Impact of Caffeine on Aquatic Ecosystems: Assessing Trophic-Level Biological Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Caffeine and Test Concentrations
2.2. Organisms, Culture Maintenance, and Bioassays
2.2.1. Aliivibrio fischeri
2.2.2. Raphidocelis subcapitata
2.2.3. Lemna minor
Sub-Individual Assessment of L. minor
2.2.4. Chironomus riparius
Sub-Individual Assessment of C. riparius
2.3. Statistical Analysis
2.4. Species Sensitivity Distribution (SSD)
3. Results
3.1. Aliivibrio fischeri
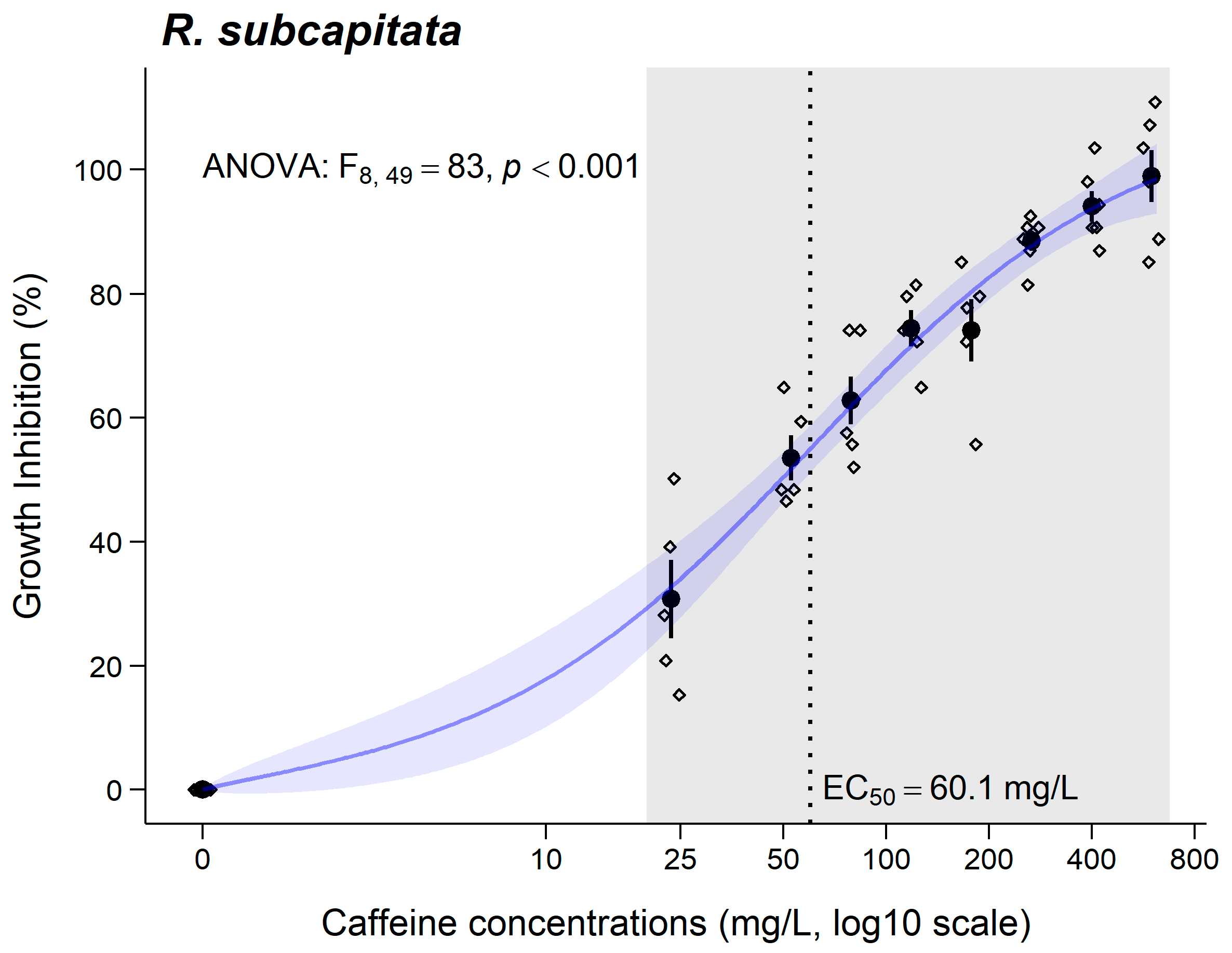
3.2. Raphidocelis subcapitata
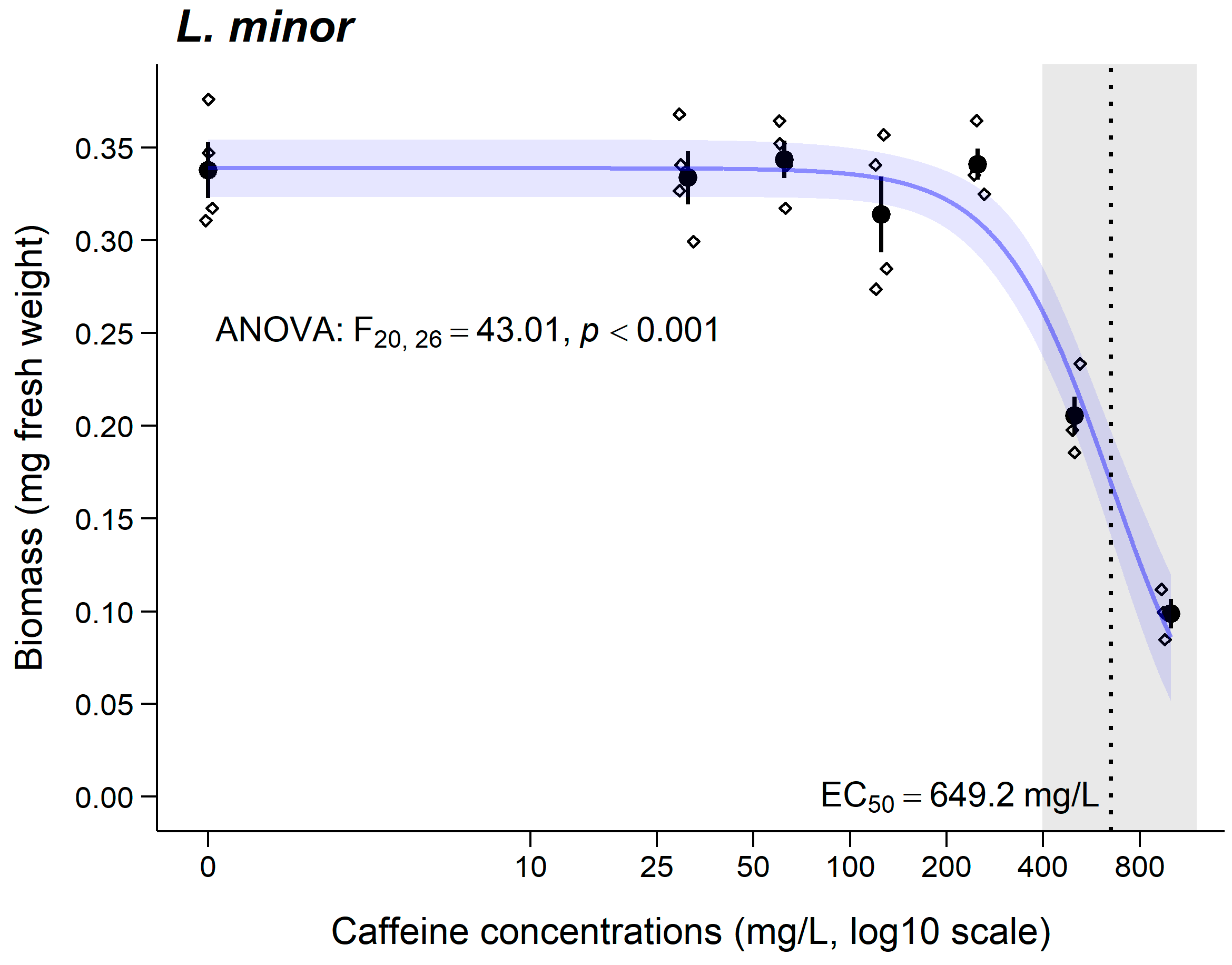
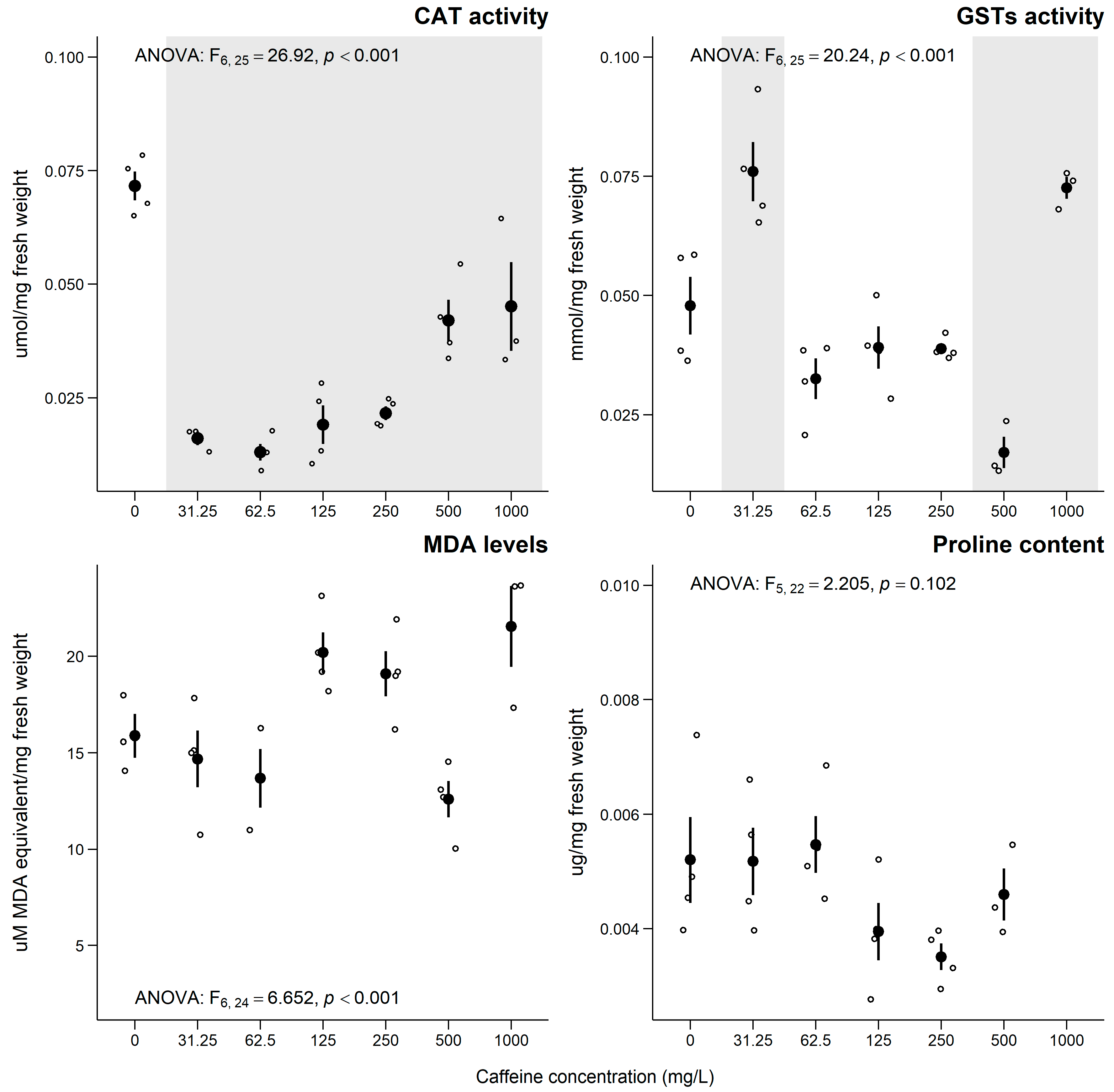
3.3. Lemna minor
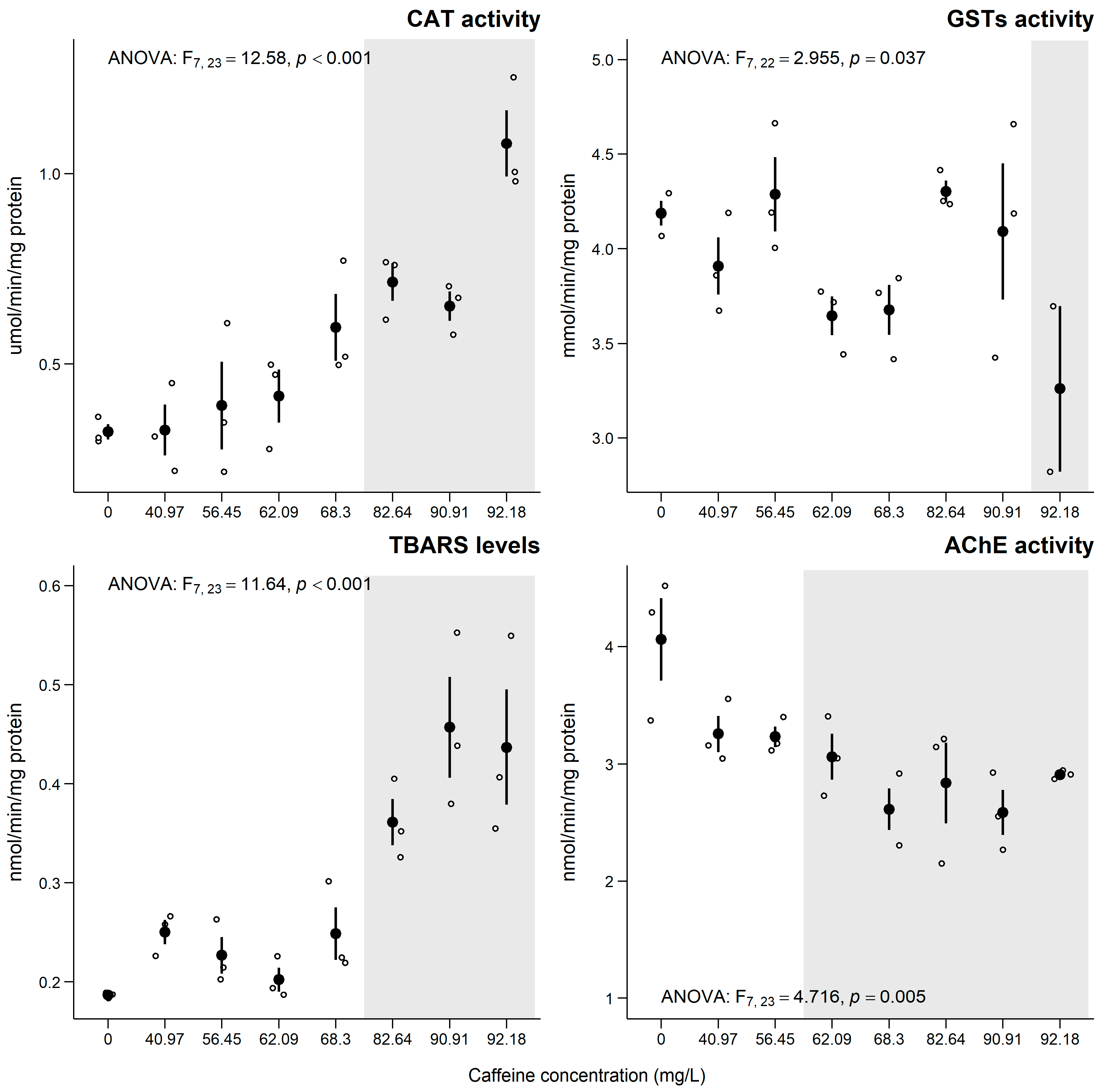
3.4. Chironomus riparius
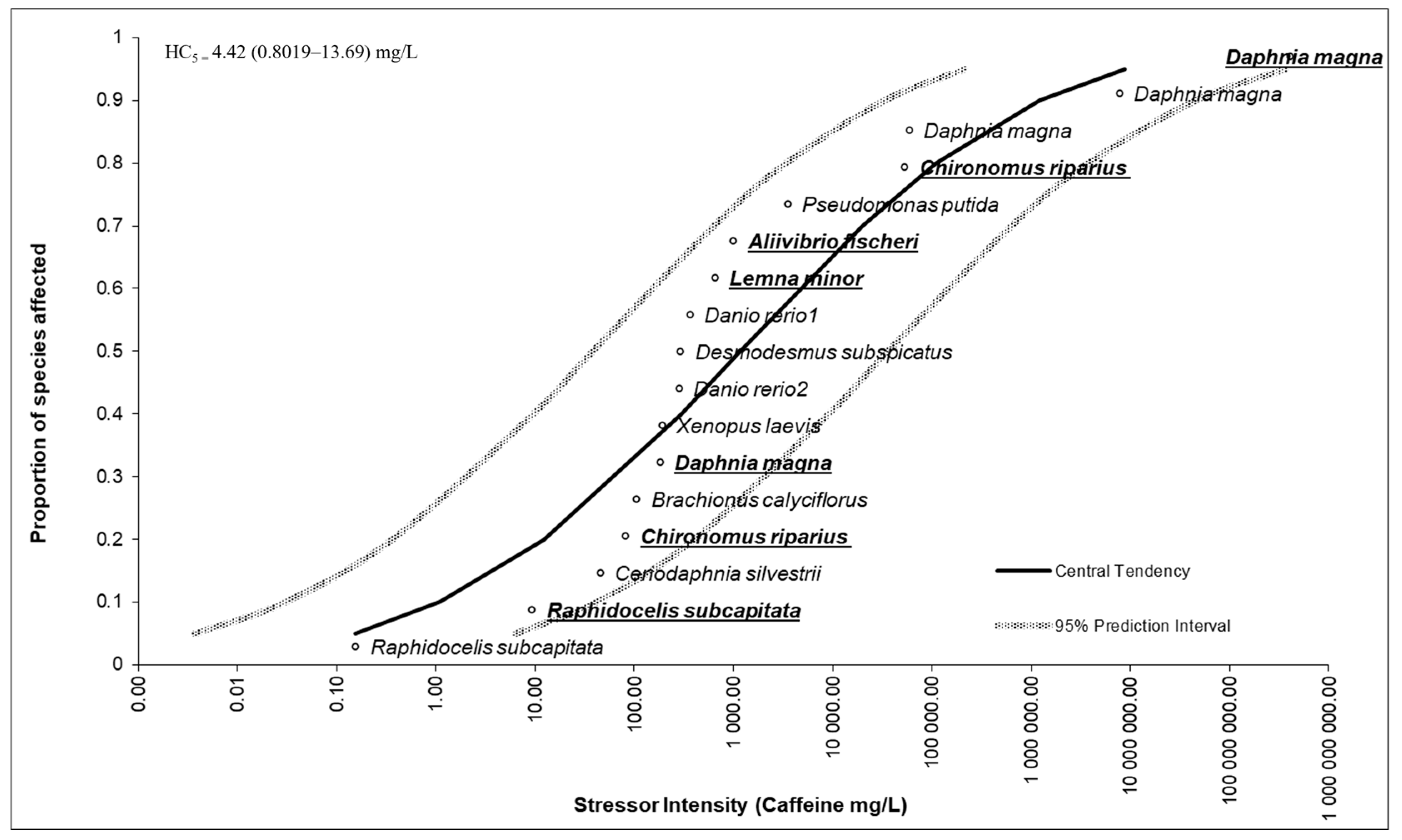
3.5. Species Sensitivity Distribution Outcomes
4. Discussion
4.1. Aliivibrio fischeri
4.2. Raphidocelis subcapitata
4.3. Lemna minor
4.4. Chironomus riparius
4.5. Species Sensitivity Distribution Outcomes
4.6. Implications for Environmental Risk Assessment and Regulatory Context
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.K.; Sharma, A.; Verma, K.K.; Gaur, P.K.; Kaushik, R.; Abdali, B. A comprehensive review on pharmacological potentials of caffeine. J. Appl. Pharm. Sci. Res. 2023, 6, 16–26. [Google Scholar] [CrossRef]
- Hawash, H.B.; Moneer, A.A.; Galhoum, A.A.; Elgarahy, A.M.; Mohamed, W.A.; Samy, M.; El-Seedi, H.R.; Gaballah, M.S.; Mubarak, M.F.; Attia, N.F. Occurrence and spatial distribution of pharmaceuticals and personal care products (PPCPs) in the aquatic environment, their characteristics, and adopted legislations. J. Water Process Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- Moore, M.T.; Greenway, S.L.; Farris, J.L.; Guerra, B. Assessing caffeine as an emerging environmental concern using conventional approaches. Arch. Environ. Contam. Toxicol. 2008, 54, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, E.; Färbinger, A.; König, J. Determination of the caffeine contents of various food items within the Austrian market and validation of a caffeine assessment tool (CAT). Food Addit. Contam. Part A 2012, 29, 1849–1860. [Google Scholar] [CrossRef]
- Korekar, G.; Kumar, A.; Ugale, C. Occurrence, fate, persistence and remediation of caffeine: A review. Environ. Sci. Pollut. Res. 2020, 27, 34715–34733. [Google Scholar] [CrossRef]
- Edwards, Q.A.; Kulikov, S.M.; O’Neale, L.D.G. Caffeine in surface and wastewaters in Barbados, West Indies. Springer Plus 2015, 4, 57. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of caffeine on mesoporous activated carbon fibers prepared from pineapple plant leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef]
- Hillebrand, O.; Nödler, K.; Licha, T.; Sauter, M.; Geyer, T. Caffeine as an indicator for the quantification of untreated wastewater in karst systems. Water Res. 2012, 46, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Edwards, Q.A.; Sultana, T.; Kulikov, S.M.; Garner-O’neale, L.D.; Yargeau, V.; Metcalfe, C.D. Contaminants of Emerging Concern in Wastewaters in Barbados, West Indies. Bull. Environ. Contam. Toxicol. 2018, 101, 1–6. [Google Scholar] [CrossRef]
- Mirasole, C.; Di Carro, M.; Tanwar, S.; Magi, E. Liquid chromatography-tandem mass spectrometry and passive sampling: Powerful tools for the determination of emerging pollutants in water for human consumption. J. Mass Spectrom. 2016, 51, 814–820. [Google Scholar] [CrossRef]
- de Jesus Gaffney, V.; Almeida, C.M.M.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Cardoso, V.V. Occurrence of pharmaceutical in a water supply system and related human health risk assessment. Water Res. 2015, 72, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2022, 424, 127284. [Google Scholar] [CrossRef]
- Li, S.; Wen, J.; He, B.; Wang, J.; Hu, X.; Liu, J. Occurrence of caffeine in the freshwater environment: Implications for ecopharmacovigilance. Environ. Pollut. 2020, 263, 114371. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.A.; Quadra, G.R.; Almeida, R.M. Sublethal effects of environmental concentrations of caffeine on a neotropical freshwater fish. Ecotoxicology 2022, 31, 161–167. [Google Scholar] [CrossRef]
- Muñoz-Peñuela, M.; Moreira, R.G.; Gomes, A.D.O.; Tolussi, C.E.; Branco, G.S.; Pinheiro, J.P.S.; Zampieri, R.A.; Nostro, F.L.L. Neurotoxic, biotransformation, oxidative stress and genotoxic effects in Astyanax altiparanae (Teleostei, Characiformes) males exposed to environmentally relevant concentrations of diclofenac and/or caffeine. Environ. Toxicol. Pharmacol. 2022, 91, 103821. [Google Scholar] [CrossRef]
- Vieira, L.R.; Soares, A.M.V.M.; Freitas, R. Caffeine as a contaminant of concern: A review on concentrations and impacts in marine coastal systems. Chemosphere 2022, 286, 131675. [Google Scholar] [CrossRef]
- Oliveira, T.S.; Murphy, M.; Mendola, N.; Wong, V.; Carlson, D.; Waring, L. Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci. Total Environ. 2015, 518, 459–478. [Google Scholar] [CrossRef]
- Nödler, K.; Tsakiri, M.; Aloupi, M.; Gatidou, G.; Stasinakis, A.S.; Licha, T. Evaluation of polar organic micropollutants as indicators for wastewater-related coastal water quality impairment. Environ. Pollut. 2016, 211, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Diogo, B.S.; Antunes, S.C.; Pinto, I.; Amorim, J.; Teixeira, C.; Teles, L.O.; Golovko, O.; Žlábek, V.; Carvalho, A.P.; Rodrigues, S. Insights into environmental caffeine contamination in ecotoxicological biomarkers and potential health effects of Danio rerio. Heliyon 2023, 9, e19875. [Google Scholar] [CrossRef]
- Zarrelli, A.; DellaGreca, M.; Iesce, M.R.; Lavorgna, M.; Temussi, F.; Schiavone, L.; Criscuolo, E.; Parrella, A.; Previtera, L.; Isidori, M. Ecotoxicological evaluation of caffeine and its derivates from a simulated shlorination step. Sci. Total Environ. 2014, 470–471, 453–458. [Google Scholar] [CrossRef]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, W. Removal of selected pharmaceuticals and personal care products in reclaime water during simulated managed aquifer recharge. Sci. Total Environ. 2018, 640–641, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Rood, S.; Smith, B.D.; Proctor, K.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Biotic phase micropollutant distribution in horizontal sub-surface flow constructed wetlands. Sci. Total Environ. 2018, 630, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Česen, M.; Heath, D.; Krivec, M.; Košmrlj, J.; Kosjek, T.; Heath, E. Seasonal and spatial variations in the occurrence, mass loadings and removal of compounds of emerging concern in the Slovene aqueous environment and environmental risk assessment. Environ. Pollut. 2018, 242, 143–144. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, J.; Li, H.; Lin, F. A study of the microstructures and mechanical properties of Ti6A14V fabricated by SLM under vacuum. Mater. Sci. Eng. Part A 2018, 724, 1–10. [Google Scholar] [CrossRef]
- ECHA. Caffeine—Substance Information. 2022. Available online: https://chem.echa.europa.eu/100.000.329/overview?searchText=caffeine (accessed on 28 January 2025).
- EPA. Caffeine—Chemical Classification and Information Database, CCID. 2022. Available online: https://www.epa.govt.nz/database-search/chemical-classification-and-information-database-ccid/view/40D455B8-C49E-4D54-968A-28BA7285843E (accessed on 28 January 2025).
- OECD. Caffeine—OECD Existing Chemicals Database. 2012. Available online: https://www.oecd.org/chemicalsafety/testing/48532204.pdf (accessed on 12 January 2025).
- Aguirre-Martínez, G.; Owuor, M.; Garrido-Pérez, C.; Salamanca, M.; Del Valls, T.; Martín-Díaz, M. Are standard tests sensitive enough to evaluate effects of human pharmaceuticals in aquatic biota? Facing changes in research approaches when performing risk assessment of drugs. Chemosphere 2015, 120, 75–85. [Google Scholar] [CrossRef]
- Cruz, D.; Almeida, Â.; Calisto, V.; Esteves, V.I.; Schneider, R.J.; Wrona, F.J.; Soares, A.M.; Figueira, E.; Freitas, R. Caffeine impacts in the clam Ruditapes philippinarum: Alterations on energy reserves, metabolic activity and oxidative stress biomarkers. Chemosphere 2016, 160, 95–103. [Google Scholar] [CrossRef]
- Pires, A.; Almeida, Â.; Calisto, V.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Soares, A.M.; Figueira, E.; Freitas, R. Hediste diversicolor as bioindicator of pharmaceutical pollution: Results from single and combined exposure to carbamazepine and caffeine. Comp. Biochem. Physicology Part C Toxicol. Pharmacol. 2016, 188, 30–38. [Google Scholar] [CrossRef]
- Pires, A.; Almeida, Â.; Calisto, V.; Schneider, R.J.; Esteves, V.I.; Wrona, F.J.; Soares, A.M.; Figueira, E.; Freitas, R. Long-term exposure of polychaetes to caffeine: Biochemical alterations induced in Diopatra neapolitana and Arenicola marina. Environ. Pollut. 2016, 214, 456–463. [Google Scholar] [CrossRef]
- Kusk, K.O.; Christensen, A.M.; Nyholm, N. Algal growth inhibition test results of 425 organic chemicals substances. Chemosphere 2018, 204, 405–412. [Google Scholar] [CrossRef]
- Lomba, L.; Lapeña, D.; Ros, N.; Aso, E.; Cannavò, M.; Errazquin, D.; Giner, B. Ecotoxicological study of six drugs in Aliivibrio fischeri, Daphnia magna and Raphidocelis subcapitata. Environ. Sci. Pollut. Res. 2020, 27, 9891–9900. [Google Scholar] [CrossRef] [PubMed]
- Diniz, V.; Rath, G.; Rath, S.; Rodrigues-Silva, C.; Guimarães, J.R.; Cunha, D.G. Long-term ecotoxicological effects of ciprofloxacin in combination with caffeine on the microalga Raphidocelis subcapitata. Toxicol. Rep. 2021, 8, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Morales, D.; Fajardo-Romero, D.; Rodríguez-Rodríguez, C.E.; Cedergreen, N. Single and mixture toxicity of selected pharmaceuticals to the aquatic macrophyte Lemna minor. Ecotoxicology 2022, 31, 714–724. [Google Scholar] [CrossRef]
- Teixeira, C.; Rodrigues, S.; Amorim, J.; Diogo, B.S.; Pinto, I.; Carvalho, A.P.; Antunes, S.C.; Teles, L.O. Comprehensive Machine Learning Assessment of Zebrafish Behaviour and Biochemical Markers in Response to Caffeine Exposure. Ecotoxicology Online ahead of print. 2025. [Google Scholar] [CrossRef] [PubMed]
- Microbics, M. Microtox Manual—A Toxicity Handbook; Microbics Corporation. Inc.: Carlsbad, CA, USA, 1992; Volume I–IV. [Google Scholar]
- Christensen, D.G.; Visick, K.L. Vibrio fischeri: Laboratory Cultivation, Storage, and Common Phenotypic Assays. Curr. Protoc. Microbiol. 2020, 57, e103. [Google Scholar] [CrossRef] [PubMed]
- Septer, A.N.; Visick, K.L. Lighting the way: How the Vibrio fischeri model microbe reveals the complexity of Earth’s "simplest" life forms. J. Bacteriol. 2024, 206, e0003524. [Google Scholar] [CrossRef]
- Fuzinatto, C.F. Avaliação da Qualidade da Água de Rios Localizados na Ilha de Santa Catarina Utilizando Parâmetros Toxciológicos e o Índice de Qualidade de Água. Doctoral Dissertation, Universidade Federal de Santa Catarina, Centro Tecnológico, Programa de Pós-Graduação em Engenharia Ambiental, Florianópolis, Brazil, 2009. [Google Scholar]
- Stein-Taylor, J.R. (Ed.) Handbook of Phycological Methods—Culture Methods and Growth Measurements; Cambridge University Press: Cambridge, UK, 1973; Volume 1. [Google Scholar]
- Pinto, I.; Rodrigues, S.; Lage, O.; Antunes, S. Assessment of water quality in Aguieira reservoir: Ecotoxicological tools in addition to the Water Framework Directive. Ecotoxicol. Environ. Saf. 2021, 208, 111583. [Google Scholar] [CrossRef]
- OECD. Freshwater Algae and Cyanobacteria, Growth Inhibition Test. No. 201; OECD: Paris, France, 2011. [Google Scholar]
- Nunes, B.; Pinto, G.; Martins, L.; Gonçalves, F.; Antunes, S.C. Biochemical and standard toxic effects of acetaminophen on the macrophyte species Lemna minor and Lemna gibba. Environ. Sci. Pollut. Res. 2014, 21, 10815–10822. [Google Scholar] [CrossRef]
- OECD. Guidelines for the Testing of Chemicals, Section 2. No. 221; OECD: Paris, France, 2006. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Doria, H.B.; Caliendo, C.; Gerber, S.; Pfenninger, M. Photoperiod is an important seasonal selection factor in Chironomus riparius (Diptera: Chironomidae). Biol. J. Linn. Soc. 2022, 135, 277–290. [Google Scholar] [CrossRef]
- Foucault, Q.; Wieser, A.; Waldvogel, A.; Pfenninger, M. Establishing laboratory cultures and performing ecological and evolutionary experiments with the emerging model species Chironomus riparius. J. Appl. Entomol. 2019, 143, 584–592. [Google Scholar] [CrossRef]
- Osborne, S.; Hurrell, S.; Simkiss, K.; Leidi, A. Factors influencing the distribution and feeding of the larvae of Chironomus riparius. Entomol. Exp. Et Appl. 2003, 94, 67–73. [Google Scholar] [CrossRef]
- OECD. Validation Report of the Chironomid Full Life-Cycle Toxicity Test. Series on Testing and Assessment No. 136, ENV/JM/MONO (2011) 35; OECD: Paris, France, 2011. [Google Scholar]
- OECD. Guidelines for the Testing of Chemicals, Section 2. No. 219; OECD: Paris, France, 2004. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Streibig, J.C. Bioassay Analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef]
- Ritz, C. Toward a unified approach to dose-response modelling in ecotoxicology. Environ. Toxicol. Chem. 2010, 29, 220–229. [Google Scholar] [CrossRef]
- EC. Commission of the European Communities: Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for Existing Substances, Part II-Environmental Risk Assessment (Brussels, Belgium). 1996. Available online: https://op.europa.eu/en/publication-detail/-/publication/212940b8-3e55-43f8-8448-ba258d0374bb (accessed on 12 January 2025).
- Aldenberg, T.; Luttik, R. Extrapolation factors for tiny toxicity data sets from species sensitivity distributions with know standard deviation. In Species Sensitivity Distributions in Ecotoxicology, 1st ed.; CRC Press: Boca Raton, FL, USA; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 127–142. [Google Scholar]
- Di Lorenzo, T.; Castaño-Sánchez, A.; Di Marzio, W.D.; García-Doncel, P.; Martínez, L.N.; Galassi, D.M.P.; Iepure, S. The role of freshwater copepods in the environmental risk assessment of caffeine and propranolol mixtures in the surface water bodies of Spain. Chemosphere 2019, 220, 227–236. [Google Scholar] [CrossRef]
- de Farias, N.O.; Andrade, T.d.S.; Santos, V.L.; Galvino, P.; Suares-Rocha, P.; Domingues, I.; Grisolia, C.K.; Oliveira, R. Neuromotor activity inhibition in zebrafish early-life stages after exposure to environmental relevant concentrations of caffeine. J. Environ. Sci. Health Part A 2021, 56, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Caldas, L.L.; Moreira, R.A.; Espíndola, E.L.G.; Novelli, A. Environmental Risk Assessment of Drugs in Tropical Freshwaters Using Ceriodaphnia silvestrii as Test Organism. Bull. Environ. Contam. Toxicol. 2023, 110, 106. [Google Scholar] [CrossRef]
- Kobetičová, K.; Losonszky, G.; Pařízek, O. Effects of caffeine on plants. Proceedings of Scientific Articles—Influence of Abiotic and Biotic Stresses on Properties of Plants; Czech University of Life Science Prague Institute of Forest Ecology SAS: Prague, Czech, 2015; pp. 166–168. [Google Scholar]
- Harm, W. Analysis of photoenzymatic repair of UV lesions in DNA by single light flashes VIII. Inhibition of photoenzymatic repair of UV lesions in E. coli DNA by caffeine. Mutat. Res. / Fundam. Mol. Mech. Mutagen. 1970, 10, 319–333. [Google Scholar] [CrossRef]
- Cali, J.J.; Niles, A.; Valley, M.P.; A O’Brien, M.; Riss, T.L.; Shultz, J. Bioluminescent assays for ADMET. Expert Opinion on Drug Metab. Toxicol. 2008, 4, 103–120. [Google Scholar] [CrossRef]
- Callahan, S.M.; Cornell, N.W.; Dunlap, P.V. Purification and properties of periplasmic 3’: 5’-cyclic nucleotide phosphodiesterase: A novel zinc-containing enzyme from the marine symbiotic bacterium Vibrio fischeri. J. Biol. Chem. 1995, 270, 17627–17632. [Google Scholar] [CrossRef]
- McCall, J.R.; Brown, A.P.; Sausman, K.T.; McCall, S.H. Microalgae nanotechnology and drug development. In Handbook of Microbial Nanotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 169–190. [Google Scholar] [CrossRef]
- Johansen, M.N. (Ed.) Microalgae: Biotechnology, Microbiology and Energy; Science Publishers: Rawalpindi, Pakistan, 2012. [Google Scholar]
- Machado, M.D.; Soares, E.V. Features of the microalga Raphidocelis subcapitata: Physiology and applications. Appl. Microbiol. Biotechnol. 2024, 108, 219. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.; Watts, C.; Boucard, T. Chronic aquatic environmental risks from exposure to human pharmaceuticals. Sci. Total Environ. 2006, 367, 23–41. [Google Scholar] [CrossRef]
- Melis, A.; Harvey, G.W. Regulation of photosystem stoichiometry, chlorophyll a and chlorophyll b content and relation to chloroplast ultrastructure. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1981, 637, 138–145. [Google Scholar] [CrossRef]
- Dale, M.P.; Causton, D.R. Use of the chlorophyll a/b ratio as a bioassay for the light environment of a plant. Funct. Ecol. 1992, 6, 190–196. [Google Scholar] [CrossRef]
- Souza, L.R.R.; Bernardes, L.E.; Barbetta, M.F.S.; da Veiga, M.A.M.S. Iron oxid nanoparticle phytotoxicity to the aquatic plant Lemna minor: Effect on reactive oxygen species (ROS) production and chlorophyll a/chlorophyll b ratio. Environ. Sci. Pollut. Res. 2019, 26, 24121–24131. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, R.C.; Rodrigues-Silva, C.; Ribeiro, A.R.; Rath, S. Tracking the occurrence of psychotropic pharmaceuticals in Brasilian wastewater treatment plants and treatment plants and surface water, with assessment of environmental risks. Sci. Total Environ. 2020, 727, 138661. [Google Scholar] [CrossRef]
- Ramírez-Morales, D.; Masís-Mora, M.; Montiel-Mora, J.R.; Cambronero-Heinrichs, J.C.; Pérez-Rojas, G.; Tormo-Budowski, R.; Méndez-Rivera, M.; Briceño-Guevara, S.; Gutiérrez-Quirós, J.A.; Arias-Mora, V.; et al. Multiresidue analysis of pharmaceuticals in water samples by liquid chromatography-mass spectrometry: Quality assessment and application to the risk assessment of urban-influenced surface waters in a metropolitan area of Central America. Process Saf. Environ. Prot. 2021, 153, 289–300. [Google Scholar] [CrossRef]
- Dogan, M.; Saygideger, S.D.; Colak, U. Effect of Lead Toxicity on Aquatic Macrophyte Elodea canadensis Michx. Bull. Environ. Contam. Toxicol. 2009, 83, 249–254. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, Q. Toxic effects of Hydrilla verticillata exposed to toluene, ethylbenzene and xylene and safety assessment for protecting aquatic macrophytes. Chemosphere 2011, 85, 1088–1094. [Google Scholar] [CrossRef]
- Fekete-Kertész, I.; Kunglné-Nagy, Z.; Gruiz, K.; Magyar, Á.; Farkas, É.; Molnár, M. Assessing Toxicity of Organic Aquatic Micropollutants Based on the Total Chlorophyll Content of Lemna minor as a Sensitive Endpoint. Period. Polytech. Chem. Eng. 2015, 59, 262–271. [Google Scholar] [CrossRef]
- Baker, A.; Lin, C.-C.; Lett, C.; Karpinska, B.; Wright, M.H.; Foyer, C.H. Catalase: A critical node in the regulation of cell fate. Free. Radic. Biol. Med. 2023, 199, 56–66. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.V.; Del Valls, T.A.; Martín-Díaz, M.L. Identification of biomarkers responsive to chronic exposure to pharmaceuticals in target tissues of Carcinus maenas. Mar. Environ. Res. 2013, 87–88, 1–11. [Google Scholar] [CrossRef]
- Li, S.; He, B.; Wang, J.; Liu, J.; Hu, X. Risks of caffeine residues in the environment: Necessity for a targeted ecopharmacovigilance program. Chemosphere 2020, 243, 125343. [Google Scholar] [CrossRef]
- Ősz, B.-E.; Jîtcă, G.; Ștefănescu, R.-E.; Pușcaș, A.; Tero-Vescan, A.; Vari, C.-E. Caffeine and Its Antioxidant Properties-It Is All about Dose and Source. Int. J. Mol. Sci. 2022, 23, 13074. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Wang, X.; Sun, G.; Gao, X.; Xu, S.; Wang, X. Effects of atrazine and chlorpyrifos on activity and transcription of glutathione S-transferase in common carp (Cyprinus carpio L.). Environ. Toxicol. Pharmacol. 2012, 33, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Raimundo, S.; De Marchi, L.; Leite, C.; Pretti, C.; Meucci, V.; Soares, A.M.V.M.; Antunes, S.C.; Freitas, R. Biochemical dynamics of the invasive clams Ruditapes philippinarum and Corbicula fluminea in response to caffeine concentrations under present and future climate scenarios. Estuar. Coast. Shelf Sci. 2025, 318, 109217. [Google Scholar] [CrossRef]
- Richardson, K.L.; Gold-Bouchot, G.; Schlenk, D. The characterization of cytosolic glutathione transferase from four species of sea turtles: Loggerhead (Caretta caretta), green (Chelonia mydas), olive ridley (Lepidochelys olivacea), and hawksbill (Eretmochelys imbricata). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 279–284. [Google Scholar] [CrossRef]
- Košpić, K.; Vitko, S.; Kobelščak, L.; Matešković, A.; Štefanić, P.P.; Dimitrov, N.; Tkalec, M.; Balen, B. Physiological responses of Lemna minor to polystyrene and polymethyl methacrylate microplastics. Curr. Plant Biol. 2025, 42, 100473. [Google Scholar] [CrossRef]
- Mattioli, R.; Constantino, P.; Trovato, M. Proline accumulation in plants: Not only stress. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Wang, C.; Liao, J.; Ni, J.; Zhang, T.; Wang, R.; Ruan, H. Growth, physiological function, and antioxidant defense system responses of Lemna minor L. to decabromodiphenyl ether (BDE-209) induced phytotoxicity. Plant Physiol. Biochem. 2019, 139, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Silva, A.M.; Antunes, S.C. Assessment of 17α-ethinylestradiol effects in Daphnia magna: Life-history traits, biochemical and genotoxic parameters. Environ. Sci. Pollut. Res. 2021, 28, 23160–23173. [Google Scholar] [CrossRef] [PubMed]
- Fiani, B.; Zhu, L.; Musch, B.L.; Briceno, S.; Andel, R.; Sadeq, N.; Ansari, A.Z. The neurophysiology of caffeine as a central nervous system stimulant and the resultant effects on cognitive function. Cureus 2021, 13, 15032. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Dobes, P. Caffeine inhibits Acetylcholinesterase, but not butyrylcholinesterase. Int. J. Mol. Sci. 2013, 14, 9873–9882. [Google Scholar] [CrossRef]
| A. fischeri | R. subcapitata | L. minor | C. riparius | ||
| Exposure time | 30 min | 3 days | 7 days | 1 day | 10 days |
| Endpoint | Bioluminescence inhibition | Growth inhibition | Biomass (fresh weight) | Mortality | Biomass (fresh weight) |
| Culture medium | Distilled water | Woods Hole MBL | Steinberg | ASTM hard water | |
| Stock solution (mg/L) | 2750 | 600 | 1000 | 2000 | 3000 |
| Nominal [CAF] (mg/L) | 4.8–2252 (dilution factor 2×) | 23.4–600 (dilution factor 1.5×) | 31.25–1000 (dilution factor 2×) | 268.4–2000 (dilution factor 1.25×) | 40.97–92.18 (dilution factor 1.1× and 1.5×) |
| Real-lowest and highest [CAF] (mg/L) | 6.0 and 2191.0 | 22.0 and 692.0 | 31.0 and 1043.0 | 206.0 and 1828.0 | 36.0 and 88.0 |
| A. fischeri | R. subcapitata | L. minor | C. riparius | ||
| E(L)C50 (CI95%) (mg/L) | 30 min 998.5 (329.9–1667) | 3 days 60.1 (34.89–85.33) | 7 days 649.2 (557.8–740.6) | 1 day LC50 = 644.5 (578.4–710.6) | 10 days 81.62 (74.27–88.97) |
| Toxicity class | Nontoxic | Hazardous | Nontoxic | - | Hazardous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, S.; Alves, R.S.; Antunes, S.C. Impact of Caffeine on Aquatic Ecosystems: Assessing Trophic-Level Biological Responses. J. Xenobiot. 2025, 15, 86. https://doi.org/10.3390/jox15030086
Rodrigues S, Alves RS, Antunes SC. Impact of Caffeine on Aquatic Ecosystems: Assessing Trophic-Level Biological Responses. Journal of Xenobiotics. 2025; 15(3):86. https://doi.org/10.3390/jox15030086
Chicago/Turabian StyleRodrigues, Sara, Rita S. Alves, and Sara C. Antunes. 2025. "Impact of Caffeine on Aquatic Ecosystems: Assessing Trophic-Level Biological Responses" Journal of Xenobiotics 15, no. 3: 86. https://doi.org/10.3390/jox15030086
APA StyleRodrigues, S., Alves, R. S., & Antunes, S. C. (2025). Impact of Caffeine on Aquatic Ecosystems: Assessing Trophic-Level Biological Responses. Journal of Xenobiotics, 15(3), 86. https://doi.org/10.3390/jox15030086








