Antioxidant Potential and Antibacterial Activities of Caucasian Endemic Plants Sempervivum transcaucasicum and Paeonia daurica subsp. mlokosewitschii Extracts and Molecular In Silico Mechanism Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Drying Processes
2.3. Extraction of Plant Samples for the Determination of Antioxidant Activity (DPPH Test and TAC Methods) and Total Phenolic and Flavonoid Content
2.4. DPPH Radical Scavenging Activity Assay
2.5. Total Antioxidant Capacity (Phosphomolybdate Assay)
2.6. Determination of Total Phenolic Content (TPC)
2.7. Determination of Total Flavonoid Content (TFC)
2.8. Extraction of Plant Samples for the Determination of Ascorbate and Glutathione Contents
2.9. Determination of Reduced (ASC) and Oxidised (DHA) Ascorbate Content
2.10. Determination of Reduced (GSH) and Oxidised (GSSG) Glutathione
2.11. Determination of Antibacterial Activity
2.12. Determination of Minimal Inhibitory Concentration (MIC)
2.13. Statistical Analysis
2.14. Molecular Docking
2.14.1. Ligand Preparation
2.14.2. HDOCK Docking
3. Results and Discussion
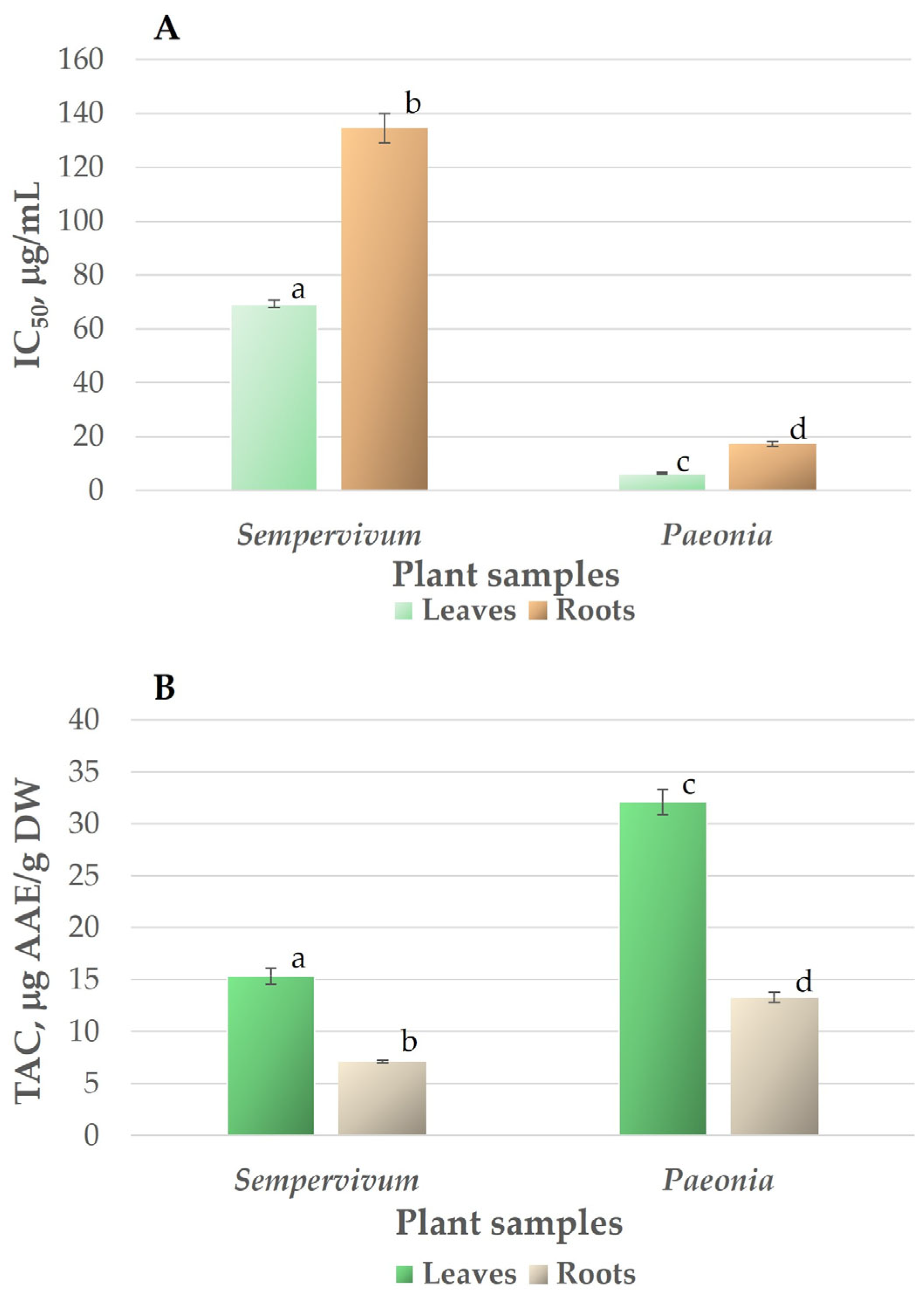
3.1. Antioxidant Activity and Total Antioxidant Capacity
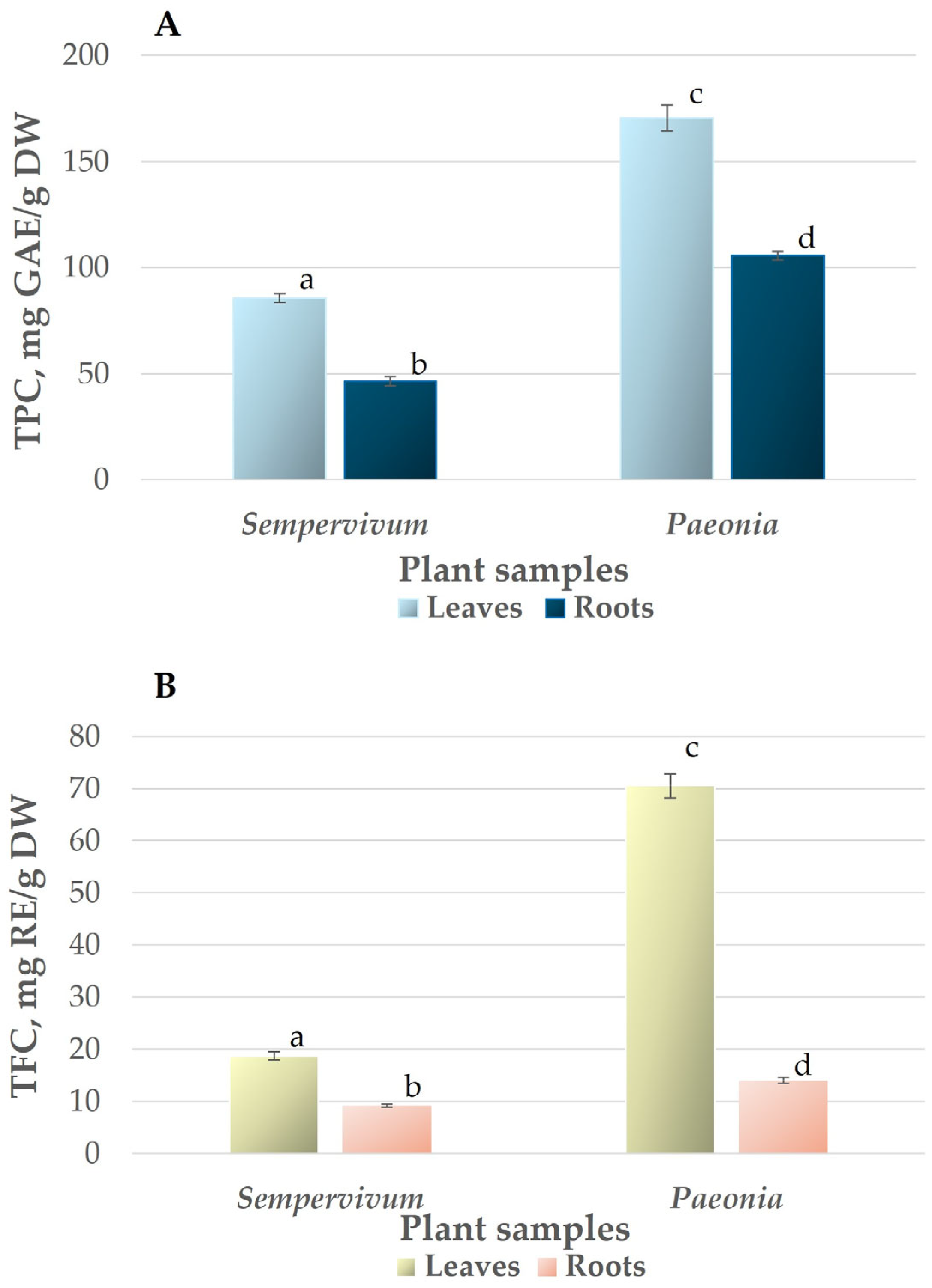
3.2. Total Phenolic and Flavonoid Content
3.3. Ascorbate and Glutathione Content
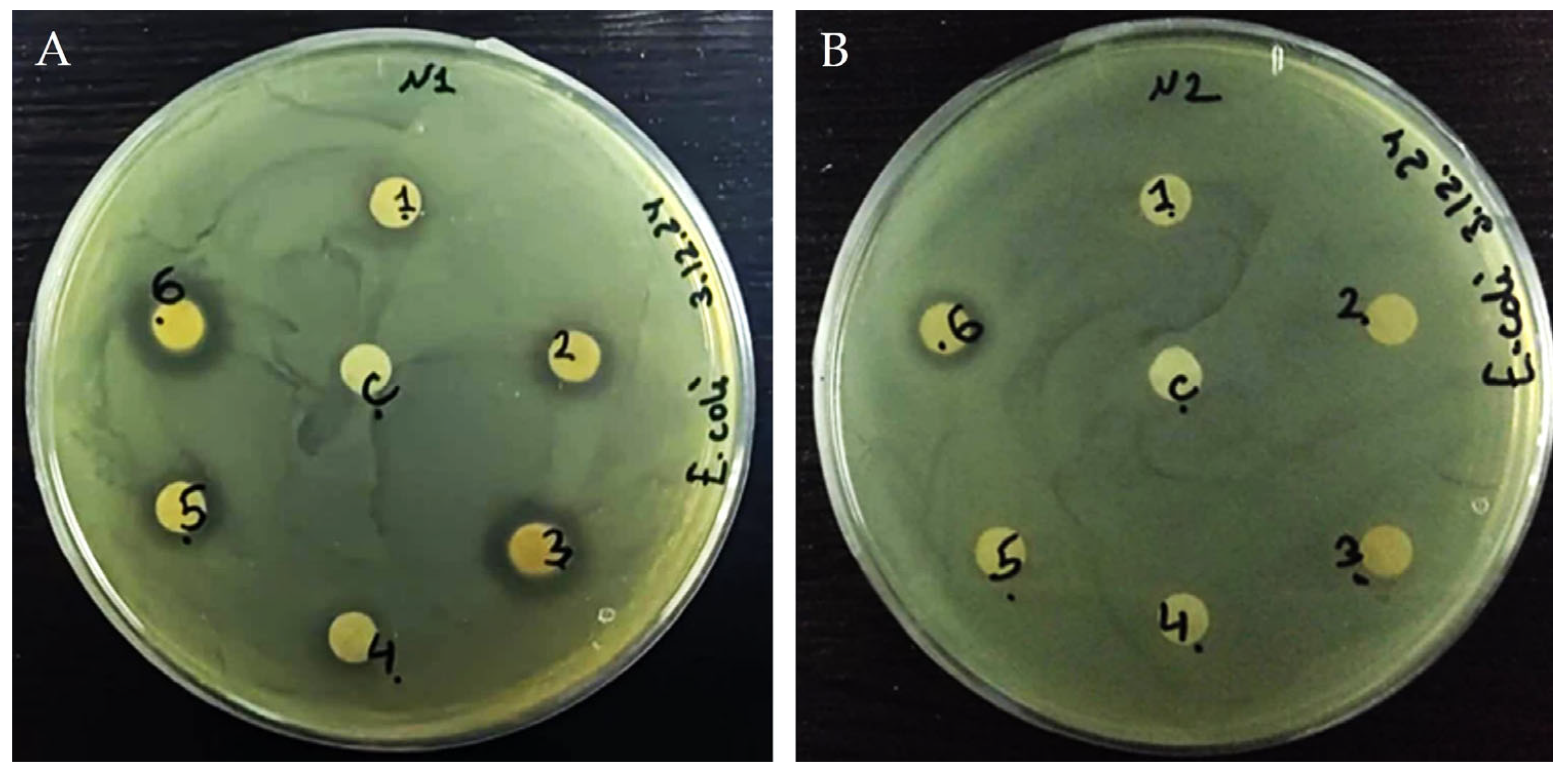
3.4. Antibacterial Activity of Plant Extracts
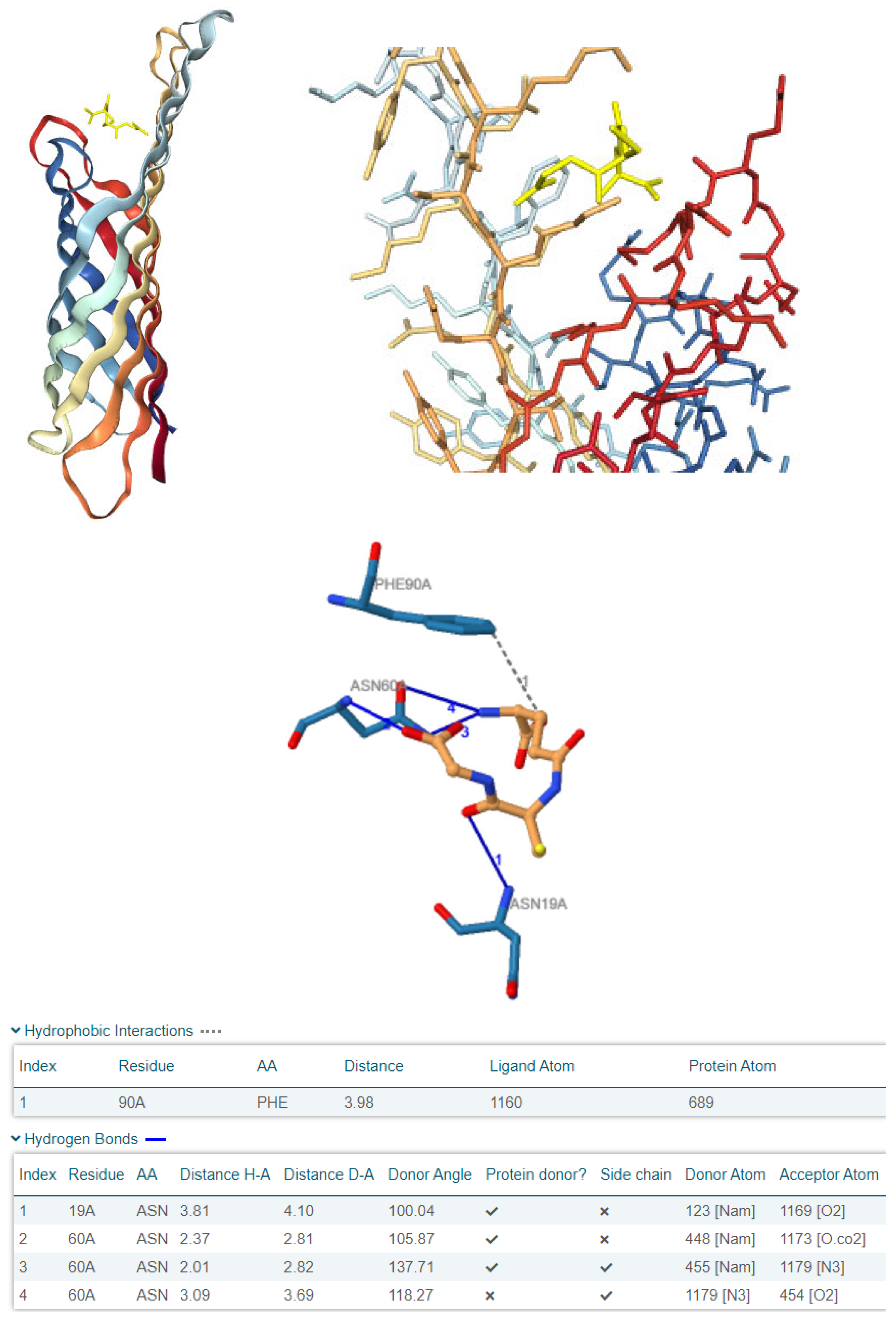
3.5. Computational Investigation of OmpX as a Molecular Target for Plant-Derived Antioxidants in E. coli
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASC | Reduced ascorbate |
| DHA | Oxidised ascorbate |
| GSH | Reduced glutathione |
| GSSG | Oxidised glutathione |
| IZ | Inhibition zone |
| MIC | Minimum inhibitory concentration |
| OmpX | Outer Membrane Protein X |
| PDB | Protein Data Bank |
| SD | Standard deviation |
| TFC | Total flavonoid content |
| TPC | Total phenolic content |
References
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative processes and xenobiotic metabolism in plants: Mechanisms of defense and potential therapeutic implications. J. Xenobiotics 2024, 14, 1541–1569. [Google Scholar] [CrossRef]
- Fryzova, R.; Pohanka, M.; Martinkova, P.; Cihlarova, H.; Brtnicky, M.; Hladky, J.; Kynicky, J. Oxidative stress and heavy metals in plants. In Reviews of Environmental Contamination and Toxicology; De Voogt, P., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Swizerland, 2017; Volume 245, pp. 129–156. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and oxidative stress: An overview of basic concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Llauradó Maury, G.; Méndez Rodríguez, D.; Hendrix, S.; Escalona Arranz, J.C.; Fung Boix, Y.; Pacheco, A.O.; García Díaz, J.; Morris-Quevedo, H.J.; Ferrer Dubois, A.; Aleman, E.I.; et al. Antioxidants in plants: A valorization potential emphasizing the need for the conservation of plant biodiversity in Cuba. Antioxidants 2020, 9, 1048. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Han, R.-M.; Zhang, J.-P.; Skibsted, L.H. Reaction dynamics of flavonoids and carotenoids as antioxidants. Molecules 2012, 17, 2140–2160. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Mittova, V.; Tsetskhladze, Z.R.; Palumbo, R.; Pastore, R.; Roviello, G.N. Georgian medicinal plants as rich natural sources of antioxidant derivatives: A review on the current knowledge and future perspectives. Curr. Med. Chem. 2024, 31, 4407–4424. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.; Mittova, V.; Motsonelidze, C.; Vakhania, M.; Vicidomini, C.; Roviello, G.N. Antimicrobial metabolites of Caucasian Medicinal plants as alternatives to antibiotics. Antibiotics 2024, 13, 487. [Google Scholar] [CrossRef]
- Sempervivum transcaucasicum. In Flora of Georgia, 2nd ed.; Chief editor N. Ketskhoveli: Tbilisi, Georgia, 1979; Volume V, pp. 240–245.
- Plants of Worlds Online. Available online: https://powo.science.kew.org/ (accessed on 5 May 2025).
- Thiede, J. Sempervivum gurgenidzeae (Crassulaceae)—An overlooked name from the Great Caucasus of Georgia. Bradleya 2017, 35, 177–179. [Google Scholar] [CrossRef]
- Bidzinashvili, R. Medicinal Plants of Spring Flora; Mtsignobari: Tbilisi, Georgia, 2021; pp. 238–239. [Google Scholar]
- Karaer, F.; Celep, F.; Eggli, U. A Taxonomic Revision of the Sempervivum davisii complex (Crassulaceae). Nord. J. Bot. 2011, 29, 49–53. [Google Scholar] [CrossRef]
- Stojković, D.; Barros, L.; Petrović, J.; Glamoclija, J.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Soković, M. Ethnopharmacological uses of Sempervivum tectorum L. in Southern Serbia: Scientific confirmation for the use against otitis linked bacteria. J. Ethnopharmacol. 2015, 176, 297–304. [Google Scholar] [CrossRef]
- Dégi, D.M.; Imre, K.; Herman, V.; Dégi, J.; Cristina, R.T.; Marcu, A.; Morariu, F.; Muselin, F. Antimicrobial activity of Sempervivum tectorum L. extract on pathogenic bacteria isolated from otitis externa of dogs. Vet. Sci. 2023, 10, 265. [Google Scholar] [CrossRef]
- Uzun, Y.; Dalar, A.; Konczak, I. Sempervivum davisii: Phytochemical composition, antioxidant and lipase-inhibitory activities. Pharm. Biol. 2017, 55, 532–540. [Google Scholar] [CrossRef]
- Paeonia mlokosewitschii. In Flora of Georgia; Metsniereba: Tbilisi, Georgia, 1973; Volume II, pp. 8–15.
- Nadiradze, T.; Eradze, N. Overview of Paeonia mlokosewitschii L. World J. Adv. Res. Rev. 2020, 6, 5–8. [Google Scholar]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukudai, Y.; Chikuda, J.; Zhang, M.; Shigemori, H.; Yazawa, K.; Kondo, S.; Shimane, T.; Shirota, T. Combinational anti-tumor effects of chemicals from Paeonia lutea leaf extract in oral squamous cell carcinoma cells. Anticancer Res. 2021, 41, 6077–6086. [Google Scholar] [CrossRef]
- Yesilada, E.; Mutlugil, A.; Sener, B. The antiinflammatory principle of the roots of Paeonia daurica. Int. J. Pharmacogn. 1992, 30, 66–70. [Google Scholar] [CrossRef]
- Ajvazi, M.; Osmani, I.; Gashi, D.; Krasniqi, E.; Zeneli, L. Radical scavenging, antioxidant and antimicrobial activity of Paeonia peregrina Mill., Paeonia mascula (L.) Mill. and Paeonia officinalis (L.). Iran. J. Chem. Chem. Eng. 2023, 42, 3835–3848. [Google Scholar]
- Tong, N.-N.; Zhou, X.-Y.; Peng, L.-P.; Liu, Z.-A.; Shu, Q.-Y. A Comprehensive study of three species of Paeonia stem and leaf phytochemicals, and their antioxidant activities. J. Ethnopharmacol. 2021, 273, 113985. [Google Scholar] [CrossRef]
- Demürboğa, G.; Demürboğa, Y.; Özbay, N. Types of Paeonia and their use in phytotherapy. Karadeniz Fen. Bilimleri. Dergisi. 2021, 11, 318–327. [Google Scholar] [CrossRef]
- Hovsepyan, R.; Stepanyan-Gandilyan, N.; Melkumyan, H.; Harutyunyan, L. Food as a marker for economy and part of identity: Traditional vegetal food of Yezidis and Kurds in Armenia. J. Ethn. Foods 2016, 3, 32–41. [Google Scholar] [CrossRef]
- Mittova, V.; Tseskhladze, Z.R.; Makalatia, K.; Bidzinashvili, R.; Mindiashvili, T.; Kobiashvili, M.; Roviello, G.N. Antioxidant and antibacterial activity of root extracts of Georgian medicinal plants obtained using different extraction methods. Mod. Issues Med. Manag. 2024, 28, 2. [Google Scholar] [CrossRef]
- Mittova, V.; Pirtskhalava, M.; Bidzinashvili, R.; Vakhania, M.; Mindiashvili, T.; Kobiashvili, M. Effects of different drying, extraction methods, and solvent polarity on the antioxidant properties of Paeonia daurica subsp. mlokosewitschii leaves. Mod. Issues Med. Manag. 2023, 26, 2. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, X. Purification, identification and evaluation of antioxidant peptides from pea protein hydrolysates. Molecules 2023, 28, 2952. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern. Med. 2012, 12, 221. [Google Scholar]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A Reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Park, H.-H.; Lee, S.; Son, H.-Y.; Park, S.-B.; Kim, M.-S.; Choi, E.-J.; Singh, T.S.K.; Ha, J.-H.; Lee, M.-G.; Kim, J.-E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef]
- Shalata, A.; Mittova, V.; Volokita, M.; Guy, M.; Tal, M. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: The root antioxidative system. Physiol. Plant. 2001, 112, 487–494. [Google Scholar] [CrossRef]
- Sahoo, S.; Awasthi, J.P.; Sunkar, R.; Panda, S.K. Determining glutathione levels in plants. In Plant Stress Tolerance; Sunkar, R., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1631, pp. 273–277. [Google Scholar]
- Mitra, S.; Bhesania Hodiwala, A.V.; Kar, H. Susceptibility and synergistic effects of guava plant extract and antimicrobial drugs on Escherichia coli. Cureus 2024, 16, e52345. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. BBA Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Lash, L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006, 163, 54–67. [Google Scholar] [CrossRef]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Alberti, Á.; Riethmüller, E.; Béni, S.; Kéry, Á. Evaluation of radical scavenging activity of Sempervivum tectorum and Corylus avellana extracts with different phenolic composition. Nat. Prod. Commun. 2016, 11, 469–474. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskienė, M.; Pukalskas, A.; Pereira, C.V.; Matias, A.A.; Venskutonis, P.R. Isolation of strong antioxidants from Paeonia officinalis roots and leaves and evaluation of their bioactivities. Antioxidants 2019, 8, 249. [Google Scholar] [CrossRef]
- Batinic, P.; Cutovic, N.; Jovanović, A.; Stojković, D.; Zengin, G.; Carević, T.; Prijić, Ž.; Marinković, A.; Marković, T. Antioxidant, antibacterial and anti-enzymatic activity of the leaf extracts of Paeonia daurica Andrews grown in Serbia. Lek. Sirovine 2023, 43, e159. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, Y.; Tao, J. Antioxidant and antibacterial activities of 13 ornamental herbaceous Peony cultivars: A comparative study with stems and leaves. N. Z. J. Crop Hortic. Sci. 2022, 50, 326–340. [Google Scholar] [CrossRef]
- Sadati Lamardi, S.N.; Taleb Kashefi, N.; Yassa, N. Phytochemical evaluation, antioxidant activity and toxicity of Paeonia daurica ssp. macrophylla root. Res. J. Pharmacogn 2018, 5, 9–15. [Google Scholar]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Jankov, M.; Ristivojević, P.; Cvijetić, I.; Milojković-Opsenica, D. Assessing radical scavenging capacity of Sempervivum tectorum L. leaf extracts: An integrated high-performance thin-layer chromatography/in silico/chemometrics approach. J. Chromatogr. A 2023, 1703, 464082. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Brahmi, F.; Lounis, N.; Mebarakou, S.; Guendouze, N.; Yalaoui-Guellal, D.; Madani, K.; Boulekbache-Makhlouf, L.; Duez, P. Impact of growth sites on the phenolic contents and antioxidant activities of three Algerian Mentha species (M. pulegium L., M. rotundifolia (L.) Huds., and M. spicata L.). Front. Pharmacol. 2022, 13, 886337. [Google Scholar] [CrossRef]
- Karabegovic, I.; Stojicevic, S.; Velickovic, D.; Nikolic, N.; Lazic, M. Direct ultrasound-assisted extraction and characterization of phenolic compounds from fresh houseleek (Sempervivum marmoreum L.) leaves. Hem. Ind. 2018, 72, 13–21. [Google Scholar] [CrossRef]
- Deeva, A.M.; Voizechovskaia, A.A.; Spiridovich, A.V.; Reshetnikov, V.V. The content of phenolic compounds in the leaves of Paeonia suffruticosa Andr. and their antioxidant activity. Proc. Natl. Acad. Sci. Belarus. Biol. Ser. 2021, 66, 205–221. [Google Scholar] [CrossRef]
- Yan, Z.; Xie, L.; Li, M.; Yuan, M.; Tian, Y.; Sun, D.; Zhang, Y.; Niu, L. Phytochemical components and bioactivities of novel medicinal food—Peony roots. Int. Food Res. 2021, 140, 109902. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Li, N.; Wu, L.; Huang, Z.; Hu, Z.; Li, X.; Qu, Z. Phytochemicals and anti-tyrosinase activities of Paeonia ostii leaves and roots. Plant. Physiol. Biochem. 2022, 181, 50–60. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kunert, K. The ascorbate–glutathione cycle coming of age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Kalendar, O.V.; Kostikova, V.A.; Kukushkina, T.A.; Erst, A.S.; Kuznetsov, A.A.; Kulikovskiy, M.S.; Vasilyeva, O.Y. Seasonal development of Paeonia obovata and Paeonia oreogeton and their contents of biologically active and reserve substances in the forest-steppe zone of Western Siberia. Horticulturae 2023, 9, 102. [Google Scholar] [CrossRef]
- Pivato, M.; Fabrega-Prats, M.; Masi, A. Low-molecular-weight thiols in plants: Functional and analytical implications. Arch. Biochem. Biophys. 2014, 560, 83–99. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Papliński, R.; Mysiak, B.; Nurzyńska-Wierdak, R. Phenolic profile and antioxidant activity of extracts from aerial parts of Thymus vulgaris L. and Sideritis scardica Griseb. Appl. Sci. 2025, 15, 3842. [Google Scholar] [CrossRef]
- Rovčanin, B.R.; Tatjana; Steševi, D.; Keki, D.; Risti, M. Antibacterial effect of Herniaria hirsuta, Prunus avium, Rubia tinctorum and Sempervivum tectorum plant extracts on multiple antibiotic resistant Escherichia coli. Biosci. J. 2015, 31, 1852–1861. [Google Scholar] [CrossRef]
- Mufti, F.U.D.; Ullah, H.; Bangash, A.; Khan, N.; Hussain, S.; Ullah, F.; Jamil, M.; Jabeen, M. Antimicrobial activities of Aerva javanica and Paeonia emodi plants. Pak. J. Pharm. Sci. 2012, 25, 565–569. [Google Scholar]
- Mahdavi Fikjvar, E.; Saghafi, E.; Shkreli, R. Antimicrobial effects of Paeonia wendlboi extracts against Escherichia coli. Int. J. BioLife Sci. 2024, 3, 95. [Google Scholar]
- Papandreou, V.; Magiatis, P.; Chinou, I.; Kalpoutzakis, E.; Skaltsounis, A.-L.; Tsarbopoulos, A. Volatiles with antimicrobial activity from the roots of Greek Paeonia taxa. J. Ethnopharmacol. 2002, 81, 101–104. [Google Scholar] [CrossRef]
- Abdoulahi, M.I.I.; Yanick Kevin, M.D.; Lauve Rachel, T.Y.; Habibou, H.H.; Sahabi, B.; Abdelkader, A.S.; Boyom, F.F.; Tidjani, I.A. Antibacterial activity of eight medicinal plants from the traditional pharmacopoeia of Niger. J. Trop. Med. 2023, 2023, 6120255. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Amábile-Cuevas, C.F. Ascorbate and antibiotics, at concentrations attainable in urine, can inhibit the growth of resistant strains of Escherichia coli cultured in synthetic human urine. Antibiotics 2023, 12, 985. [Google Scholar] [CrossRef]
- Hamed, S.; Emara, M. Antibacterial and antivirulence activities of acetate, zinc oxide nanoparticles, and vitamin C against E. coli O157:H7 and P. aeruginosa. Curr. Microbiol. 2023, 80, 57. [Google Scholar]
- Hassuna, N.A.; Rabie, E.M.; Mahd, W.K.M.; Refaie, M.M.M.; Yousef, R.K.M.; Abdelraheem, W.M. Antibacterial fffect of Vitamin C against uropathogenic E. coli in vitro and in vivo. BMC Microbiol. 2023, 23, 112. [Google Scholar] [CrossRef]
- Alharbe, R.; Almansour, A.; Kwon, D.H. Antibacterial activity of exogenous glutathione and its synergism on antibiotics sensitize carbapenem-associated multidrug resistant clinical isolates of Acinetobacter baumannii. Int. J. Med. Microbiol. 2017, 307, 409–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, K. Glutathione exhibits antibacterial activity and increases tetracycline efficacy against Pseudomonas aeruginosa. Sci. China Ser. C 2009, 52, 501–505. [Google Scholar] [CrossRef]
- Hermansen, S.; Ryoo, D.; Orwick-Rydmark, M.; Saragliadis, A.; Gumbart, J.C.; Linke, D. The role of extracellular loops in the folding of outer membrane protein X (OmpX) of Escherichia coli. Front. Mol. Biosci. 2022, 9, 918480. [Google Scholar] [CrossRef]
- Sierra-Vargas, M.P.; Montero-Vargas, J.M.; Debray-García, Y.; Vizuet-de-Rueda, J.C.; Loaeza-Román, A.; Terán, L.M. Oxidative stress and air pollution: Its impact on chronic respiratory diseases. Int. J. Mol. Sci. 2023, 24, 853. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Wong, D.; Patani, A.; Paital, B.; Yadav, V.K.; Patel, A.; Jergens, A.E. Exploring the role of antioxidants in sepsis-associated oxidative stress: A comprehensive review. Front. Cell. Infect. Microbiol. 2024, 14, 1348713. [Google Scholar] [CrossRef]
- Lv, C.; Huang, L. Xenobiotic receptors in mediating the effect of sepsis on drug metabolism. Acta Pharm. Sin. B 2020, 10, 33–41. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Aisa-Álvarez, A.; Casarez-Alvarado, S.; Borrayo, G.; Márquez-Velasco, R.; Guarner-Lans, V.; Manzano-Pech, L.; Cruz-Soto, R.; Gonzalez-Marcos, O.; Fuentevilla-Álvarez, G.; et al. Impact of treatment with antioxidants as an adjuvant to standard therapy in patients with septic shock: Analysis of the correlation between cytokine storm and oxidative stress and therapeutic effects. Int. J. Mol. Sci. 2023, 24, 16610. [Google Scholar] [CrossRef]


| Plant/Organ | Reduced and Oxidised Ascorbate Content | ||
|---|---|---|---|
| ASC, mg/100 g DW | DHA, mg/100 g DW | ASC/DHA | |
| Sempervivum | |||
| Leaves | 78.36 ± 4.80 a | 63.39 ± 6.45 a | 1.24 ± 0.11 a |
| Roots | 33.70 ± 2.09 b | 29.99 ± 2.54 b | 1.26± 0.14 a |
| Paeonia | |||
| Leaves | 359.12 ± 12.80 c | 46.96 ± 3.47 c | 7.696 ± 0.76 b |
| Roots | 117.27 ± 6.97 d | 52.93 ± 3.65 d | 2.23 ± 0.0.22 c |
| Plant/Organ | Reduced and Oxidised Glutathione Content | ||
|---|---|---|---|
| GSH, mg/100 g DW | GSSG, mg/100 g DW | GSH/GSSG | |
| Sempervivum | |||
| Leaves | 3.00 ± 0.21 a | 1.58 ± 0.13 a | 1.92 ± 0.20 a |
| Roots | 2.16 ± 0.20 b | 0.35 ± 0.02 b | 6.19 ± 0.43 b |
| Paeonia | |||
| Leaves | 29.95 ± 2.92 c | 2.78 ± 0.27 c | 10.84 ± 1.18 c |
| Roots | 23.56 ± 1.63 d | 1.74 ± 0.07 d | 13.61 ± 1.28 d |
| Plant Species/Organ and Control (Amoxicillin) | Diameter of Inhibition Zone, mm at 100 µg/disk | MIC, mg/mL |
|---|---|---|
| Sempervivum transcaucasicum | ||
| Leaves | NI | NI |
| Roots | 8.8 ± 0.3 a | 5 |
| Paeonia daurica subsp. mlokosewitschii | ||
| Leaves | 13.3 ± 0.3 b | 2 |
| Roots | 13.1 ± 0.5 b | 3 |
| Amoxicillin | 27.8 ± 0.8 c | 0.004 |
| GSH | ASC | |
|---|---|---|
| Top-1 pose score | −110.66 | −95.86 |
| Average score (top 1–3) | −108.65 | −91.54 |
| SD of top 1–3 scores | 1.76 | 3.46 |
| OmpX interactions | Hydrophobic interaction with Phe90 (distance: 3.98 Å); H bond with Asn19 (H-A distance: 3.80 Å); multiple H bonds with Asn60 (H-A distances: 2.37 Å, 2.01 Å, 3.09 Å) | H bond with Ile65 (H-A distance: 2.60 Å) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mittova, V.; Pirtskhalava, M.; Tsetskhladze, Z.R.; Makalatia, K.; Loladze, A.; Bebiashvili, I.; Barblishvili, T.; Gogoladze, A.; Roviello, G.N. Antioxidant Potential and Antibacterial Activities of Caucasian Endemic Plants Sempervivum transcaucasicum and Paeonia daurica subsp. mlokosewitschii Extracts and Molecular In Silico Mechanism Insights. J. Xenobiot. 2025, 15, 109. https://doi.org/10.3390/jox15040109
Mittova V, Pirtskhalava M, Tsetskhladze ZR, Makalatia K, Loladze A, Bebiashvili I, Barblishvili T, Gogoladze A, Roviello GN. Antioxidant Potential and Antibacterial Activities of Caucasian Endemic Plants Sempervivum transcaucasicum and Paeonia daurica subsp. mlokosewitschii Extracts and Molecular In Silico Mechanism Insights. Journal of Xenobiotics. 2025; 15(4):109. https://doi.org/10.3390/jox15040109
Chicago/Turabian StyleMittova, Valentina, Marina Pirtskhalava, Zurab R. Tsetskhladze, Khatuna Makalatia, Alexander Loladze, Irakli Bebiashvili, Tinatin Barblishvili, Ana Gogoladze, and Giovanni N. Roviello. 2025. "Antioxidant Potential and Antibacterial Activities of Caucasian Endemic Plants Sempervivum transcaucasicum and Paeonia daurica subsp. mlokosewitschii Extracts and Molecular In Silico Mechanism Insights" Journal of Xenobiotics 15, no. 4: 109. https://doi.org/10.3390/jox15040109
APA StyleMittova, V., Pirtskhalava, M., Tsetskhladze, Z. R., Makalatia, K., Loladze, A., Bebiashvili, I., Barblishvili, T., Gogoladze, A., & Roviello, G. N. (2025). Antioxidant Potential and Antibacterial Activities of Caucasian Endemic Plants Sempervivum transcaucasicum and Paeonia daurica subsp. mlokosewitschii Extracts and Molecular In Silico Mechanism Insights. Journal of Xenobiotics, 15(4), 109. https://doi.org/10.3390/jox15040109









