Ecotoxicological Effects of Environmentally Relevant Concentrations of Nickel Nanoparticles on Aquatic Organisms from Three Trophic Levels: Insights from Oxidative Stress Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Test Solutions
2.2. Test Organisms and Acclimation
2.2.1. Polychaetes (Hediste diversicolor)
2.2.2. Mussels (Mytilus spp.)
2.2.3. Fish (Sparus aurata)
2.3. Experimental Design and Exposure Conditions
2.4. Euthanasia and Collection of Biological Samples
2.5. Biochemical Determinations
2.5.1. Determination of Glutathione S-Transferases (GSTs) Activity
2.5.2. Determination of Catalase (CAT) Activity
2.5.3. Quantification of Lipid Peroxidation
2.5.4. Quantification of Total Soluble Protein
2.6. Statistical Analysis
3. Results
3.1. Glutathione S-Transferases (GSTs)
3.2. Catalase (CAT)
3.3. Thiobarbituric Acid Reactive Substances (TBARS)
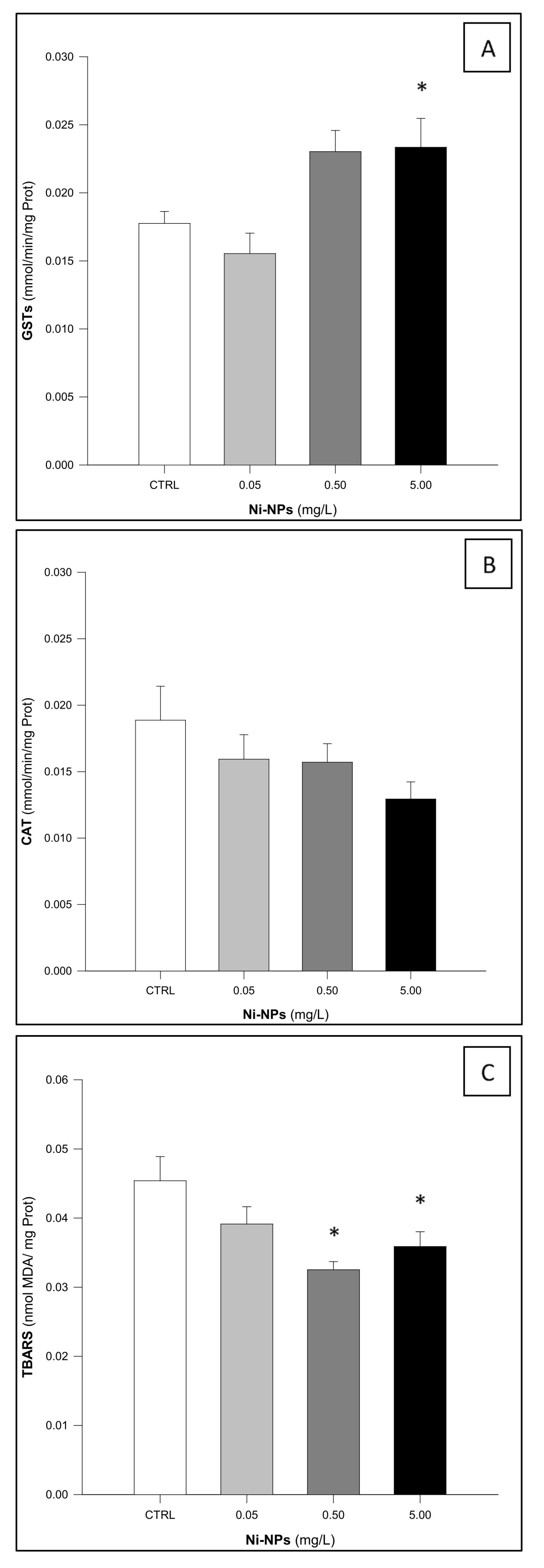
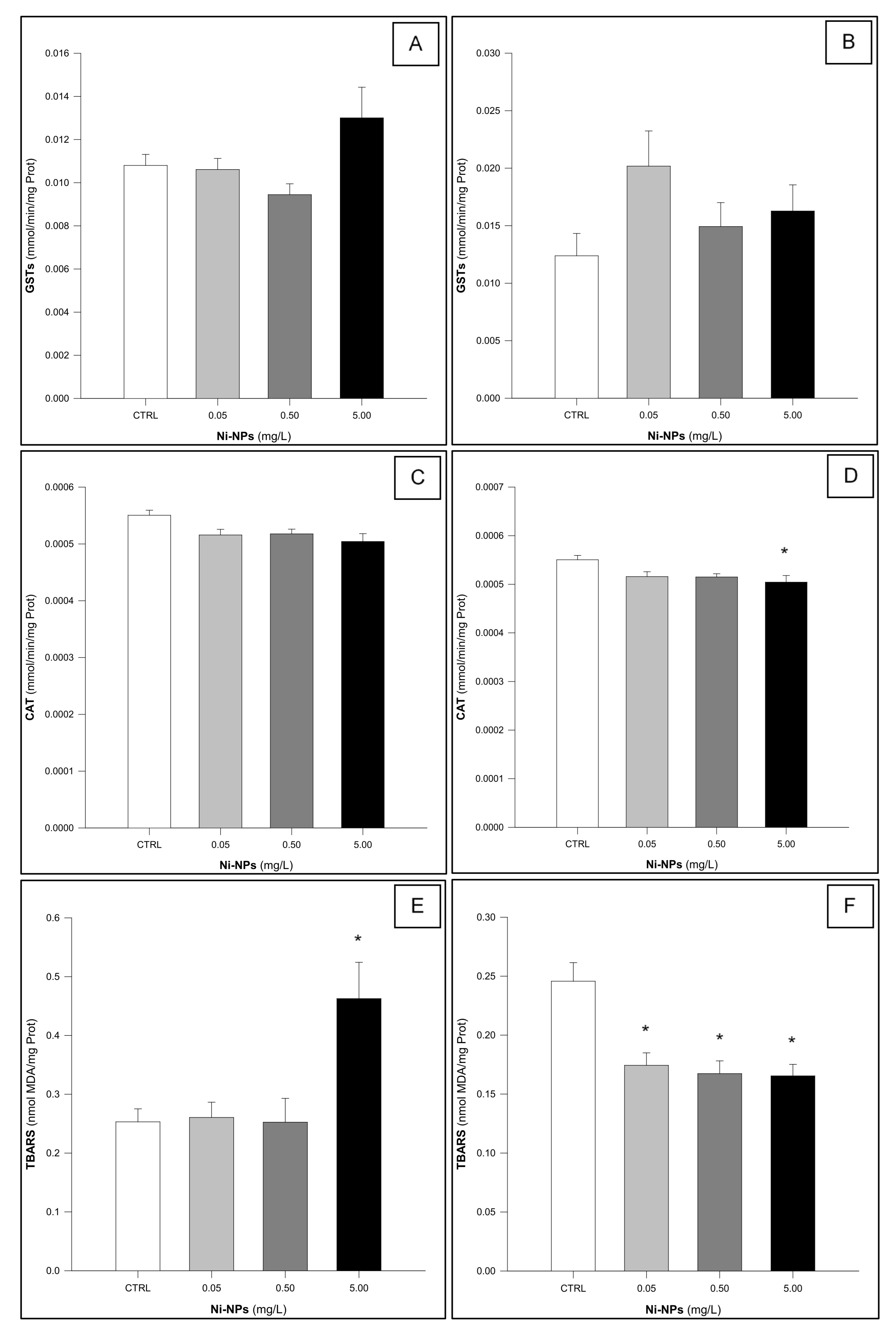
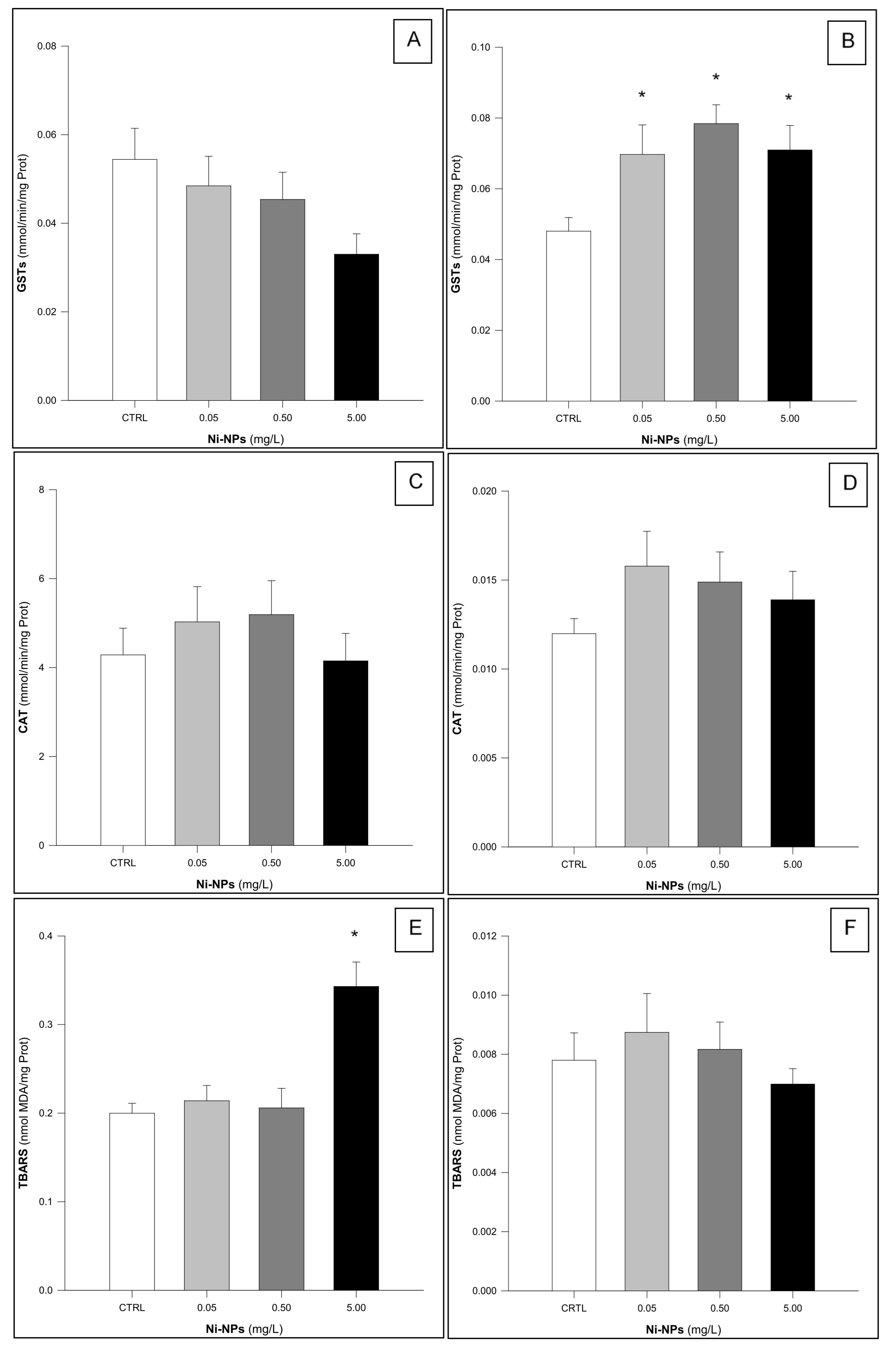
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Soininen, J.; Heino, J. Ecological indicators for aquatic biodiversity, ecosystem functions, human activities and climate change. Ecol. Indic. 2021, 132, 108250. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef]
- Häder, D.P.; Banaszak, A.T.; Villafañe, V.E.; Narvarte, M.A.; González, R.A.; Helbling, E.W. Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci. Total Environ. 2020, 713, 136586. [Google Scholar] [CrossRef]
- Coscia, I.; Kaiser, M. The impact of the human activities on aquatic ecosystems. J. Fish Biol. 2022, 101, 331–332. [Google Scholar] [CrossRef]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology; Hakeem, K., Bhat, R., Qadri, H., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Sengupta, S.; Roy, U.; Chowdhary, S.; Das, P. New Bioremediation Technologies to Remove Heavy Metals and Radionuclides. In Removal of Emerging Contaminants Through Microbial Processes; Shah, M.P., Ed.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Qin, J.; Vasseghian, Y. Occurrences and removal of pharmaceutical and personal care products from aquatic systems using advanced treatment—A review. Environ. Res. 2022, 204, 112298. [Google Scholar] [CrossRef]
- El-Kalliny, A.S.; Abdel-Wahed, M.S.; El-Zahhar, A.A.; Hamza, I.A.; Gad-Allah, T.A. Nanomaterials: A review of emerging contaminants with potential health or environmental impact. Disc. Nano 2023, 18, 68. [Google Scholar] [CrossRef]
- Ju-Nam, Y.; Lead, J.R. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [CrossRef]
- Felix, L.C.; Ortega, V.A.; Ede, J.D.; Goss, G.G. Physicochemical characteristics of polymer-coated metal-oxide nanoparticles and their toxicological effects on zebrafish (Danio rerio) development. Environ. Sci. Technol. 2013, 47, 130603125147001. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, H.Z.; Lu, G.H. Effects of selected metal oxide nanoparticles on multiple biomarkers in Carassius auratus. Biomed. Environ. Sci. 2013, 26, 742–749. [Google Scholar] [CrossRef]
- Shilpa, M.; Baranidharan, S. Fate, transport, and toxicity of nanoparticles: An emerging pollutant on biotic factors. Proc. Saf. Environ. Prot. 2023, 174, 595–607. [Google Scholar] [CrossRef]
- Chen, X.; O’Halloran, J.; Jansen, M.A. The toxicity of zinc oxide nanoparticles to Lemna minor (L.) is predominantly caused by dissolved Zn. Aq. Toxicol. 2016, 174, 46–53. [Google Scholar] [CrossRef]
- Bordin, E.R.; Ramsdorf, W.A.; Domingos, L.M.L.; Miranda, L.P.S.; Filho, N.P.M.; Cestari, M.M. Ecotoxicological effects of zinc oxide nanoparticles (ZnO-NPs) on aquatic organisms: Current research and emerging trends. J. Environ. Manag. 2024, 349, 119396. [Google Scholar] [CrossRef]
- Farkas, J.; Christian, P.; Gallego-Urrea, J.; Roos, N.A.; Hassellöv, M.; Tollefsen, K.E.; Thomas, K.V. Uptake and effects of manufactured silver nanoparticles in rainbow trout (Oncorhynchus mykiss) gill cells. Aquat. Toxicol. 2011, 101, 117–125. [Google Scholar] [CrossRef]
- Vali, S.; Majidiyan, N.; Yalsuyi, A.M.; Vajargah, M.F.; Prokić, M.D.; Faggio, C. Ecotoxicological Effects of Silver Nanoparticles (Ag-NPs) on Parturition Time, Survival Rate, Reproductive Success and Blood Parameters of Adult Common Molly (Poecilia sphenops) and Their Larvae. Water 2022, 14, 144. [Google Scholar] [CrossRef]
- Correia, A.T.; Rebelo, D.; Marques, J.; Nunes, B. Effects of the chronic exposure to cerium dioxide nanoparticles in Oncorhynchus mykiss: Assessment of oxidative stress, neurotoxicity and histological alterations. Environ Toxicol. Pharmacol. 2019, 68, 27–36. [Google Scholar] [CrossRef]
- Correia, A.T.; Rodrigues, S.; Ferreira-Martins, D.; Nunes, B.N.; Ribeiro, M.I.; Antunes, S.C. Multi-biomarker approach to assess the acute effects of cerium dioxide nanoparticles in gills, liver and kidney of Oncorhynchus mykiss. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 238, 108842. [Google Scholar] [CrossRef]
- Liu, S.; Cui, M.; Li, X.; Thuyet, D.Q.; Fan, W. Effects of hydrophobicity of titanium dioxide nanoparticles and exposure scenarios on copper uptake and toxicity in Daphnia magna. Water Res. 2019, 154, 162–170. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yu, W.S.; Liu, G.C.; Hung, S.C.; Chang, J.H.; Chang, J.C.; Cheng, C.L.; Sun, D.S.; Lin, M.D.; Lin, W.Y.; et al. Opportunistic gill infection is associated with TiO2 nanoparticle-induced mortality in zebrafish. PLoS ONE 2021, 16, e0247859. [Google Scholar] [CrossRef]
- Al Ghais, S.; Bhardwaj, V.; Kumbhar, P.; Shehhi, O.A. Effect of copper nanoparticles and organometallic compounds (dibutyltin) on tilapia fish. JoBAZ 2019, 80, 32. [Google Scholar] [CrossRef]
- Yang, L.; He, Z.; Li, X.; Jiang, Z.; Xuan, F.; Tang, B.; Bian, X. Behavior and toxicity assessment of copper nanoparticles in aquatic environment: A case study on redwamp crayfish. J. Environ. Manag. 2022, 313, 114986. [Google Scholar] [CrossRef]
- Kanold, J.M.; Wang, J.; Brümmer, F.; Šiller, L. Metallic nickel nanoparticles and their effect on the embryonic development of the sea urchin Paracentrotus lividus. Environ. Pollut. 2016, 212, 224–229. [Google Scholar] [CrossRef]
- Data Intelo. Nickel Nanoparticule Market: Global Industry Analysis, Size, Share, Growth, Trends and Forecast. MC-521759. 2025. 283 Pages. Available online: https://dataintelo.com/report/nickel-nanoparticle-market (accessed on 30 June 2025).
- Verified Markets Reports. Nickel Nano Powder Market. 2025. Report Nº 601144. 210 Pages. Available online: https://www.verifiedmarketreports.com/product/nickel-nano-powder-market/ (accessed on 30 June 2025).
- Wu, Y.; Kong, L. Advance on toxicity of metal nickel nanoparticles. Environ. Geochem. Health 2020, 42, 2277–2286. [Google Scholar] [CrossRef]
- Bhaduri, G.A.; Šiller, L. Nickel nanoparticles catalyse reversible hydration of carbon dioxide for mineralization carbon capture and storage. Catal. Sci. Technol. 2013, 3, 1234–1239. [Google Scholar] [CrossRef]
- Morgaleva, T.; Morgalev, Y.; Gosteva, I.; Morgalev, S. Research of nickel nanoparticles toxicity with use of aquatic organisms. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012012. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental transformation and nano-toxicity of engineered nanoparticles (ENPs) in aquatic and terrestrial organisms. Crit. Rev. Environ. Sci. Technol. 2020, 23, 2523–2581. [Google Scholar] [CrossRef]
- Brix, K.V.; Schlekat, C.E.; Garman, E.R. The mechanisms of nickel toxicity in aquatic environments: An adverse outcome pathway analysis. Environ. Toxicol. Chem. 2017, 36, 1128–1137. [Google Scholar] [CrossRef]
- Meyer, J.S.; Lyons-Darden, T.; Garman, E.R.; Middleton, E.T.; Schlekat, C.E. Toxicity of Nanoparticulate Nickel to Aquatic Organisms: Review and Recommendations for Improvement of Toxicity Tests. Environ. Toxicol Chem. 2020, 39, 1861–1883. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Ramkumar, R.; Perumal, P.; Rajakumar, G.; Kirthi, A.V.; Santhoshkumar, T.; Marimuthu, S. Effect of sub-acute exposure to nickel nanoparticles on oxidative stress and histopathological changes in Mozambique tilapia, Oreochromis mossambicus. Ecotoxicol. Environ. Saf. 2014, 107, 220–228. [Google Scholar] [CrossRef]
- Samim, A.R.; Singh, V.K.; Vaseem, H. Assessment of hazardous impact of nickel oxide nanoparticles on biochemical and histological parameters of gills and liver tissues of Heteropneustes fossilis. J. Trace Elem. Med. Biol. 2022, 74, 127059. [Google Scholar] [CrossRef]
- Samim, A.R.; Vaseem, H. Exposure to Nickel Oxide Nanoparticles Induces Alterations in Antioxidant System, Metabolic Enzymes and Nutritional Composition in Muscles of Heteropneustes fossilis. Bull. Environ. Contam. Toxicol. 2023, 110, 79. [Google Scholar] [CrossRef]
- Yokota, S.; Nakamura, K.; Kamata, R. A comparative study of nickel nanoparticle and ionic nickel toxicities in zebrafish: Histopathological changes and oxidative stress. J. Toxicol. Sci. 2019, 44, 737–751. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through theredox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K.I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Nunes, B. Acute and chronic effects of erythromycin exposure on oxidative stress and genotoxicity parameters of Oncorhynchus mykiss. Sci. Total Environ. 2016, 545–546, 591–600. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, S.; Yue, M.; Zhu, S.; Liu, Q.; Zhao, X.E. Recent advances in analytical methods of oxidative stress biomarkers induced by environmental pollutant exposure. TrAC Trends Anal. Chem. 2023, 160, 116978. [Google Scholar] [CrossRef]
- Poornavaishnavi, C.; Gowthami, R.; Srikanth, K.; Bramhachari, P.V.; Venkatramaiah, N. Nickel nanoparticles induces cytotoxicity, cell morphology and oxidative stress in bluegill sunfish (BF-2) cells. Appl. Surf. Sci. 2019, 483, 1174–1181. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.J.; van den Berg, S.J.P.; Dingemans, M.M.L.; Van den Brink, P.J. (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: Facts, challenges, and future. Sci. Total Environ. 2021, 795, 148776. [Google Scholar] [CrossRef]
- Scaps, P. A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor. Hydrobiology 2002, 470, 203–218. [Google Scholar] [CrossRef]
- Gillet, P.; Mouloud, M.; Durou, C.; Deutsch, B. Response of Nereis diversicolor population (Polychaeta, Nereididae) to the pollution impact–Authie and Seine estuaries (France). ECSS 2008, 76, 201–210. [Google Scholar] [CrossRef]
- Maranho, L.A.; Baena-Nogueras, R.M.; Lara-Martín, P.A.; Delvalls, T.A.; Martín-Díaz, M.L. Bioavailability, oxidative stress, neurotoxicity and genotoxicity of pharmaceuticals bound to marine sediments. The use of the polychaete Hediste diversicolor as bioindicator species. Environ. Res. 2014, 134, 353–365. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Ghribi, R.; Correia, A.T.; Elleuch, B.; Nunes, B. Effects of chronic exposure to sediments from the Zarzis area, Gulf of Gabes, measured in the mussel (Mytilus spp.): A multi-biomarker approach involving oxidative stress and neurotoxicity. Soil Sediment Contam. An Int. J. 2020, 29, 744–769. [Google Scholar] [CrossRef]
- Franch, R.; Louro, B.; Tsalavouta, M.; Chatziplis, D.; Tsidgenopoulos, C.S.; Sarropoulou, E.; Antonello, J.; Magoulas, A.; Mylonas, C.C.; Babbucci, M.; et al. A genetic linkage map of the hermaphrodite teleost fish Sparus aurata L. Genetics 2006, 174, 851–861. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Nunes, B. Ecotoxicological evaluation of gilthead seabream (Sparus aurata) exposed to the antibiotic oxytetracycline using a multibiomarker approach. Mar. Environ. Res. 2018, 141, 233–246. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Caccamo, L.; Pansera, L.; Oteri, M.; Chiofalo, B.; Maricchiolo, G. Influence of Hermetia illucens larvae meal dietary inclusion on growth performance, gut histological traits and stress parameters in Sparus aurata. Animals 2023, 13, 339. [Google Scholar] [CrossRef]
- Carmona, E.R.; García-Rodríguez, A.; Marcos, R. Genotoxicity of copper and nickel nanoparticles in somatic cells of Drosophila melanogaster. J. Toxicol. 2018, 1, 7278036. [Google Scholar] [CrossRef]
- SFT. Guidelines for classification of environmental quality in fjords and coastal areas. Revision of classification of metals and organic contaminants in water and sediment. Norwegian Statens Forurensnings Tilsyn (SFT), 2007, Norwegian Pollution Control Authority SFT TA-2229/2007. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/klifsft/publikasjoner/2229/ta2229.pdf (accessed on 30 June 2025).
- Ates, M.; Demir, V.; Arslan, Z.; Camas, M.; Celik, F. Toxicity of Engineered Nickel Oxide and Cobalt Oxide Nanoparticles to Artemia salina in Seawater. Water Air Soil Pollut. 2016, 227, 70. [Google Scholar] [CrossRef]
- Gürkan, S.E. Impact of Nickel Oxide Nanoparticles (NiO) on Oxidative Stress Biomarkers and Hemocyte Counts of Mytilus galloprovincialis. Biol. Trace Elem. Res. 2022, 200, 3429–3441. [Google Scholar] [CrossRef]
- ASTM E1562-00; Standard Guide for Conducting Acute, Chronic, and Life-Cycle Aquatic Toxicity Tests with Polychaetous Annelids. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM E2455-22; Standard Guide for Conducting Laboratory Toxicity Tests with Freshwater Mussels. ASTM International: West Conshohocken, PA, USA, 2022.
- OECD. Test No. 215: Fish, Juvenile Growth Test, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2000. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 6, 105–121. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of micrograma quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Iqbal, S.; Jabeen, F.; Chaudhry, A.S.; Shah, M.A.; Batiha, G.E. Toxicity assessment of metallic nickel nanoparticles in various biological models: An interplay of reactive oxygen species, oxidative stress, and apoptosis. Toxicol. Ind. Health 2021, 37, 635–651. [Google Scholar] [CrossRef]
- Aziz, S.; Abdullah, S.; Anwar, H.; Latif, F.; Mustfa, W. Effect of Engineered Nickel Oxide Nanoparticles on Antioxidant Enzymes. Pak.Vet. J. 2021, 41, 424–428. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Ghobadi, S.; Vatandoust, S.; Manouchehri, H.; Changizi, R. Investigating the effect of nickel oxide nanoparticles on cellular oxidative stress in Carassius auratus. Exp. Animal Biol. 2023, 1, 1–14. Available online: https://eab.journals.pnu.ac.ir/article_10129_e538e86a828af96569907bfc414b22ce.pdf?lang=en (accessed on 30 June 2025).
- Oukarroum, A.; Barhoumi, L.; Samadani, M.; Dewez, D. Toxic Effects of Nickel Oxide Bulk and Nanoparticles on the Aquatic Plant Lemna gibba L. Biomed. Res. Int. 2015, 2015, 501326. [Google Scholar] [CrossRef]
- Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Toxic effects of nickel oxide (NiO) nanoparticles on the freshwater alga Pseudokirchneriella subcapitata. Aquat. Toxicol. 2018, 204, 80–90. [Google Scholar] [CrossRef]
- Djebbi, E.; Bonnet, D.; Pringault, O.; Tlili, K.; Yahia, M.N.D. Effects of nickel oxide nanoparticles on survival, reproduction, and oxidative stress biomarkers in the marine calanoid copepod Centropages ponticus under short-term exposure. Environ. Sci. Pollut. Res. 2021, 28, 21978–21990. [Google Scholar] [CrossRef]
- Mouneyrac, C.; Buffet, P.E.; Poirier, L.; Zalouk-Vergnoux, A.; Guibbolini, M.; Faverney, C.R.D.; Gilliland, D.; Berhanu, D.; Dybowska, A.; Châtel, A.; et al. Fate and effects of metal-based nanoparticles in two marine invertebrates, the bivalve mollusc Scrobicularia plana and the annelid polychaete Hediste diversicolor. Environ. Sci. Pollut. Res. 2014, 21, 7899–7912. [Google Scholar] [CrossRef]
- Zhang, J.F.; Liu, H.; Sun, Y.Y.; Wang, X.R.; Wu, J.C.; Xue, Y.Q. Responses of the antioxidant defenses of the goldfish Carassius auratus, exposed to 2,4-dichlorophenol. Environ. Toxicol. Pharmacol. 2005, 19, 185–190. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Oliva, M.; Vicente, J.J.; Gravato, C.; Guilhermino, L.; María Dolores Galindo-Riaño, M.D. Oxidative stress biomarkers in Senegal sole, Solea senegalensis, to assess the impact of heavy metal pollution in a Huelva estuary (SW Spain): Seasonal and spatial variation. Ecotox. Environ. Saf. 2012, 75, 151–162. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alrokayan, S.A. MgO nanoparticles cytotoxicity caused primarily by GSH depletion in human lung epithelial cells. J. Trace Elem. Med. Biol. 2018, 50, 283–290. [Google Scholar] [CrossRef]
- Kulasza, M.; Lidia Skuza, L. Changes of Gene Expression Patterns from Aquatic Organisms Exposed to Metal Nanoparticles. Int. J. Environ. Res. Public Health. 2021, 18, 8361. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Larsen, P.S. Physiologically regulated valve-closure makes mussels long-term starvation survivors: Test of hypothesis. J. Molluscan Stud. 2015, 81, 303–307. [Google Scholar] [CrossRef]
- Fdil, A.; Mouabad, M.; Outzourhit, A.; Benhra, A.; Maarouf, A.; Pihan, J.C. Valve movement response of the mussel Mytilus galloprovincialis to metals (Cu, Hg, Cd and Zn) and phosphate industry effluents from Moroccan Atlantic coast. Ecotoxicology 2006, 15, 477–486. [Google Scholar] [CrossRef]
- Ayad, M.A.; Fdil, M.A.; Mouabad, A. Effects of Cypermethrin (Pyrethroid Insecticide) on the Valve Activity Behavior, Byssal Thread Formation, and Survival in Air of the Marine Mussel Mytilus galloprovincialis. Arch. Environ. Contam. Toxicol. 2011, 60, 462–470. [Google Scholar] [CrossRef]
- Redmond, K.J.; Berry, M.; Pampanin, D.M.; Andersen, O.K. Valve gape behaviour of mussels (Mytilus edulis) exposed to dispersed crude oil as an environmental monitoring endpoint. Mar. Poll. Bull. 2017, 117, 330–339. [Google Scholar] [CrossRef]
- Durier, G.; Nadalini, J.B.; Saint-Louis, R.; Genard, B.; Comeau, L.A.; Tremblay, R. Sensitivity to oil dispersants: Effects on the valve movements of the blue mussel Mytilus edulis and the giant scallop Placopecten magellanicus, in sub-arctic conditions. Aquat. Toxicol. 2021, 234, 105797. [Google Scholar] [CrossRef]
- Canli, E.G.; Canli, M. Antioxidant system biomarkers of freshwater mussel (Unio tigridis) respond to nanoparticles (Al2O3, CuO, TiO2) exposures. Biomarkers 2021, 26, 434–442. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox. Environ. Safe 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Livingstone, D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001, 42, 656–666. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Sousa, V.S.; Teixeira, M.R.; Pinheiro, J.P.; Bebianno, M.J. Effects of silver nanoparticles exposure in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2014, 101, 208–214. [Google Scholar] [CrossRef]
- Gambardella, C.; Mesarič, T.; Milivojević, T.; Sepčić, K.; Gallus, L.; Carbone, S.; Ferrando, S.; Faimali, M. Effects of selected metal oxide nanoparticles on Artemia salina larvae: Evaluation of mortality and behavioural and biochemical responses. Environ. Monit. Assess. 2014, 186, 4249–4259. [Google Scholar] [CrossRef]
- Janssens, B.J.; Childress, J.J.; Baguet, F.; Rees, J.F. Reduced enzymatic antioxidative defense in deep-sea fish. J. Exp. Biol. 2000, 203, 3717–3725. [Google Scholar] [CrossRef]
- Nunes, B.; Vidal, D.; Barbosa, I.; Soares, A.M.V.M.; Freitas, R. Pollution efects on biochemical pathways determined in the polychaete Hediste diversicolor collected in three Portuguese estuaries. Environ. Sci. Processes Impacts 2016, 18, 1208–1219. [Google Scholar] [CrossRef]
- Ahamed, M. Toxic response of nickel nanoparticles in human lung epithelial A549 cells. Toxicol. Vitr. 2011, 25, 930–936. [Google Scholar] [CrossRef]
- Horie, M.; Fukui, H.; Nishio, K.; Endoh, S.; Kato, H.; Fujita, K.; Miyauchi, A.; Nakamura, A.; Shichiri, M.; Ishida, N.; et al. Evaluation of Acute Oxidative Stress Induced by NiO Nanoparticles In Vivo and In Vitro. J. Occup. Health 2011, 53, 64–74. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, D.; Alakhtani, S.; Suhaibani, E.S.; Al-Qahtani, A. Reactive Oxygen Species-Mediated DNA Damage and Apoptosis in Human Skin Epidermal Cells After Exposure to Nickel Nanoparticles. Biol. Trace Elem. Res. 2014, 157, 84–93. [Google Scholar] [CrossRef]
- Misra, M.; Rodriguez, R.E.; Kasprzak, K.S. Nickel induced lipid peroxidation in the rat: Correlation with nickel effect on antioxidant defense systems. Toxicology 1990, 64, 1–17. [Google Scholar] [CrossRef]
- Anoosha, F.; Seyedalipour, B.; Hoseini, S. Toxicity of Nickel Nanoparticles and Nickel Chloride on Activity of Antioxidant Enzymes and Level of Lipid Peroxidation in Liver and Serum of Rats. Iran South Med. J. 2020, 23, 14–26. Available online: https://ismj.bpums.ac.ir/article-1-1239-en.html (accessed on 30 June 2025). [CrossRef]
- Gallo, A.; Boni, R.; Buttino, I.; Tosti, E. Spermiotoxicity of nickel nanoparticles in the marine invertebrate Ciona intestinalis (ascidians). Nanotoxicol. 2016, 10, 1096–1104. [Google Scholar] [CrossRef]
- Gong, N.; Shao, K.; Che, C.; Sun, Y. Stability of nickel oxide nanoparticles and its influence on toxicity to marine algae Chlorella vulgaris. Mar. Poll. Bull. 2019, 149, 110532. [Google Scholar] [CrossRef]
- Richter, C. Biophysical consequences of lipid peroxidation in membranes. Chem. Phys. Lipids 1987, 44, 175–189. [Google Scholar] [CrossRef]
- Catalá, A.; Díaz, M. Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. Available online: https://www.frontiersin.org/journals/physiology/articles/10.3389/fphys.2016.00423/full (accessed on 30 June 2025). [CrossRef]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Feher, C.; Sasson, S.; et al. Pathological aspects of lipid peroxidation. Free. Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, A.T.; Motta, E.; Daniel, D.; Nunes, B.; Neves, J. Ecotoxicological Effects of Environmentally Relevant Concentrations of Nickel Nanoparticles on Aquatic Organisms from Three Trophic Levels: Insights from Oxidative Stress Biomarkers. J. Xenobiot. 2025, 15, 112. https://doi.org/10.3390/jox15040112
Correia AT, Motta E, Daniel D, Nunes B, Neves J. Ecotoxicological Effects of Environmentally Relevant Concentrations of Nickel Nanoparticles on Aquatic Organisms from Three Trophic Levels: Insights from Oxidative Stress Biomarkers. Journal of Xenobiotics. 2025; 15(4):112. https://doi.org/10.3390/jox15040112
Chicago/Turabian StyleCorreia, Alberto Teodorico, Eduardo Motta, David Daniel, Bruno Nunes, and José Neves. 2025. "Ecotoxicological Effects of Environmentally Relevant Concentrations of Nickel Nanoparticles on Aquatic Organisms from Three Trophic Levels: Insights from Oxidative Stress Biomarkers" Journal of Xenobiotics 15, no. 4: 112. https://doi.org/10.3390/jox15040112
APA StyleCorreia, A. T., Motta, E., Daniel, D., Nunes, B., & Neves, J. (2025). Ecotoxicological Effects of Environmentally Relevant Concentrations of Nickel Nanoparticles on Aquatic Organisms from Three Trophic Levels: Insights from Oxidative Stress Biomarkers. Journal of Xenobiotics, 15(4), 112. https://doi.org/10.3390/jox15040112






