interspecies Comparison of Mercury Inhibition on Muscarinic Acetylcholine Receptor Binding in the Cerebral Cortex and Cerebellum. Toxicol. Appl. Pharmacol. 2005, 205, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Syversen, T. Methylmercury: Recent advances in the understanding of its neurotoxicity. Ther. Drug Monit. 2005, 27, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Bottini, C.L.J.; MacDougall-Shackleton, S.A. Methylmercury Effects on Avian Brains. Neurotoxicology 2023, 96, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Y.; Shi, L.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H. Heavy Metals Induced Mitochondrial Dysfunction in Animals: Molecular Mechanism of Toxicity. Toxicology 2022, 469, 153136. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, B.; Ke, T.; Li, S.; Yang, X.; Aschner, M.; Chen, P. Mechanisms of Metal-Induced Mitochondrial Dysfunction in Neurological Disorders. Toxics 2021, 9, 142. [Google Scholar] [CrossRef]
- Henry, K.A.; Cristol, D.A.; Varian-Ramos, C.W.; Bradley, E.L. Oxidative Stress in Songbirds Exposed to Dietary Methylmercury. Ecotoxicology 2015, 24, 520–526. [Google Scholar] [CrossRef]
- van den Brink, N.W.; Scheiber, I.B.R.; de Jong, M.E.; Braun, A.; Arini, A.; Basu, N.; van den Berg, H.; Komdeur, J.; Loonen, M.J.J.E. Mercury Associated Neurochemical Response in Arctic Barnacle Goslings (Branta leucopsis). Sci. Total Environ. 2018, 624, 1052–1058. [Google Scholar] [CrossRef]
- Rutkiewicz, J.; Nam, D.-H.; Cooley, T.; Neumann, K.; Padilla, I.B.; Route, W.; Strom, S.; Basu, N. Mercury Exposure and Neurochemical Impacts in Bald Eagles across Several Great Lakes States. Ecotoxicology 2011, 20, 1669–1676. [Google Scholar] [CrossRef]
- Rutkiewicz, J.; Scheuhammer, A.; Crump, D.; Jagla, M.; Basu, N. Investigation of Spatial Trends and Neurochemical Impacts of Mercury in Herring Gulls across the Laurentian Great Lakes. Environ. Pollut. 2010, 158, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Braune, B.M.; Scheuhammer, A.M.; Crump, D.; Jones, S.; Porter, E.; Bond, D. Toxicity of Methylmercury Injected into Eggs of Thick-Billed Murres and Arctic Terns. Ecotoxicology 2012, 21, 2143–2152. [Google Scholar] [CrossRef]
- Rutkiewicz, J.; Bradley, M.; Mittal, K.; Basu, N. Methylmercury Egg Injections: Part 2—Pathology, Neurochemistry, and Behavior in the Avian Embryo and Hatchling. Ecotoxicol. Environ. Saf. 2013, 93, 77–86. [Google Scholar] [CrossRef]
- Rutkiewicz, J.; Basu, N. Methylmercury Egg Injections: Part 1-Tissue Distribution of Mercury in the Avian Embryo and Hatchling. Ecotoxicol. Environ. Saf. 2013, 93, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Bourdineaud, J.P.; Marumoto, M.; Yasutake, A.; Fujimura, M. Dietary Mercury Exposure Resulted in Behavioral Differences in Mice Contaminated with Fish-Associated Methylmercury Compared to Methylmercury Chloride Added to Diet. Biomed. Res. Int. 2012, 2012, 681016. [Google Scholar] [CrossRef]
- Heinz, G.H.; Hoffman, D.J.; Klimstra, J.D.; Stebbins, K.R.; Kondrad, S.L. Toxicity of Methylmercury Injected into Eggs When Dissolved in Water versus Corn Oil. Environ. Toxicol. Chem. 2011, 30, 2103–2106. [Google Scholar] [CrossRef] [PubMed]
- Swaddle, J.P.; Diehl, T.R.; Taylor, C.E.; Fanaee, A.S.; Benson, J.L.; Huckstep, N.R.; Cristol, D.A. Exposure to Dietary Mercury Alters Cognition and Behavior of Zebra Finches. Curr. Zool. 2017, 63, 213–219. [Google Scholar] [CrossRef]
- Adams, E.M.; Frederick, P.C.; Larkin, I.L.V.; Guillette, L.J. Sublethal Effects of Methylmercury on Fecal Metabolites of Testosterone, Estradiol, and Corticosterone in Captive Juvenile White Ibises (Eudocimus albus). Environ. Toxicol. Chem. 2009, 28, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, M.D.; Lane, O.P.; Evers, D.C.; Reed, J.M.; Hoskins, B.; Romero, L.M. The Corticosterone Stress Response and Mercury Contamination in Free-Living Tree Swallows, Tachycineta Bicolor. Ecotoxicology 2009, 18, 514–521. [Google Scholar] [CrossRef]
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The Endocrine Effects of Mercury in Humans and Wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef]
- Moore, C.S.; Cristol, D.A.; Maddux, S.L.; Varian-Ramos, C.W.; Bradley, E.L. Lifelong Exposure to Methylmercury Disrupts Stress-Induced Corticosterone Response in Zebra Finches (Taeniopygia guttata). Environ. Toxicol. Chem. 2014, 33, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Buntin, J.D. Neural and Hormonal Control of Parental Behavior in Birds. Adv. Study Behav. 1996, 25, 161–213. [Google Scholar] [CrossRef]
- Tartu, S.; Goutte, A.; Bustamante, P.; Angelier, F.; Moe, B.; Clément-Chastel, C.; Bech, C.; Gabrielsen, G.W.; Bustnes, J.O.; Chastel, O. To Breed or Not to Breed: Endocrine Response to Mercury Contamination by an Arctic Seabird. Biol. Lett. 2013, 9, 2013–2016. [Google Scholar] [CrossRef]
- Scoville, S.A.; Lane, O.P. Cerebellar Abnormalities Typical of Methylmercury Poisoning in a Fledged Saltmarsh Sparrow, Ammodramus Caudacutus. Bull. Environ. Contam. Toxicol. 2013, 90, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Abe, O.; Nakamura, M.; Inui, S.; Uetani, H.; Ueda, M.; Azuma, M. Brain Structural Changes in Patients with Chronic Methylmercury Poisoning in Minamata. Brain Res. 2023, 1805, 148278. [Google Scholar] [CrossRef] [PubMed]
- Pass, D.A.; Little, P.B.; Karstad, L.H. The Pathology of Subacute and Chronic Methyl Mercury Poisoning of the Mallard Duck (Anas platyrhynchos). J. Comp. Pathol. 1975, 85, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Heinz, G.H.; Locke, L.N. Brain Lesions in Mallard Ducklings from Parents Fed Methylmercury. Avian Dis. 1976, 20, 9–17. [Google Scholar] [CrossRef]
- Evans, H.L.; Garman, R.; Laties, V. Neurotoxicity of Methylmercury in the Pigeon. Neurotoxicology 1982, 3, 21–36. [Google Scholar]
- Loerzel, S.; Samuelson, D.; Szabo, N. Ocular Effects of Methylmercury in Juvenile Double-Crested Cormorants (Phalacrocorax auritus). Investig. Ophthalmol. Vis. Sci. 1999, 126, 117–127. [Google Scholar]
- Wolf, S.E.; Swaddle, J.P.; Cristol, D.A.; Buchser, W.J. Methylmercury Exposure Reduces the Auditory Brainstem Response of Zebra Finches (Taeniopygia guttata). JARO J. Assoc. Res. Otolaryngol. 2017, 18, 569. [Google Scholar] [CrossRef]
- Yu, M.S.; Eng, M.L.; Williams, T.D.; Guigueno, M.F.; Elliott, J.E. Assessment of Neuroanatomical and Behavioural Effects of in Ovo Methylmercury Exposure in Zebra Finches (Taeniopygia guttata). Neurotoxicology 2017, 59, 33–39. [Google Scholar] [CrossRef]
- Bennett, R.S.; French, J.B.; Rossmann, R.; Haebler, R. Dietary Toxicity and Tissue Accumulation of Methylmercury in American Kestrels. Arch. Environ. Contam. Toxicol. 2009, 56, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Fimreite, N.; Karstad, L. Effects of Dietary Methyl Mercury on Red-Tailed Hawks. J. Wildl. Manag. 1971, 35, 293. [Google Scholar] [CrossRef]
- Cooper, A.J.L. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Cerebral Ammonia Homeostasis. Neurochem. Res. 2012, 37, 2439–2455. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Basu, N.; Burgess, N.M.; Elliott, J.E.; Campbell, G.D.; Wayland, M.; Champoux, L.; Rodrigue, J. Relationships among Mercury, Selenium, and Neurochemical Parameters in Common Loons (Gavia immer) and Bald Eagles (Haliaeetus leucocephalus). Ecotoxicology 2008, 17, 93–101. [Google Scholar] [CrossRef]
- Hamilton, M.; Scheuhammer, A.; Basu, N. Mercury, Selenium and Neurochemical Biomarkers in Different Brain Regions of Migrating Common Loons from Lake Erie, Canada. Ecotoxicology 2011, 20, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, L.; Snoeijs, T.; Van Duyse, E.; Eens, M. Heavy Metal Pollution Affects Dawn Singing Behaviour in a Small Passerine Bird. Oecologia 2005, 145, 504–509. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.L.; Maher, C.R. Relationship between Blood Mercury Levels and Components of Male Song in Nelson’s Sparrows (Ammodramus nelsoni). Ecotoxicology 2012, 21, 2391–2397. [Google Scholar] [CrossRef]
- Hallinger, K.K.; Zabransky, D.J.; Kazmer, K.A.; Cristol, D.A. Birdsong Differs between Mercury-Polluted and Reference Sites. Auk 2010, 127, 156–161. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Bouton, S.N.; Frederick, P.C.; Spalding, M.G.; McGill, H. Effects of Chronic, Low Concentrations of Dietary Methylmercury on the Behavior of Juvenile Great Egrets. Environ. Toxicol. Chem. 1999, 18, 1934–1939. [Google Scholar] [CrossRef]
- Scheuhammer, A.M. Chronic Dietary Toxicity of Methylmercury in the Zebra Finch, Poephila guttata. Bull. Environ. Contam. Toxicol. 1988, 40, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Laties, V.G.; Evans, H.L. Methylmercury-Induced Changes in Operant Discrimination by the Pigeon. J. Pharmacol. Exp. Ther. 1980, 214, 3. [Google Scholar]
- Wolfe, M.F.; Schwarzbach, S.; Sulaiman, R.A. Effects of Mercury on Wildlife: A Comprehensive Review. Environ. Toxicol. Chem. 1998, 17, 146–160. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Effects of Lead on Learning in Herring Gulls: An Avian Wildlife Model for Neurobehavioral Deficits. Neurotoxicology 2005, 26, 615–624. [Google Scholar] [CrossRef]
- Henny, C.J.; Hill, E.F.; Grove, R.A.; Chelgren, N.D.; Haggerty, P.K. Mercury and Drought along the Lower Carson River, Nevada: IV. Snowy Egret Post-Fledging Dispersal, Timing of Migration and Survival, 2002–2004. Ecotoxicol. Environ. Saf. 2017, 135, 358–367. [Google Scholar] [CrossRef]
- Provencher, J.F.; Forbes, M.R.; Hennin, H.L.; Love, O.P.; Braune, B.M.; Mallory, M.L.; Gilchrist, H.G. Implications of Mercury and Lead Concentrations on Breeding Physiology and Phenology in an Arctic Bird. Environ. Pollut. 2016, 218, 1014–1022. [Google Scholar] [CrossRef]
- Olsen, B.; Evers, D.; DeSorbo, C. Effect of Methylated Mercury on the Diving Frequency of the Common Loon. J. Ecol. Res. 2000, 2, 67–72. [Google Scholar]
- Jenko, K.; Karouna-Renier, N.K.; Hoffman, D.J. Gene Expression, Glutathione Status, and Indicators of Hepatic Oxidative Stress in Laughing Gull (Larus atricilla) Hatchlings Exposed to Methylmercury. Environ. Toxicol. Chem. 2012, 31, 2588–2596. [Google Scholar] [CrossRef]
- Ackerman, J.T.; Peterson, S.H.; Herzog, M.P.; Yee, J.L. Methylmercury Effects on Birds: A Review, Meta-Analysis, and Development of Toxicity Reference Values for Injury Assessment Based on Tissue Residues and Diet. Environ. Toxicol. Chem. 2024, 43, 1195–1241. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.T.; Herzog, M.P.; Evers, D.C.; Cristol, D.A.; Kenow, K.P.; Heinz, G.H.; Lavoie, R.A.; Brasso, R.L.; Mallory, M.L.; Provencher, J.F.; et al. Synthesis of Maternal Transfer of Mercury in Birds: Implications for Altered Toxicity Risk. Environ. Sci. Technol. 2020, 54, 2878–2891. [Google Scholar] [CrossRef] [PubMed]
- Paris, O.J.; Swaddle, J.P.; Cristol, D.A. Exposure to Dietary Methyl-Mercury Solely during Embryonic and Juvenile Development Halves Subsequent Reproductive Success in Adult Zebra Finches. Environ. Sci. Technol. 2018, 52, 3117–3124. [Google Scholar] [CrossRef] [PubMed]
- Eagles-Smith, C.A.; Ackerman, J.T.; De La Cruz, S.E.W.; Takekawa, J.Y. Mercury Bioaccumulation and Risk to Three Waterbird Foraging Guilds Is Influenced by Foraging Ecology and Breeding Stage. Environ. Pollut. 2009, 157, 1993–2002. [Google Scholar] [CrossRef]
- Ou, L.; Varian-Ramos, C.W.; Cristol, D.A. Effect of Laying Sequence on Egg Mercury in Captive Zebra Finches: An Interpretation Considering Individual Variation. Environ. Toxicol. Chem. 2015, 34, 1787–1792. [Google Scholar] [CrossRef]
- Evers, D.C.; Taylor, K.M.; Major, A.; Taylor, R.J.; Poppenga, R.H.; Scheuhammer, A.M. Common Loon Eggs as Indicators of Methylmercury Availability in North America. Ecotoxicology 2003, 12, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Herring, G.; Ackerman, J.T.; Eagles-Smith, C.A. Embryo Malposition as a Potential Mechanism for Mercury-induced Hatching Failure in Bird Eggs. Environ. Toxicol. Chem. 2010, 29, 1788–1794. [Google Scholar] [CrossRef]
- Leaphart, J.C.; Chinn, S.M.; Beasley, J.C. Impact of Developmental Methylmercury Exposure on Avian Embryonic Development, Hatchling Growth, and Survival. Environ. Toxicol. Chem. 2025, 44, 432–443. [Google Scholar] [CrossRef]
- Weiss, B.; Clarkson, T.W.; Simon, W. Silent Latency Periods in Methylmercury Poisoning and in Neurodegenerative Disease. Environ. Health Perspect. 2002, 110, 851–854. [Google Scholar] [CrossRef]
- Nocera, J.J.; Taylor, P.D. In situ behavioral response of common loons associated with elevated mercury (Hg) exposure. Conserv. Ecol. 1998, 2, 10. Available online: http://www.consecol.org/vol2/iss2/art10/ (accessed on 9 March 2025).
- Kenow, K.P.; Hines, R.K.; Meyer, M.W.; Suarez, S.A.; Gray, B.R. Effects of Methylmercury Exposure on the Behavior of Captive-Reared Common Loon (Gavia immer) Chicks. Ecotoxicology 2010, 19, 933–944. [Google Scholar] [CrossRef]
- Kenow, K.P.; Meyer, M.W.; Rossmann, R.; Gendron-Fitzpatrick, A.; Gray, B.R. Effects of Injected Methylmercury on the Hatching of Common Loon (Gavia immer) Eggs. Ecotoxicology 2011, 20, 1684–1693. [Google Scholar] [CrossRef]
- Heinz, G. Effects of Methylmercury on Approach and Avoidance Behavior of Mallard Ducklings. Bull. Environ. Contam. Toxicol. 1975, 13, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Heinz, G.H. Methylmercury: Second-Year Feeding Effects on Mallard Reproduction and Duckling Behavior. J. Wildl. Manag. 1976, 40, 82. [Google Scholar] [CrossRef]
- Heinz, G.H. Methylmercury: Reproductive and Behavioral Effects on Three Generations of Mallard Ducks. J. Wildl. Manag. 1979, 43, 394. [Google Scholar] [CrossRef]
- Grace, J.; Duran, E.; Ann Ottinger, M.; Maness, T. Sublethal Effects of Early-Life Exposure to Common and Emerging Contaminants in Birds. Curr. Res. Toxicol. 2024, 7, 100190. [Google Scholar] [CrossRef] [PubMed]
- Kenow, K.P.; Gutreuter, S.; Hines, R.K.; Meyer, M.W.; Fournier, F.; Karasov, W.H. Effects of Methyl Mercury Exposure on the Growth of Juvenile Common Loons. Ecotoxicology 2003, 12, 171–181. [Google Scholar] [CrossRef]
- Bateson, P.; Laland, K.N. Tinbergen’s Four Questions: An Appreciation and an Update. Trends Ecol. Evol. 2013, 28, 712–718. [Google Scholar] [CrossRef]
- Seewagen, C.L. Blood Mercury Levels and the Stopover Refueling Performance of a Long-Distance Migratory Songbird. Can. J. Zool. 2013, 91, 41–45. [Google Scholar] [CrossRef]
- Seewagen, C.L.; Cristol, D.A.; Gerson, A.R. Mobilization of Mercury from Lean Tissues during Simulated Migratory Fasting in a Model Songbird. Sci. Rep. 2016, 6, 25762. [Google Scholar] [CrossRef]
- Seewagen, C.L.; Ma, Y.; Morbey, Y.E.; Guglielmo, C.G. Stopover Departure Behavior and Flight Orientation of Spring-Migrant Yellow-Rumped Warblers (Setophaga coronata) Experimentally Exposed to Methylmercury. J. Ornithol. 2019, 160, 617–624. [Google Scholar] [CrossRef]
- Ritschard, M.; Brumm, H. Zebra Finch Song Reflects Current Food Availability. Evol. Ecol. 2012, 26, 801–812. [Google Scholar] [CrossRef]
- Chin, S.Y.; Hopkins, W.A.; Cristol, D.A. Mercury Alters Initiation and Construction of Nests by Zebra Finches, but Not Incubation or Provisioning Behaviors. Ecotoxicology 2017, 26, 1271–1283. [Google Scholar] [CrossRef]
- Albers, P.H.; Koterba, M.T.; Rossmann, R.; Link, W.A.; French, J.B.; Bennett, R.S.; Bauer, W.C. Effects of Methylmercury on Reproduction in American Kestrels. Environ. Toxicol. Chem. 2007, 26, 1856–1866. [Google Scholar] [CrossRef]
- Varian-Ramos, C.W.; Swaddle, J.P.; Cristol, D.A. Mercury Reduces Avian Reproductive Success and Imposes Selection: An Experimental Study with Adult- or Lifetime-Exposure in Zebra Finch. PLoS ONE 2014, 9, e95674. [Google Scholar] [CrossRef]
- Heinz, G.H.; Hoffman, D.J.; Klimstra, J.D.; Stebbins, K.R.; Kondrad, S.L.; Erwin, C.A. Species Differences in the Sensitivity of Avian Embryos to Methylmercury. Arch. Environ. Contam. Toxicol. 2009, 56, 129–138. [Google Scholar] [CrossRef]
- Lewis, C.A.; Cristol, D.A.; Swaddle, J.P.; Varian-Ramos, C.W.; Zwollo, P. Decreased Immune Response in Zebra Finches Exposed to Sublethal Doses of Mercury. Arch. Environ. Contam. Toxicol. 2013, 64, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; van den Berg, H.; Loonen, M.J.J.E.; Mateo, R.; van den Brink, N.W. Mercury-Modulated Immune Responses in Arctic Barnacle Goslings (Branta leucopsis) upon a Viral-Like Immune Challenge. Environ. Sci. Technol. 2023, 57, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Hawley, D.M.; Hallinger, K.K.; Cristol, D.A. Compromised Immune Competence in Free-Living Tree Swallows Exposed to Mercury. Ecotoxicology 2009, 18, 499–503. [Google Scholar] [CrossRef]
- Kose, M.; Møller, A.P. Sexual Selection, Feather Breakage and Parasites: The Importance of White Spots in the Tail of the Barn Swallow (Hirundo rustica). Behav. Ecol. Sociobiol. 1999, 45, 430–436. [Google Scholar] [CrossRef]
- Herring, G.; Eagles-Smith, C.A.; Ackerman, J.T. Mercury Exposure May Influence Fluctuating Asymmetry in Waterbirds. Environ. Toxicol. Chem. 2017, 36, 1599–1605. [Google Scholar] [CrossRef]
- Hartman, C.A.; Ackerman, J.T.; Herzog, M.P. Mercury Exposure and Altered Parental Nesting Behavior in a Wild Songbird. Environ. Sci. Technol. 2019, 53, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, K.; Binkowski, Ł.J.; Dudzik, K. Spatial and Temporal Trends in Mercury Levels in the down of Black Stork Chicks in Central Europe. Environ. Pollut. 2021, 274, 116571. [Google Scholar] [CrossRef] [PubMed]
- Furness, R.W.; Muirhead, S.J.; Woodburn, M. Using Bird Feathers to Measure Mercury in the Environment: Relationships between Mercury Content and Moult. Mar. Pollut. Bull. 1986, 17, 27–30. [Google Scholar] [CrossRef]
- Thompson, D.R.; Stewart, F.M.; Furness, R.W. Using Seabirds to Monitor Mercury in Marine Environments: The Validity of Conversion Ratios for Tissue Comparisons. Mar. Pollut. Bull. 1990, 21, 339–342. [Google Scholar] [CrossRef]
- Honda, K.; Nasu, T.; Tatsukawa, R. Seasonal Changes in Mercury Accumulation in the Black-Eared Kite, Milvus migrans Lineatus. Environ. Pollut. Ser. A Ecol. Biol. 1986, 42, 325–334. [Google Scholar] [CrossRef]
- Stoewsand, G.S.; Bache, C.A.; Lisk, D.J. Dietary Selenium Protection of Methylmercury Intoxication of Japanese Quail. Bull. Environ. Contam. Toxicol. 1974, 11, 152–156. [Google Scholar] [CrossRef]
- Spallholz, J.E.; Hoffman, D.J. Selenium Toxicity: Cause and Effects in Aquatic Birds. Aquat. Toxicol. 2002, 57, 27–37. [Google Scholar] [CrossRef]
- Heinz, G.H.; Hoffman, D.J.; Klimstra, J.D.; Stebbins, K.R. A Comparison of the Teratogenicity of Methylmercury and Selenomethionine Injected into Bird Eggs. Arch. Environ. Contam. Toxicol. 2012, 62, 519–528. [Google Scholar] [CrossRef]
- Klimstra, J.D.; Yee, J.L.; Heinz, G.H.; Hoffman, D.J.; Stebbins, K.R. Interactions between Methylmercury and Selenomethionine Injected into Mallard Eggs. Environ. Toxicol. Chem. 2012, 31, 579–584. [Google Scholar] [CrossRef]
- Buck, K.A.; Varian-Ramos, C.W.; Cristol, D.A.; Swaddle, J.P. Blood Mercury Levels of Zebra Finches Are Heritable: Implications for the Evolution of Mercury Resistance. PLoS ONE 2016, 11, e0162440. [Google Scholar] [CrossRef]
- Basu, N.; Goodrich, J.; Head, J. Ecogenetics of Mercury: From Genetic Polymorphisms and Epigenetics to Risk Assessment and Decision-making. Environ. Toxicol. Chem. 2014, 33, 1248–1258. [Google Scholar] [CrossRef]
- Shumway, S.E.; Allen, S.M.; Boersma, P.D. Marine Birds and Harmful Algal Blooms: Sporadic Victims or under-Reported Events? Harmful Algae 2003, 2, 1–17. [Google Scholar] [CrossRef]
- Su, T.; He, C.; Jiang, A.; Xu, Z.; Goodale, E.; Qiu, G. Passerine Bird Reproduction Does Not Decline in a Highly-Contaminated Mercury Mining District of China. Environ. Pollut. 2021, 286, 117440. [Google Scholar] [CrossRef]
- Hallinger, K.K.; Cristol, D.A. The Role of Weather in Mediating the Effect of Mercury Exposure on Reproductive Success in Tree Swallows. Ecotoxicology 2011, 20, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.; Ferguson, L.; Lanctot, R.B.; Stenhouse, I.J.; Kendall, S.; Brown, S.; Gates, H.R.; Hall, J.O.; Regan, K.; Evers, D.C. Mercury Exposure and Risk in Breeding and Staging Alaskan Shorebirds. Condor Ornithol. Appl. 2016, 118, 571–582. [Google Scholar] [CrossRef]
- Jackson, A.K.; Evers, D.C.; Etterson, M.A.; Condon, A.M.; Folsom, S.B.; Detweiler, J.; Schmerfeld, J.; Cristol, D.A. Mercury Exposure Affects the Reproductive Success of a Free-Living Terrestrial Songbird, the Carolina Wren (Thryothorus ludovicianus). Auk 2011, 128, 759–769. [Google Scholar] [CrossRef]
- Rewi, S.T.; Fessardi, M.; Landers, T.J.; Lyver, P.O.B.; Taylor, G.A.; Bury, S.J.; Dunphy, B.J. Feather Mercury Content of Grey-Faced Petrels (Pterodroma gouldi): Relationships with Age, Breeding Success, and Foraging Behaviour, in Known Age Individuals. Sci. Total Environ. 2024, 951, 175778. [Google Scholar] [CrossRef]
- Santos, C.S.A.; Blondel, L.; Sotillo, A.; Müller, W.; Stienen, E.W.M.; Boeckx, P.; Soares, A.M.V.M.; Monteiro, M.S.; Loureiro, S.; de Neve, L.; et al. Offspring Hg Exposure Relates to Parental Feeding Strategies in a Generalist Bird with Strong Individual Foraging Specialization. Sci. Total Environ. 2017, 601–602, 1315–1323. [Google Scholar] [CrossRef]
- Grunst, A.S.; Grunst, M.L.; Grémillet, D.; Kato, A.; Bustamante, P.; Albert, C.; Brisson-Curadeau, É.; Clairbaux, M.; Cruz-Flores, M.; Gentès, S.; et al. Mercury Contamination Challenges the Behavioral Response of a Keystone Species to Arctic Climate Change. Environ. Sci. Technol. 2023, 57, 2054–2063. [Google Scholar] [CrossRef]
- Carlson, J.R.; Cristol, D.; Swaddle, J.P. Dietary Mercury Exposure Causes Decreased Escape Takeoff Flight Performance and Increased Molt Rate in European Starlings (Sturnus vulgaris). Ecotoxicology 2014, 23, 1464–1473. [Google Scholar] [CrossRef]
- Blévin, P.; Tartu, S.; Ellis, H.I.; Chastel, O.; Bustamante, P.; Parenteau, C.; Herzke, D.; Angelier, F.; Gabrielsen, G.W. Contaminants and Energy Expenditure in an Arctic Seabird: Organochlorine Pesticides and Perfluoroalkyl Substances Are Associated with Metabolic Rate in a Contrasted Manner. Environ. Res. 2017, 157, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bourdineaud, J.P.; Fujimura, M.; Laclau, M.; Sawada, M.; Yasutake, A. Deleterious Effects in Mice of Fish-Associated Methylmercury Contained in a Diet Mimicking the Western Populations’ Average Fish Consumption. Environ. Int. 2011, 37, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Fuchsman, P.C.; Brown, L.E.; Henning, M.H.; Bock, M.J.; Magar, V.S. Toxicity Reference Values for Methylmercury Effects on Avian Reproduction: Critical Review and Analysis. Environ. Toxicol. Chem. 2017, 36, 294–319. [Google Scholar] [CrossRef] [PubMed]
- Blévin, P.; Carravieri, A.; Jaeger, A.; Chastel, O.; Bustamante, P.; Cherel, Y. Wide Range of Mercury Contamination in Chicks of Southern Ocean Seabirds. PLoS ONE 2013, 8, e54508. [Google Scholar] [CrossRef]
- Scheuhammer, A.M. Effects of Acidification on the Availability of Toxic Metals and Calcium to Wild Birds and Mammals. Environ. Pollut. 1991, 71, 329–375. [Google Scholar] [CrossRef]
- Pulford, I.D.; Watson, C. Phytoremediation of Heavy Metal-Contaminated Land by Trees—A Review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]




| Species | Study Type | Tissue | Hg Concentration (µg·g−1) | Observed Effect | Reference |
|---|---|---|---|---|---|
| Common loon, Gavia immer | Field | Blood | 3.00 | Increased lethargy, reduced incubation time | Evers et al. [22] |
| Mallard, Anas platyrhynchos | Lab | Dietary | 0.50 | Reduced clutch size, altered behaviour | Heinz [167] |
| Zebra finch, Taeniopygia guttata | Lab | Dietary | 0.30–2.40 | Reduced reproductive success, impaired foraging | Varian-Ramos et al. [177] |
| Tree swallow, Tachycineta bicolor | Field | Blood/Egg | 0.07–3.03 | Reduced fledging success, altered incubation | Hallinger and Cristol [197], Hartman et al. [184] |
| Species | Study Type | Tissue | Hg Concentration (µg·g−1) | Observed Effect | Reference |
|---|---|---|---|---|---|
| Common loon, Gavia immer | Field | Feathers | ≥40 | Developmental instability (asymmetrical feathers) | Evers et al. [22] |
| Black-legged kittiwake, Rissa tridactyla | Field | Blood | 1.80 | Increased likelihood of skipping breeding | Tartu et al. [51] |
| Tree swallow, Tachycineta bicolor | Field | Eggs | 0.07–0.53 | Altered incubation behaviour, reduced success | Hartman et al. [184,199] |
| Carolina wren, Thryothorus ludovicianus | Field | Blood/Feathers/ Eggs | 0.70–3.00 (blood, feathers), 0.11 (eggs) | 10% reduction in nest success | Jackson et al. [199] |
| Grey-faced petrel, Pterodroma gouldi | Field | Feathers | 38.2–39.50 | No significant effect on breeding outcomes | Rewi et al. [200] |
| Species | Study Type | Tissue | Hg Concentration (µg·g−1) | Observed Effect | Reference |
|---|---|---|---|---|---|
| Lesser black-backed gull, Larus fuscus | Field | Egg Yolk, Feathers | 0.37–2.77 | No observable behavioural effects | Santos et al. [201] |
| Little auk, Alle alle | Field | Blood | 0.50–2.00 | Prolonged post-dive recovery (physiological stress) | Grunst et al. [202] |
| Zebra finch, Taeniopygia guttata | Lab | Dietary | 0.30–2.40 | Reduced reproductive success, impaired foraging | Varian-Ramos et al. [177] |
| European starling, Sturnus vulgaris | Lab | Dietary | 0.75–1.50 | Reduced flight escape response, increased moulting | Carlson et al. [203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjedov, D.; Sudarić Bogojević, M.; Bernal-Alviz, J.; Klobučar, G.; Bourdineaud, J.-P.; Aarif, K.M.; Mikuška, A. An Integrative Approach to Assessing the Impact of Mercury (Hg) on Avian Behaviour: From Molecule to Movement. J. Xenobiot. 2025, 15, 117. https://doi.org/10.3390/jox15040117
Bjedov D, Sudarić Bogojević M, Bernal-Alviz J, Klobučar G, Bourdineaud J-P, Aarif KM, Mikuška A. An Integrative Approach to Assessing the Impact of Mercury (Hg) on Avian Behaviour: From Molecule to Movement. Journal of Xenobiotics. 2025; 15(4):117. https://doi.org/10.3390/jox15040117
Chicago/Turabian StyleBjedov, Dora, Mirta Sudarić Bogojević, Jorge Bernal-Alviz, Goran Klobučar, Jean-Paul Bourdineaud, K. M. Aarif, and Alma Mikuška. 2025. "An Integrative Approach to Assessing the Impact of Mercury (Hg) on Avian Behaviour: From Molecule to Movement" Journal of Xenobiotics 15, no. 4: 117. https://doi.org/10.3390/jox15040117
APA StyleBjedov, D., Sudarić Bogojević, M., Bernal-Alviz, J., Klobučar, G., Bourdineaud, J.-P., Aarif, K. M., & Mikuška, A. (2025). An Integrative Approach to Assessing the Impact of Mercury (Hg) on Avian Behaviour: From Molecule to Movement. Journal of Xenobiotics, 15(4), 117. https://doi.org/10.3390/jox15040117