Abstract
Climate change, mining activities, pollution and other human impacts on the natural environment cause significant changes in the concentrations and mixtures of metallic elements found in different ecosystems. Metals such as cadmium, copper, lead and mercury affect multiple aspects of host–pathogen interactions, influencing the risk of infectious diseases caused by various classes of pathogens. Notably, exposure to metals in doses and combinations toxic to the immune system can favor the dissemination of pathogens in natural environments, threatening the reproduction, well-being and survival of varied animal species. However, these problems remain neglected, since the influences of metals on infectious diseases are studied with a primary focus on human medicine. Therefore, this article aims to review the influence of metals/metalloids (e.g., arsenic, cadmium, chromium, copper, iron, lead, mercury, nickel, zinc) on infectious and parasitic diseases in animals living in natural environments. The potential impact of metals on the risk of zoonotic spillover events is also discussed. Metal pollution tends to increase as the demand for elements used in the manufacture of industrial products, batteries, and electronic devices increases globally. This problem can aggravate the biodiversity crisis and facilitate the emergence of infectious diseases. Considering the interconnections between pollution and immunity, measures to limit metal pollution are necessary to protect human health and biodiversity from the risks posed by pathogens. This review helps fill the gap in the literature regarding the connections between metal pollution and various aspects of infectious diseases.
1. Introduction
Metal pollution has anthropogenic and natural (geogenic) causes [1,2]. Metal emissions from natural events, such as volcanic activity, rock weathering, and wildfires in fire-dependent ecosystems, are beyond human control [1,3]. The impossibility of totally controlling natural events and their consequences makes it even more crucial to limit the anthropogenic emissions of metals to reduce their impact on the environment [3]. Metal pollution has become a problem of increasing concern as human impacts on the environment intensified from the 1950s onwards [4], especially considering the intensive mining and agricultural practices (e.g., the use of fertilizers and pesticides containing metals), poor management of electronic waste and batteries, increasing industrial processes, and release of metals from automotive components and traffic (e.g., tire, fuel, road runoff), among other anthropogenic sources and activities [1,5,6,7]. Metal pollution currently affects the soil on every continent, putting between 0.9 and 1.4 billion people at risk of suffering severe health consequences from exposure to metals [2].
The growing demand for electronic devices and efforts towards a green energy transition indicate an upward trend in mining activities globally [8]. Industrial and artisanal mining are responsible for a large portion of the metal pollution observed in several places around the world, either due to by-products or mining procedures or due to accidental spills and other mining-related disasters. In Brazil, the rupture of two mining tailings dams in the state of Minas Gerais in 2015 (Mariana city) and 2019 (Brumadinho city) led to the contamination of different ecosystems with several toxic metals [9,10]. In the Amazon region, artisanal gold mining causes mercury (Hg) contamination of forest ecosystems, leading to the bioaccumulation of this metal in fish [11]. In addition to these metal pollution-related case studies located in Latin America, Table 1 presents other examples [12,13,14,15,16,17,18,19,20,21,22] of pollution associated with mining activities in various regions of the world, showing that this is a problem on a global scale. Artisanal battery recycling in Africa, Latin America and Asia is also responsible for releasing large amounts of metals into the environment [23]. Furthermore, climate change and extreme weather events affect the distribution, concentration and bioavailability of metals in soils, water bodies and the atmosphere [24].

Table 1.
Examples of pollution caused by mining activities in various regions of the world.
The mixtures and concentration of chemical elements observed in ecosystems, whether caused by human actions or by natural geographic variations in soil elemental concentrations, may have important effects on human infectious diseases. For example, it has already been hypothesized that the levels of selenium (Se), a non-metal element, found in soils in different regions of the world influence the risk of viral infections in human populations [25]. Also, it was demonstrated that the amount of the metal vanadium (V) present in water sources from Hawai’i increases the risk of pulmonary bacterial infections in humans [26].
While the impact of metals on human infectious diseases is better established [27,28], there is limited data on their effect on infection risk in nature, especially concerning non-human vertebrates [29]. Evidence since the 1980s highlights the significant influence of environmental metals on animal infection rates, as discussed by Kosoy and Biggins [30]. Also, it is well known that the levels of metals present in soils may affect wildlife health status and the risk of infectious diseases in animals, especially those that live in direct contact with the soil, such as earthworms, snails and insects [31]. However, this study topic remains overlooked in the field of disease ecology. Thus, the aim of this article is to review the influences of metals on infectious diseases in animals living in natural environments, including birds, mollusks, non-human mammals, reptiles, fish and bees. Also, how these influences affect the risk of zoonotic spillover events will be discussed.
2. Methodological Notes
This is a narrative review intended to highlight a gap in the literature regarding the effects of metal exposure on infection-related outcomes in non-human animals. The documents included in this review come from non-systematic searches in PubMed [32] and Google Scholar [33] in 2024 and 2025 using keywords such as “metal”, “infection”, “infectious diseases”, “wildlife”, “environment”, “animal”, “spillover” and “zoonosis” in different combinations. The literature cited in the selected documents was also used as a research source. Finally, the authors’ virtual library was consulted for supplementary sources relevant to the topics addressed in this review. Considering the scarcity of references on the effects of metals and infectious diseases in natural environments, no limitation was applied regarding the year of publication of the works for inclusion in the review. In addition to studies carried out with animals sampled in nature, some experimental and theoretical studies were included in the review as they provide relevant information to explain the mechanisms of metal toxicity and their relationship to infectious processes.
Section 3 shows a summarized view of the effects of metals on animal infection. Sequentially, the detailed results of studies evaluating the impacts of metals on infections in invertebrates such as bees (Section 3.1) and mollusks (Section 3.2), and vertebrates such as fish (Section 3.3), reptiles (Section 3.4), birds (Section 3.5) and non-human mammals (Section 3.6), is presented and discussed. A synthesis of the mechanisms involved in the influence of metals on infectious diseases is presented in Section 4, and the potential impacts on zoonotic spillover events are described in Section 5. A general discussion is presented in Section 6. Finally, Section 7 presents a summary of the main results of the reviewed articles, concluding the article.
3. Impacts of Metal Exposure on Infection-Related Outcomes in Animals
Table 2 summarizes the main effects of metals on infections in animals sampled in nature based on the main studies [29,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52] reviewed in this article. The following sections provide more detailed descriptions of these studies, supplemented by a discussion covering additional field, theoretical and laboratory investigations.

Table 2.
Main effects of metals on infections in animals sampled in nature.
3.1. Impacts on Insects (Bees)
Invertebrates may be exposed to metal pollution through contact with the environment (e.g., earthworms through metals present in soil) or through food chains (e.g., phytophagous insects through metals present in plants) [53,54]. Metals affect microbe–insect interactions by regulating insect immune systems, affecting the host microbiome, promoting physiological stress and regulating the expression of genes that act in defenses against pathogens, among other mechanisms [54,55,56,57].
Szentgyörgyi et al. [34] investigated the accumulation of metals (i.e., lead (Pb), cadmium (Cd), zinc (Zn)), species diversity and parasite load (Microsporidia Nosema bombi) in bumblebees. The study was performed in Poland and Russia, considering different sites and including three metal-polluted locations (i.e., Guryevsk metallurgic plant (Russia), Belovo Zn smelter (Russia) and Olkusz Zn smelter (Poland)) and two control regions (the mountains of Kouznetskiy Alatau and Gornaya Shoria, both in Russia). The levels of Pb and Cd in bumblebee tissues correlated with the content of these metals detected in soil samples from the Guryevsk and Belovo locations. A significantly higher percentage of infected bees was found at the Kouznetskiy Alatau control region (29.4%) compared to a specific site in Guryevsk (6.1%). However, the metal pollution indexes did not significantly impact species diversity parameters or correlate with infection rates, as reported by Szentgyörgyi et al. [34]. Further studies evaluating infection rates by other microorganisms in bees and other insects exposed to different levels of metal pollution are needed to determine the intensity with which this animal group is affected by metals and how this is reflected in infection outcomes.
3.2. Impacts on Mollusks
Analyzing cockles (Cerastoderma edule) collected at Banc d’Arguin, south-west France, Baudrimont et al. [36] found no significant difference in levels of Cd, Pb, mercury (Hg), Zn and copper (Cu) between bivalves parasitized with Labratrema minimus, a digenean parasite, and those without infection. Notably, metal concentrations were generally very low, likely due to the mollusk collection site being relatively unpolluted, as suggested by the low content (<2%) of organic matter [36]. In Ireland, a study evaluated Littorina littorea periwinkles that were also infected with digenean parasites and found reduced levels of iron (Fe), Cu and nickel (Ni) compared to uninfected periwinkles. Obliteration of the hepatopancreas caused by digenean parasites may result in decreased metal storage, potentially explaining these results [35]. Also, digenean parasite infection can modulate metallothionein synthesis in mollusks, impacting metal metabolism by altering the detoxification of toxic elements and the homeostasis of essential metals [36].
Some parasites accumulate large amounts of metals [58]; therefore, parasite–metal–host interactions have complex effects on infection outcomes. In a study performed in Egypt, Cd water levels were positively associated with Schistosoma mansoni infection rate in the offspring of Biomphalaria alexandrina snails, while Pb, Cu and Hg water levels were negatively correlated with infection rate [37]. In an experimental study, B. glabrata snails infected with Schistosoma mansoni parasites and concomitantly exposed to metals (Cd, Hg and Pb) showed increased mortality rates compared to uninfected snails exposed to metals [59]. Similarly, another experimental study [60] described that co-exposure to Cd and S. mansoni affect the growth and reproduction of B. alexandrina snails, in addition to reducing snail survival rate. Taken together, these results indicate that metals affect mollusks’ parasitic infection response and can enhance the deleterious effects of pathogens.
3.3. Impacts on Fish
In the 1960s, Pippi and Hare [38] reported an epizootic outbreak affecting Atlantic salmon (Salmo salar) and suckers (Catostomus commersonii) caused by the bacteria Aeromonas liquefaciens in Miramichi River, New Brunswick, Canada. The epizootic outbreak was linked to the combined effects of two environmental stressors: river pollution by Cu and Zn and elevated river temperatures. These two factors made the fish more susceptible to infection (i.e., “weakened” immune defenses) and provide conditions conducive to the development of bacteria (i.e., near-optimum temperature), triggering the epizootic outbreak [38]. Indeed, metals can modulate the immune system and physiology of fish, thus affecting disease susceptibility [61].
In a study performed with European chubs (Squalius cephalus) from Sava River, Croatia, fish infected with the acanthocephalan Pomphorhynchus laevis showed lower levels of Cu, Cd and Pb compared to uninfected fish [40]. In South Africa, infected fish from Hex River showed (non-significant: p > 0.05) lower metal concentrations (e.g., Cd, chromium (Cr), Ni, platinum (Pt) and Pb) compared to uninfected fish. It was also observed that fish with a higher infection load had lower levels of metals in their tissues in comparison to fish with reduced infection intensity [41]. Similar results were observed in Egypt, where Hassanine and Al-Hasawi [39] detected reduced levels of Cd or Pb in liver samples of fish (Siganus rivulatus) infected with the intestinal acanthocephalan Sclerocollum rubrimaris compared to uninfected fish.
Sures and Siddal [62] showed experimentally that acanthocephalan parasite P. laevis parasitizing the intestine of chub (Leuciscus cephalus) accumulated high Pb content (much higher than those levels detected in host tissues). Marijić et al. [40] detected higher levels of many metals (i.e., Cu, manganese (Mn), silver (Ag), Cd, Pb) in acanthocephalans than those observed in fish gastrointestinal tissue samples. When analyzing parasitized fish, the cestode Atractolytocestus huronensis and the nematode Contracaecum sp. accumulated higher metal levels than those detected in host tissues [41]. Adding to this evidence are the recent findings of Mijošek et al. [42], which observed higher content of several metals, including the highly toxic Pb, Cd and thallium (Tl), in acanthocephalans (Dentitruncus truttae) as compared to those detected in the intestine of fish (Salmo trutta) samples at Krka River, Croatia. Inverse relationships between parasite abundance and metal levels in fish were observed for several metals and other elements, specifically As, Cr, Mn, Se, V, molybdenum (Mo), barium (Ba), Cu and Fe. This result reinforces the protective role of parasites against metal intoxication in fish [42].
Parasites can capture bile-bound metal complexes that form in the liver and are released through the bile duct into the intestine, thereby reducing the availability of metals for reabsorption by the host’s intestinal cells. Furthermore, parasites tend to accumulate significantly higher levels of metals compared to those present in their environment. This high bioaccumulation capacity makes parasites good bioindicators of metal pollution. Also, the bioaccumulation capacity of some parasite groups (i.e., acanthocephalans and cestodes) may reduce metal accumulation in fish through metal sequestration. In practical terms, this body of information suggests that, in addition to potentially protecting fish from metal toxicity, parasites may also play a valuable role in environmental monitoring. The abundance and diversity of parasite species in fish, along with the concentrations of metals accumulated in these parasites, can serve as complementary indicators for monitoring metal pollution in biological systems [39,40,41,58,62].
Finally, methylmercury (MeHg+), a toxic form of organic Hg, enters the wildlife food chain primarily through bioaccumulation in fish and shellfish. Therefore, it is not only harmful to fish themselves but also poses a serious threat to birds, mammals and other animal groups that feed on fish and shellfish [3]. Although the entry of Hg into the food chain through fish/shellfish is a well-known problem, with widely reported toxic effects on humans [63], the effects of Hg on infectious diseases are still unknown. Considering the immunotoxic and immunodisruptive activities of Hg [64,65], the potential effects of this element on infectious processes represent an interesting research question.
3.4. Impacts of Reptiles
A study performed with Chalcides ocellatus lizards collected from industrial, rural and urban sites from Egypt evaluated the prevalence of parasitic infection and the levels of metals (i.e., Cu, Ni, Cd, Pb) in parasite and intestinal lizard tissue samples [43]. One cestode (Oochoristica tuberculata) and five nematode species (Thelandros alatus, Parapharyngodon micipsae, Pharyngodon mamillatus, Spauligodon auziensis and Cosmocerca ornata) were found infecting the lizards. Nematode and cestode infection intensity correlated positively with Cu, Cd and Pb levels at all sites studied. The cestode O. tuberculata accumulates more metal levels, particularly Cu and Cd, compared to nematodes. Also, lizards infected with O. tuberculata showed increased metal intestinal levels. These findings indicate that O. tuberculata may serve as a biomarker for metal pollution [43]. Considering the direct interaction that reptiles have with the environment, especially soil, more research on the effect of metal pollution on the immune system and infectious processes in reptiles is welcome.
3.5. Impacts on Birds
Mashima et al. [45] investigated an avian cholera outbreak caused by Pasteurella multocida in oldsquaws (Clangula hyemalis) in the Chesapeake Bay (USA) in 1994. Their findings indicated that elevated Cd levels in liver and kidney samples were linked to a higher risk of infection. The authors also observed that Se and Hg levels were lower in liver samples from birds that died of cholera compared to control birds [45].
Studying house sparrow (Passer domesticus) populations in France, Bichet et al. [29] evaluated whether the concentration of Pb, Cd and Zn in feathers was associated with the infection prevalence of Plasmodium relictum, the agent of avian malaria. Infection prevalence was negatively associated with Cd concentrations. Sub-chronic exposure to some metals may have a stimulatory effect on the immune system, potentially explaining this result. Alternatively, this result could be explained by a lethal toxic effect of Cd on the parasite. On the other hand, Pb concentrations were positively correlated with P. relictum prevalence, potentially due to the immunotoxic effect of lead on the immune systems of the sparrows. Considering that Pb concentrations were higher in urban/polluted habitats, another potential explanation for the observed results is the increased exposure of these animals to malaria-infected mosquitoes (a valid explanation for areas with urban circulation of malaria parasites) [29]. Also, in France, high levels of Pb in feathers were associated with an increased intensity of Haemosporidian blood infection in Columba livia, the urban pigeon [46].
In agreement with these previous studies, a recent work by Khan et al. [44] on house sparrows (Passer domesticus) from two islands of Norway (Hestmannøy and Gjerøy) found that increased blood Pb levels were linked to higher infection rates of Syngamus trachea, a parasitic nematode commonly known as “gapeworm”, the causative agent of syngamosis, a respiratory tract disease. This result was also attributed to the immunotoxic effect of Pb [44]. The relationship between Pb exposure and increased infection risk observed in nature is reinforced by some experimental data. In a study with red-legged partridges (Alectoris rufa), increased experimental Pb exposure showed a season-dependent impairment on T-independent humoral response, decreased levels of natural antibodies and lysozymes, and was non-significantly associated with a higher prevalence of the parasite Heterakis gallinarum [66]. Finally, Khan et al. [44] also found that infected sparrows from Gjerøy had higher magnesium (Mg) concentrations and lower levels of V compared to non-infected birds.
In addition to the toxic and immunosuppressive effects of metals, it is important to consider the beneficial effects of some metals, such as Zn, on the immune system and protection from infectious disease. Analyzing urban pigeons (Columba livia) from France, Gasparini et al. [46] found that increased Zn levels in feathers were associated with a lower intensity of Haemosporidian parasites in blood as well as a lower infection prevalence of Chlamydiaceae bacteria, the causative agent of ornithosis disease. Conversely, higher Pb levels were associated with increased Haemosporidian infection, as mentioned previously [46].
3.6. Impacts on Non-Human Mammals
A review of a set of ecological investigations performed in the 20th century by the Russian researcher Evgeny Rothschild showed that the types and quantities of metals observed in nature influence the rate of Yersinia pestis infection in free-ranging rodents [30]. This information is corroborated by additional evidence. In the south of Antwerp, Belgium, Tersago et al. [48] observed that wood mice (Apodemus sylvaticus) resistance to the endoparasite Heligmosomoides polygyrus decreased with increasing exposure to Ag, arsenic (As), Cd, cobalt (Co) and Pb. Notably, the number of H. polygyrus was positively correlated with Cd levels measured in host liver samples, and the number of fleas observed in the animals was positively correlated with Pb levels. These results suggest an immunotoxic effect of metals on the immune defenses of rodents [48].
Other mechanisms behind the relationship between metals and infection in rodents have begun to be elucidated more recently. Experimental Cd exposure facilitated Salmonella infection in C57BL/6 mice. These results were linked to higher inflammation and damage in the intestinal mucosal barrier, along with other dysfunctions caused by Cd [67]. Also, in an experimental study performed with male Wistar rats, co-exposure to Cd and infection with the intestinal parasite Moniliformis moniliformis (Acanthocephala) disrupted hormone homeostasis, potentially harming animals’ health [68].
In a study performed in the Czech Republic, Jankovská et al. [51] found decreased levels of Pb in kidney samples of foxes (Vulpes vulpes) infected with the parasites Toxascaris leonina (Nematoda) and Mesocestoides spp. (Cestoda) compared to non-infected foxes. Similarly, also in the Czech Republic, Brožová et al. [50] reported lower levels of Cd and Pb in the small intestinal tissue of red foxes (Vulpes vulpes) infected with the tapeworm Echinococcus multilocularis compared to non-infected foxes. In contrast, levels of other metals increased, specifically Cr, Cu, Fe, Mn, Ni and Zn; however, despite marked differences in metal levels between groups, only Fe showed a statistically significant difference [50]. Jankovská et al. [51] also reported statistically significantly increased levels of Mn and Cu in liver samples of parasitized foxes compared to non-infected animals, as well as higher Pb, Mn, Cu, Ni and Zn accumulation in parasites than in host tissues. These results support the capacity of parasites to sequester some metals [51], thereby decreasing their concentrations in host tissues, while remobilizing other elements, increasing their concentrations in the host [50]. Importantly, the taxonomic groups of parasites with which the host is infected (e.g., cestodes or nematodes) may influence the levels of metals in the host tissues, as shown by a study permed with small rodents [49]. In brief, certain parasite species, in natural environments, may confer protection to their hosts against the toxic effects of metals. This finding contributes to a broader understanding of the potential ecological roles of parasites and challenges the common predominantly negative perception of parasites.
In a study performed with carcasses of harbor porpoises (Phocoena phocoena) from the coastal waters of England and Wales, Bennett et al. [47] observed increased liver concentrations of Hg, Se and Zn in the porpoises that died of infectious disease compared to porpoises that died from physical trauma (e.g., entrapment in fishing nets), as determined by field data and pathological investigations. Infected animals were considered to be those with evidence of viral, parasitic, bacterial or fungal infections, with parasitic pneumonia associated with secondary bacterial infections being the most frequent observation. The Hg:Se molar ratio was also increased in infected animals compared to controls. The immunosuppressive effects of Hg may explain the increased infection rate, while higher Zn levels may be explained by the redistribution of this element due to infection. However, factors such as age and nutritional status have been identified as potential confounding factors that may complicate the associations between infection status and metal levels. No significant difference was found between the groups concerning liver concentrations of Pb, Cd, Cu and Cr [47]. Finally, we underscore that the impact of metals on infection in non-human mammals may vary across different taxonomic groups. For example, in bats, the effects of Hg on infection risk are varied and complex, differing among species [52].
4. Synthesizing the Mechanisms
The immunomodulatory effect of metals is a primary mechanism by which these elements influence infectious diseases, with metal exposure being increased or decreased by geogenic events and anthropogenic activities in natural environments. Human activities that enrich soils with toxic metals, such as mining, can promote immunotoxicity due to the toxic effects of a specific metal (e.g., Hg, As, Pb) or the synergistic effects of multiple toxic metals [24]. On the other hand, soil depletion caused by human activities (e.g., monoculture, deforestation, irrigation, erosion) can contribute to the production of foods deficient in essential metals like Zn and Fe, which are crucial for proper immune function [24]. For instance, Zn deficiency is associated with elevated levels of inflammation and dysregulated immune function, while excess Zn is associated with the suppression of T and B cells and stimulation of regulatory T cells [69].
In brief, the poor functioning of the immune defenses, both due to a deficiency and excess of metals, may contribute to increased host susceptibility to infections [24]. Furthermore, metals can activate or suppress the expression of genes that regulate immune function, affecting both susceptibility to infections and the pathogenesis of infectious diseases [24,69,70]. Recently, using a toxicogenomic approach, we reported that As, Hg and Pb affect the activity of thousands of genes across different species. Many of these genes are involved in infectious processes related to viruses, bacteria and parasites [71].
In addition to immunomodulation, other mechanisms may explain how metals affect the risk of infectious diseases. For example, many microorganisms, such as bacteria, require metals to maintain their metabolic, catalytic and structural functions. Vertebrate hosts have evolved several mechanisms (termed nutritional immunity) to limit the access of those pathogenic bacteria to trace metals, thus combating infections [72]. Nutritional immunity involves strategies such as metal sequestration, the redistribution of metals across cells and organ systems, and the genetic regulation of bioavailable metal levels. Collectively, these mechanisms constitute critical defenses against bacterial infection by limiting microbial access to metallic nutrients [72]. The deposition of air pollution particles containing metals in the lungs may serve as good sources of metals for microorganisms, favoring respiratory infections [24]. Furthermore, metals affect the survival and reproduction of parasites, thus influencing host–parasite interactions and the course of parasitic infections in animal populations [73,74]. Metal-induced damage to mucosal barriers also increases susceptibility to pathogens [67].
Metal exposure can cause reductions in body mass, growth and fitness [48,75]. Metabolizing toxic metals has a physiological cost [59] that can impact different aspects of animal fitness and their ability to deal with pathogens [48,76]. An exacerbated demand on the immune system, especially in the face of infectious threats, can lead to losses in other important functions, such as growth and reproduction [60].
Associations between metal levels in animals and infectious diseases may not always be directly linked to immunological factors, and confounding factors should be taken into account when interpreting results from field studies. For example, it is possible that animals living in polluted sites (and therefore more exposed to metals) may also be exposed to a greater burden and diversity of pathogens, which proliferate precisely due to the high pollution levels [29].
It is also essential to emphasize that the toxic effects of a particular metal should be evaluated within the context of its potential interactions with other elements. For example, Se, an essential trace element for the proper immune system function, exhibits a strong affinity for Hg. Consequently, Hg exposure in the context of Se deficiency can exacerbate Hg toxicity due to the reduced Se’s inhibitory effects on Hg while also impairing the activity of Se-dependent enzymes (selenoenzymes). Furthermore, Hg intoxication can result in Se retention within tissues, potentially leading to Se toxicity. On the other hand, higher Se levels in the body may reduce Hg toxicity without compromising the function of selenoenzymes. These Se–Hg interactions are particularly pronounced in brain tissues. In brief, the toxic effects of Hg and the protective role of Se in the immune system are largely influenced by the Se:Hg molar ratio [3]. This relationship underscores the complexity of metal toxicity, which is often difficult to associate with a particular health effect or outcome, especially in organisms exposed to a diverse mixture of metals, as is commonly observed in polluted environments. These aspects may help explain unexpected or difficult-to-interpret effects observed in studies involving metals, the immune system and infections in natural environments.
It is also important to consider the protective effects against metal poisoning exerted by some groups of parasites, such as acanthocephalans, on hosts. The reduced uptake of metals by hosts with a high parasite load associated with the “dilution” of the metal load among the host–parasite mass is a phenomenon called “biodilution” [42]. We speculate that this mechanism may protect the host against the toxic effects of metals on the immune system, preventing infections by other classes of pathogens, such as viruses and bacteria.
Ecological mechanisms also explain the association between metals and infectious diseases. Environmental characteristics, such as temperature, humidity, pH and pollutant concentration, influence the presence and distribution of pathogens in habitats and hosts [66,77]. In this sense, metals affect the communities, structure and function of soil microbiome, which can modify disease risk [78,79,80]. Figure 1 summarizes the main immune, physiological and ecological mechanisms that explain how metals affect the risk of infectious diseases in wildlife.
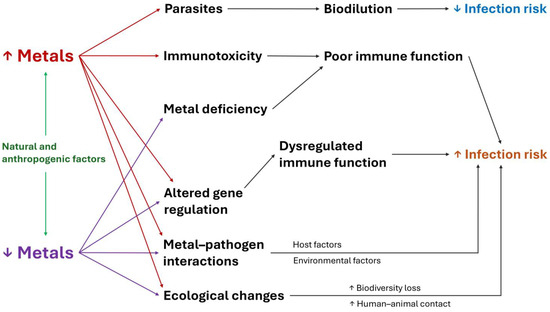
Figure 1.
Mechanisms that explain how metals affect the risk of infectious diseases in wildlife.
The ecological effects of metals on infectious diseases may be indirect and must also be highlighted (Figure 2). Mining activities driven by the growing demand for metals by human societies cause ecological changes that favor the spread of pathogens. For example, deforestation in the Amazon due to mining and other anthropogenic activities promotes the loss of biodiversity, which is associated with the greater spread of pathogens among wild species [81]. Mining activities in highly biodiverse ecosystems such as the Amazon forest also increase human contact with wild animals and disease vectors (e.g., mosquitoes), which facilitates the exchange of pathogens between humans and animals, favoring both zoonoses and anthropozoonoses [82,83]. In summary, metals are at the center of direct and indirect ecological relationships that, together with their physiological and immunological effects, affect the risk of infectious diseases in different ways.
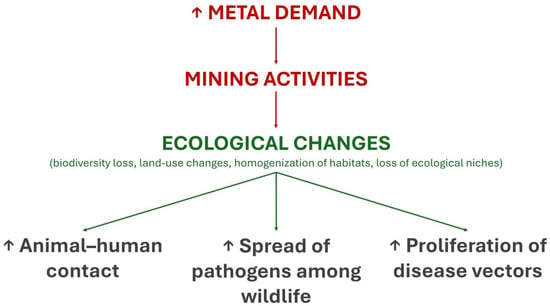
Figure 2.
Indirect ecological effects of metals on infectious diseases.
5. Potential Impacts of Metals on Zoonotic Spillover Events
Pollution is currently one of the main causes of ecological changes [84]. A recent meta-analysis by Mahon et al. [85] identified chemical pollution, including metal contamination, as a major factor contributing to the global emergence of infectious diseases. However, the mechanisms by which metals interfere in the emergence of infectious diseases still represent an open question. To begin unraveling this issue, it is necessary to explore the role of metals in mediating pathogen transmission, both between individual hosts and across species barriers.
Zoonotic spillover is a term commonly used to describe the transmission of a pathogenic microorganism, whether a virus, fungus, parasite or bacteria, from a wild species to the human population. Transmission can be direct (from an animal to a human) or indirect, involving the environment (animal to environment to human) or an intermediate host (animal to intermediate host, vertebrate or invertebrate, and then to human). Spillover events are the starting point for the majority (~70%) of emerging infectious diseases that affect humans. Several ecological and biological aspects of both hosts and pathogens determine the risk of spillover events occurring, such as the frequency of contact between different species, the phylogenetic distance between species and the ability of pathogens to interact with different cell types [86].
Using viral pathogens as an example, it is known that immunological factors define both the viral load of host sources and the susceptibility of recipient hosts to viral infections. For example, the episodes and amount of viral shedding of Hendra virus by bats, and consequent viral environmental contamination and spillover risk, are influenced by immunological characteristics and nutritional stress [87]. Animals under nutrient restriction-related immune deficiencies may be more susceptible to infections and favor the spread of pathogens in nature [88,89]. Humans with nutrition-related immunosuppression are more susceptible to zoonotic infections [90,91]. Individuals with Fe deficiency or overload show increased susceptibility to a variety of pathogens [92,93]. Taken together, this body of evidence shows that the susceptibility of a species to a given pathogen is influenced by nutritional/metal-related immune function, which also affects the number of pathogens expelled by hosts.
Metals may also influence the likelihood of spillover events involving parasites. In fish, greater metal availability may be associated with greater parasitemia, as discussed previously in the Section “Impacts on fish”. In red foxes, metal levels affect host interactions with Echinococcus multilocularis, the parasite responsible for alveolar echinococcosis, a zoonotic disease [50]. This suggests that variations in host metal levels could influence fox–parasite dynamics, potentially affecting the risk of zoonotic transmission of both known and emerging diseases to humans.
Metal-related ecological mechanisms also affect the risk of spillover events. Mineral licks are places in nature that concentrate a large quantity and variability of minerals, including metals, attracting animals that seek to supplement their diet with soil rich in these nutrients. The concentration of varied species in mineral licks facilitates the transmission of pathogens between animal species once animals deposit feces, saliva and other potentially contaminated secretions in these places, in addition to possible physical interaction between different species. Because many animals congregate there, mineral licks are also places that attract hunters, thus increasing the potential for animal–human interactions and consequently facilitating the transmission of pathogens to humans [94].
Considering (I) the critical and multiple immunoregulatory roles of metals on immune function in both animals and humans, (II) the variable amount of both essential and toxic metals found in the environment and (III) the direct and indirect ecological influences of metals on pathogen–animal–human interactions, we suggest that metals may affect the risk of pathogen spillover and disease emergence. In this sense, Figure 3 summarizes how metals can affect the main models of zoonotic spillover events. Studies evaluating the effects of specific metals, as well as metal mixtures, on zoonotic diseases are essential to clarify the relationship between metal exposure and zoonotic spillover.
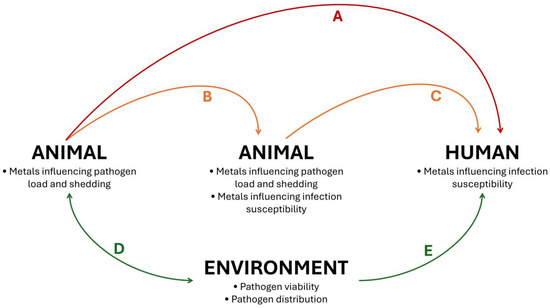
Figure 3.
Potential effects of metals on zoonotic spillover events. Metals influence pathogen load and shedding, as well as infection susceptibility, mediating the risk of direct spillover events, without intermediate hosts (A) and indirect spillover events, with intermediate hosts (B,C). Furthermore, metals present in the environment affect the viability and distribution of pathogens in media such as soil and water, affecting the outcomes of environmental contamination by pathogens (D) and the risks of environmental-borne human infections (E). Of note, although the term “zoonotic spillover” is used in different contexts in the literature, in this figure and review the expression refers to the transmission of pathogens from wildlife to humans.
6. Discussion
The effects of toxic metals are commonly studied from a human health-centered perspective, often overlooking the multiple negative impacts of metal pollution on wildlife. The Minamata Bay disaster is a notable example of Hg pollution’s impact on wildlife. The industrial discharge of Hg compounds into the Japanese bay from 1932 to 1968 led to the widespread formation of toxic methylmercury, which bioaccumulated in fish and shellfish as a result of environmental pollution [95,96]. This contamination caused severe neurological damage and mortality in marine animals, terrestrial species such as cats and predatory birds, resulting in population declines [95,96]. Of note, Minamata disease, the term for the human health issues caused by Hg poisoning, was first identified only in 1956, after children began exhibiting Hg-related neurological symptoms [96]. This highlights the high degree of permanence and bioaccumulation of Hg in the environment, in addition to drawing attention to the fact that metal pollution has been affecting humans and animals for many decades. The Minamata Bay disaster emphasizes the importance of considering wildlife effects in heavy metal pollution assessments. Our review expanded the understanding of the effects of metals on wildlife by providing a discussion of how metals influence the dynamics of infectious diseases.
Studying the effects of metals on infectious processes is complex, especially in natural environments. Pollutants can have varying effects on infectious diseases, increasing or decreasing the risk of infection in different biological contexts [52]. For example, in bats, Hg can impair proper immune function and facilitate infection. On the other hand, Hg can, through its cellular toxic effects, decrease the number of cells available for pathogens to infect, thus decreasing the infection rate. Reduced infection rates can also be explained by the direct toxic effects of Hg on the survival of pathogens. Furthermore, these effects may vary between different bat taxa [52]. Indeed, the studies reviewed in this paper showed varying effects of metals on different species (Table 2).
Our review also explored the potential mechanisms by which metals may affect the risk of spillover events (Figure 3), highlighting that the influence of metals on infection dynamics in wildlife species may reverberate over human health. In addition to the direct impacts of zoonoses on human populations (e.g., deaths, morbidity, epidemics), the burden of infectious diseases triggered by metal-induced spillover events may add to the burden of chronic diseases caused by metal pollution around the world. For example, the neurotoxic effects of Pb may have compounded the neuroimmune effects of SARS-CoV-2 during the COVID-19 pandemic, significantly impairing children’s development in socially vulnerable communities [97].
The complexity of studying metal–infection relationships increases even further when more than one metal is evaluated together. Although challenging, this multi-metal approach is important because, in nature, especially in highly anthropized environments, organisms are often exposed to mixtures of metals [71]. Another aspect that needs to be taken into consideration is related to the dose of each metal to which the organisms were exposed and the particularities of the tolerance of each organism. Using humans as an example, the safe dose range (i.e., exposure through food) for essential elements such as Fe and Zn is relatively wide [98,99]; thus, it is not common to observe deleterious effects caused by exposure to these metals. However, for other non-essential elements, such as Pb, no exposure dose is considered safe due to the high toxic effects of this metal [100]. These complexities related to exposure dose are particular to each species, and little is known about metal tolerance and poisoning effects (e.g., safety doses, sub-lethal outcomes) in non-human animals. Furthermore, different chemical forms of the same metal can exhibit distinct toxicological effects on cells and biological systems, including the immune system. These variations further complicate the interpretation and comparison of findings across studies that investigate different compounds of the same metal [71]. These limitations and biological factors must be taken into account when interpreting studies involving metals and infectious diseases.
Our review showed that metal pollution impacts different places around the world, especially regions with mining activities (Table 1). Therefore, the remediation of metal-contaminated soils is essential to mitigate the negative impacts on ecosystems. Conventional remediation methods for metal-contaminated soils (e.g., vitrification, soil incineration, excavation and landfill, soil washing, soil flushing, solidification and stabilization, electro-kinetic systems) often involve high costs, energy consumption, and potential secondary pollution [101,102]. In contrast, phytoremediation has emerged as a cheaper and environmentally friendly alternative, offering a “green” solution to mitigate metal pollution through the use of plants to extract, stabilize or degrade contaminants in the soil and water [101].
Different types of phytoremediation methods can be applied to reduce metal contamination in soil and water, including phytoextraction, phytovolatilization, phytostabilization and phytofiltration. These methods are based on distinct physiological processes performed by plants [103]. Depending on the type and form of contaminants, as well as the medium involved, different plant species may utilize one or a combination of these mechanisms to effectively remediate polluted environments [104]. The plant species Noccaea caerulescens, Noea mucronata, Prosopis laevigata, Siegesbeckia orientalis, Sedum plumbizincicola, Alyssoides utriculata and Plantago major are considered metal hyperaccumulators, and are potentially good choices for phytoremediation strategies [105]. Moreover, plant physiology and the success of phytoremediation are influenced not only by abiotic factors such as soil properties and climate but also by biotic interactions with insects, pests, and microorganisms. These factors need to be considered in phytoremediation strategies. Additionally, site management practices play a crucial role in determining the overall outcome [106].
Phytoremediation depends on the establishment and growth of vegetation, which in turn requires favorable conditions such as a suitable climate, soil type and agronomic practices, and the careful selection of plant species. Considering that soil characteristics and weather conditions vary significantly between sites, the effectiveness of phytoremediation observed with a particular plant species in one location may not be replicated elsewhere, making the process highly site-specific [106]. In this sense, it is recommended to use plant species native or “naturalized” to the region being remediated [105]. Additionally, phytoremediation generally requires a longer time and tends to be less effective compared to conventional remediation methods. However, its efficiency can be improved through genetic engineering, and the use of beneficial microorganisms and soil amendments [106]. Therefore, a comprehensive understanding of these multiple factors is essential to optimize phytoremediation strategies and enhance their effectiveness in diverse environmental conditions.
In addition to strategies focused on remediating metal pollution, it is necessary to reduce the amount of metals released into the environment. This is the most relevant action to reduce metal pollution in ecosystems. This goal can be achieved by developing mining technologies that generate less mining waste. However, the most needed action is to reduce society’s demand for metals/mining by promoting more sustainable lifestyles, which involve reducing consumption patterns, improving public transportation and recycling electronic products that contain metals. In other words, it is necessary to promote economic systems that value social well-being and healthy human development rather than focusing on economic development as the main objective [107,108]. Moreover, considering the metal load present in pesticides [5,109], it is required to strengthen projects focused on the production of foods and other agricultural products that are free of pesticides, such as agroecological systems and organic “family” farming, limiting the expansion of monocultures and other forms of production that degrade ecosystems and contribute to metal pollution globally. Finally, it is important to invest in monitoring metals in humans, the environment and animals in order to identify the locations and species most vulnerable to metal pollution through a One Health approach. These strategies will help reduce the undesirable effects of metals on infectious diseases in an integrated and realistic way.
7. Conclusions
As evidenced in Table 2 and discussed throughout this article, there is a clear need for further studies investigating how metals influence the risk of infections in animals living in natural environments, especially for certain animal groups, such as insects and reptiles. These studies are essential to add pieces to the puzzle of zoonotic diseases in the Anthropocene, when metal pollution is increasing rapidly, harming wildlife and humans.
Exposure to toxic metals from the environment or through food sources impairs the immune system of different animal species, facilitating infectious processes in natural environments. Poor immune function caused by nutritional deficiencies in essential metals also facilitates infections. Other metal-mediated mechanisms, such as the regulation of immune system genes, host–pathogen interactions and metal-induced damage to mucosal barriers, could also explain how metals influence the risk of infectious processes. Interestingly, some parasites can protect hosts from metal toxicity through the process of biodilution.
In addition to physiological and immunological mechanisms, direct and indirect ecological processes explain, at least in part, the association between metals and infectious diseases. We suggest that animal exposure to toxic metals may facilitate zoonotic spillover events and the emergence of new diseases in human populations. Moreover, due to the complex interactions between metals, the environment and different animals, as well as considering that both low and high levels of a given element can affect the host immune system, the identification of ranges of exposure and the association between such ranges and specific outcomes is mandatory to obtain a better comprehension of metal-related infectious processes. Finally, controlling metal pollution is essential to preserve the health of humans and non-human animals, in addition to protecting ecosystems.
Author Contributions
Conceptualization, J.H.E.; validation, M.Z.; writing—original draft preparation, J.H.E.; writing—review and editing, M.Z. and J.A.B.C.; visualization, J.H.E.; supervision, J.A.B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, Brazil (J.H.E., postoctoral fellowship, Programa Institucional de Pós-Doutorado, Finance Code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, Brazil (M.Z., doctoral fellowship; J.A.B.C., Bolsa de Produtividade em Pesquisa, nível 1A), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul—FAPERGS, Brazil (J.A.B.C., Programa Pesquisador Gaúcho).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The figures and the graphical abstract were created using Microsoft 365.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nnaji, N.D.; Onyeaka, H.; Miri, T.; Ugwa, C. Bioaccumulation for heavy metal removal: A review. SN Appl. Sci. 2023, 5, 125. [Google Scholar] [CrossRef]
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.G.; Hu, Q.; Zhao, F.J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Berry, M.J.; Ralston, N.V. Mercury toxicity and the mitigating role of selenium. Ecohealth 2008, 5, 456–459. [Google Scholar] [CrossRef]
- Nriagu, J.O. A History of Global Metal Pollution. Science 1996, 272, 223–224. [Google Scholar] [CrossRef]
- Naccarato, A.; Vommaro, M.L.; Amico, D.; Sprovieri, F.; Pirrone, N.; Tagarelli, A.; Giglio, A. Triazine Herbicide and NPK Fertilizer Exposure: Accumulation of Heavy Metals and Rare Earth Elements, Effects on Cuticle Melanization, and Immunocompetence in the Model Species Tenebrio molitor. Toxics 2023, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Naccarato, A.; Vommaro, M.L.; Elliani, R.; Babczyńska, A.; Tagarelli, A.; Giglio, A. Tyre and road wear particles as carriers of metals and rare earth elements: Evidence of bioaccumulation in Tenebrio molitor. J. Hazard. Mater. 2025, 494, 138556. [Google Scholar] [CrossRef]
- Polukarova, M.; Gaggini, E.L.; Rødland, E.; Sokolova, E.; Bondelind, M.; Gustafsson, M.; Strömvall, A.M.; Andersson-Sköld, Y. Tyre wear particles and metals in highway roadside ditches: Occurrence and potential transport pathways. Environ. Pollut. 2025, 372, 125971. [Google Scholar] [CrossRef]
- Jasansky, S.; Lieber, M.; Giljum, S.; Maus, V. An open database on global coal and metal mine production. Sci. Data 2023, 10, 52. [Google Scholar] [CrossRef]
- Hatje, V.; Pedreira, R.M.A.; de Rezende, C.E.; Schettini, C.A.F.; de Souza, G.C.; Marin, D.C.; Hackspacher, P.C. The environmental impacts of one of the largest tailing dam failures worldwide. Sci. Rep. 2017, 7, 10706. [Google Scholar] [CrossRef] [PubMed]
- Vergilio, C.S.; Lacerda, D.; Oliveira, B.C.V.; Sartori, E.; Campos, G.M.; Pereira, A.L.S.; Aguiar, D.B.; Souza, T.D.S.; Almeida, M.G.; Thompson, F.; et al. Metal concentrations and biological effects from one of the largest mining disasters in the world (Brumadinho, Minas Gerais, Brazil). Sci. Rep. 2020, 10, 5936. [Google Scholar] [CrossRef] [PubMed]
- Domingues, V.S.; Colmenero, C.; Vinograd, M.; Oliveira-da-Costa, M.; Balbueno, R. Mercury Dynamics and Bioaccumulation Risk Assessment in Three Gold Mining-Impacted Amazon River Basins. Toxics 2024, 12, 599. [Google Scholar] [CrossRef]
- Luo, X.; Ren, B.; Hursthouse, A.S.; Thacker, J.R.M.; Wang, Z. Soil from an Abandoned Manganese Mining Area (Hunan, China): Significance of Health Risk from Potentially Toxic Element Pollution and Its Spatial Context. Int. J. Environ. Res. Public Health 2020, 17, 6554. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A.; Lubaba Nkulu, C.B.; Mwitwa, J.; Kampemba, F.M.; Nabuyanda, M.M. Impacts of Trace Metals Pollution of Water, Food Crops, and Ambient Air on Population Health in Zambia and the DR Congo. J. Environ. Public Health 2022, 2022, 4515115. [Google Scholar] [CrossRef]
- Das, P.K.; Das, B.P.; Dash, P. Chromite mining pollution, environmental impact, toxicity and phytoremediation: A review. Environ. Chem. Lett. 2021, 19, 1369–1381. [Google Scholar] [CrossRef]
- Olías, M.; Nieto, J.M. Background Conditions and Mining Pollution throughout History in the Río Tinto (SW Spain). Environments 2015, 2, 295–316. [Google Scholar] [CrossRef]
- Saunders, R.L.; Sprague, J.B. Effects of copper-zinc mining pollution on a spawning migration of atlantic salmon. Water Res. 1967, 1, 419–432. [Google Scholar] [CrossRef]
- Soe, P.S.; Kyaw, W.T.; Arizono, K.; Ishibashi, Y.; Agusa, T. Mercury Pollution from Artisanal and Small-Scale Gold Mining in Myanmar and Other Southeast Asian Countries. Int. J. Environ. Res. Public Health 2022, 19, 6290. [Google Scholar] [CrossRef]
- Beck, K.; Mariani, M.; Fletcher, M.; Schneider, L.; Aquino-López, M.; Gadd, P.; Heijnis, H.; Saunders, K.; Zawadzki, A. The impacts of intensive mining on terrestrial and aquatic ecosystems: A case of sediment pollution and calcium decline in cool temperate Tasmania, Australia. Environ. Pollut. 2020, 265, 114695. [Google Scholar] [CrossRef]
- Winde, F.; Sandham, L.A. Uranium pollution of South African streams—An overview of the situation in gold mining areas of the Witwatersrand. Geo J. 2004, 61, 131–149. [Google Scholar] [CrossRef]
- Ramani, S.; Dragun, Z.; Kapetanović, D.; Kostov, V.; Jordanova, M.; Erk, M.; Hajrulai-Musliu, Z. Surface Water Characterization of Three Rivers in the Lead/Zinc Mining Region of Northeastern Macedonia. Arch. Environ. Contam. Toxicol. 2014, 66, 514–528. [Google Scholar] [CrossRef]
- Stamatis, G.; Voudouris, K.; Karefilakis, F. Groundwater Pollution by Heavy Metals in Historical Mining Area of Lavrio, Attica, Greece. Water Air Soil. Pollut. 2001, 128, 61–83. [Google Scholar] [CrossRef]
- Maravelias, C.; Hatzakis, A.; Katsouyanni, K.; Trichopoulos, D.; Koutselinis, A.; Ewers, U.; Brockhaus, A. Exposure to lead and cadmium of children living near a lead smelter at Lavrion, Greece. Sci. Total Environ. 1989, 84, 61–70. [Google Scholar] [CrossRef] [PubMed]
- OMS-Organização Mundial da Saúde. Children and digital dumpsites. In E-Waste Exposure and Child Health; Organização Mundial da Saúde: Geneva, Switzerland, 2022. [Google Scholar]
- Ackland, M.L.; Bornhorst, J.; Dedoussis, G.V.; Dietert, R.R.; Nriagu, J.O.; Pacyna, J.M.; Pettifor, J.M. Metals in the Environment as Risk Factors for Infectious Diseases: Gaps and Opportunities. In Trace Metals and Infectious Diseases; Nriagu, J.O., Skaar, E.P., Eds.; MIT Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Harthill, M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Lipner, E.M.; French, J.P.; Nelson, S.; Falkinham Iii, J.O.; Mercaldo, R.A.; Blakney, R.A.; Daida, Y.G.; Frankland, T.B.; Messier, K.P.; Honda, J.R.; et al. Vanadium in groundwater aquifers increases the risk of MAC pulmonary infection in O’ahu, Hawai’i. Environ. Epidemiol. 2022, 6, e220. [Google Scholar] [CrossRef]
- Skaar, E.P.; Raffatellu, M. Metals in infectious diseases and nutritional immunity. Metallomics 2015, 7, 926–928. [Google Scholar] [CrossRef]
- Weiss, G.; Carver, P.L. Role of divalent metals in infectious disease susceptibility and outcome. Clin. Microbiol. Infect. 2018, 24, 16–23. [Google Scholar] [CrossRef]
- Bichet, C.; Scheifler, R.; Cœurdassier, M.; Julliard, R.; Sorci, G.; Loiseau, C. Urbanization, trace metal pollution, and malaria prevalence in the house sparrow. PLoS ONE 2013, 8, e53866. [Google Scholar] [CrossRef]
- Kosoy, M.; Biggins, D. Plague and Trace Metals in Natural Systems. Int. J. Environ. Res. Public Health 2022, 19, 9979. [Google Scholar] [CrossRef]
- Weinberg, E.D. The influence of soil on infectious disease. Experientia 1987, 43, 81–87. [Google Scholar] [CrossRef]
- NIH—National Institutes of Health, National Library of Medicine, National Center for Biotechnology Information. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 16 April 2025).
- Google. Google Scholar. Available online: https://scholar.google.com/ (accessed on 16 April 2025).
- Szentgyörgyi, H.; Blinov, A.; Eremeeva, N.; Luzyanin, S.; Grześ, I.M.; Woyciechowski, M. Bumblebees (Bombidae) along pollution gradient–Heavy metal accumulation, species diversity, and Nosema bombi infection level. Pol. J. Ecol. 2011, 59, 599–610. [Google Scholar]
- Evans, D.W.; Irwin, S.W.B.; Fitzpatrick, S. The effect of digenean (Platyhelminthes) infections on heavy metal concentrations in Littorina littorea. J. Mar. Biol. Assoc. UK 2001, 81, 349–350. [Google Scholar] [CrossRef]
- Baudrimont, M.; de Montaudouin, X.; Palvadeau, A. Impact of digenean parasite infection on metallothionein synthesis by the cockle (Cerastoderma edule): A multivariate field monitoring. Mar. Pollut. Bull. 2006, 52, 494–502. [Google Scholar] [CrossRef]
- Sharaf El-Din, A.T.; Mohamed, M.A.; Mohamed, A.H.; El-Hommossany, K.M.; Habib, M.R. Relationship between Some Heavy Metals and Schistosoma mansoni Infection Rates in Biomphalaria alexandrina Snails Collected from Different Egyptian Localities. World Appl. Sci. J. 2010, 11, 38–43. [Google Scholar]
- Pippi, J.H.C.; Hare, G.M. Relationship of River Pollution to Bacterial Infection in Salmon (Salmo salar) and Suckers (Catostomus commersoni). Trans. Am. Fish. Soc. 1969, 4, 685–690. [Google Scholar] [CrossRef]
- Hassanine, R.; Al-Hasawi, Z. Acanthocephalan Worms Mitigate the Harmful Impacts of Heavy Metal Pollution on Their Fish Hosts. Fishes 2021, 6, 49. [Google Scholar] [CrossRef]
- Marijić, V.F.; Smrzlić, I.V.; Raspor, B. Effect of acanthocephalan infection on metal, total protein and metallothionein concentrations in European chub from a Sava River section with low metal contamination. Sci. Total Environ. 2013, 463–464, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, J.H.; Wepener, V.; Nachev, M.; Zimmermann, S.; Malherbe, W.; Sures, B.; Smit, N.J. The role of fish helminth parasites in monitoring metal pollution in aquatic ecosystems: A case study in the world’s most productive platinum mining region. Parasitol. Res. 2020, 119, 2783–2798. [Google Scholar] [CrossRef]
- Mijošek, T.; Šariri, S.; Kljaković-Gašpić, Z.; Fiket, Ž.; Filipović Marijić, V. Interrelation between environmental conditions, acanthocephalan infection and metal(loid) accumulation in fish intestine: An in-depth study. Environ. Pollut. 2024, 356, 124358. [Google Scholar] [CrossRef]
- Soliman, M.F. Heavy metal pollution across sites affecting the intestinal helminth communities of the Egyptian lizard, Chalcides ocellatus (Forskal, 1775). Environ. Monit. Assess. 2012, 184, 7677–7685. [Google Scholar] [CrossRef]
- Khan, E.A.; Greve, M.; Russell, I.; Ciesielski, T.M.; Lundregan, S.; Jensen, H.; Rønning, B.; Bones, A.M.; Asimakopoulos, A.G.; Waugh, C.A.; et al. Lead exposure is related to higher infection rate with the gapeworm in Norwegian house sparrows (Passer. domesticus). Environ. Pollut. 2024, 344, 123443. [Google Scholar] [CrossRef]
- Mashima, T.Y.; Fleming, W.J.; Stoskopf, M.K. Metal concentrations in oldsquaw (Clangula hyemalis) during an outbreak of avian cholera, Chesapeake Bay, 1994. Ecotoxicology 1998, 7, 107–111. [Google Scholar] [CrossRef]
- Gasparini, J.; Jacquin, L.; Laroucau, K.; Vorimore, F.; Aubry, E.; Castrec-Rouëlle, M.; Frantz, A. Relationships between metals exposure and epidemiological parameters of two pathogens in urban pigeons. Bull. Environ. Contam. Toxicol. 2014, 92, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.M.; Jepson, P.D.; Law, R.J.; Jones, B.R.; Kuiken, T.; Baker, J.R.; Rogan, E.; Kirkwood, J.K. Exposure to heavy metals and infectious disease mortality in harbour porpoises from England and Wales. Environ. Pollut. 2001, 112, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tersago, K.; De Coen, W.; Scheirs, J.; Vermeulen, K.; Blust, R.; Van Bockstaele, D.; Verhagen, R. Immunotoxicology in wood mice along a heavy metal pollution gradient. Environ. Pollut. 2004, 132, 385–394. [Google Scholar] [CrossRef]
- Jankovská, I.; Langrová, I.; Bejcek, V.; Miholová, D.; Vadlejch, J.; Petrtyl, M. Heavy metal accumulation in small terrestrial rodents infected by cestodes or nematodes. Parasite 2008, 15, 581–588. [Google Scholar] [CrossRef]
- Brožová, A.; Jankovská, I.; Miholová, D.; Scháňková, Š.; Truněčková, J.; Langrová, I.; Kudrnáčová, M.; Vadlejch, J. Heavy metal concentrations in the small intestine of red fox (Vulpes vulpes) with and without Echinococcus multilocularis infection. Environ. Sci. Pollut. Res. Int. 2015, 22, 3175–3179. [Google Scholar] [CrossRef]
- Jankovská, I.; Miholová, D.; Bejcek, V.; Vadlejch, J.; Sulc, M.; Száková, J.; Langrová, I. Influence of parasitism on trace element contents in tissues of red fox (Vulpes vulpes) and its parasites Mesocestoides spp. (Cestoda) and Toxascaris leonina (Nematoda). Arch. Environ. Contam. Toxicol. 2010, 58, 469–477. [Google Scholar] [CrossRef]
- Becker, D.J.; Speer, K.A.; Korstian, J.M.; Volokhov, D.V.; Droke, H.F.; Brown, A.M.; Baijnauth, C.L.; Padgett-Stewart, T.; Broders, H.G.; Plowright, R.K.; et al. Disentangling interactions among mercury, immunity and infection in a Neotropical bat community. J. Appl. Ecol. 2021, 58, 879–889. [Google Scholar] [CrossRef]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, M.; Guo, Q.; Yan, S. Transfer of heavy metal along food chain: A mini-review on insect susceptibility to entomopathogenic microorganisms under heavy metal stress. Pest. Manag. Sci. 2021, 77, 1115–1120. [Google Scholar] [CrossRef]
- Sorvari, J.; Rantala, L.M.; Rantala, M.J.; Hakkarainen, H.; Eeva, T. Heavy metal pollution disturbs immune response in wild ant populations. Environ. Pollut. 2007, 145, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, A.; Iatsenko, I. The roles of metals in insect-microbe interactions and immunity. Curr. Opin. Insect Sci. 2022, 49, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Lang, M. A Comprehensive Review on the Roles of Metals Mediating Insect-Microbial Pathogen Interactions. Metabolites 2023, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Sures, B.; Siddall, R.; Taraschewski, H. Parasites as accumulation indicators of heavy metal pollution. Parasitol. Today 1999, 15, 16–21. [Google Scholar] [CrossRef]
- Abd Allah, A.T.; Wanas, M.Q.S.; Thompson, S.N. Effects of heavy metals on survival and growth of Biomphalaria glabrata say (Gastropoda: Pulmonata) and interaction with Schistosome infection. J. Molluscan Stud. 1997, 63, 79–86. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Energy allocation patterns in Biomphalaria alexandrina snails in response to cadmium exposure and Schistosoma mansoni infection. Exp. Parasitol. 2006, 112, 31–36. [Google Scholar] [CrossRef]
- Bagheri, S.; Gholamhosseini, A.; Hoseinifar, S.H.; Banaee, M. Investigation of the effects of heavy metals (copper, cobalt, manganese, selenium, and zinc) on fish immune systems—An overview. Ann. Anim. Sci. 2024, 24, 1025–1035. [Google Scholar] [CrossRef]
- Sures, B.; Siddall, R. Pomphorhynchus laevis (Palaeacanthocephala) in the intestine of chub (Leuciscus cephalus) as an indicator of metal pollution. Int. J. Parasitol. 2003, 33, 65–70. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Brazil’s heavy metal pollution harms humans and ecosystems. Sci. One Health 2023, 2, 100019. [Google Scholar] [CrossRef]
- Das, K.; Siebert, U.; Gillet, A.; Dupont, A.; Di-Poï, C.; Fonfara, S.; Mazzucchelli, G.; De Pauw, E.; De Pauw-Gillet, M.C. Mercury immune toxicity in harbour seals: Links to in vitro toxicity. Environ. Health 2008, 7, 52. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The toxicology of mercury: Current research and emerging trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Coll, N.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Vidal, D.; Mateo, R. Sublethal Pb exposure produces season-dependent effects on immune response, oxidative balance and investment in carotenoid-based coloration in red-legged partridges. Environ. Sci. Technol. 2015, 49, 3839–3850. [Google Scholar] [CrossRef]
- Xie, S.; Jiang, L.; Wang, M.; Sun, W.; Yu, S.; Turner, J.R.; Yu, Q. Cadmium ingestion exacerbates Salmonella infection, with a loss of goblet cells through activation of Notch signaling pathways by ROS in the intestine. J. Hazard. Mater. 2020, 391, 122262. [Google Scholar] [CrossRef] [PubMed]
- Sures, B.; Scheef, G.; Klar, B.; Kloas, W.; Taraschewski, H. Interaction between cadmium exposure and infection with the intestinal parasite Moniliformis moniliformis (Acanthocephala) on the stress hormone levels in rats. Environ. Pollut. 2002, 119, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Hsu, K.S.; Goodale, B.C.; Ely, K.H.; Hampton, T.H.; Stanton, B.A.; Enelow, R.I. Single-cell RNA-seq Analysis Reveals That Prenatal Arsenic Exposure Results in Long-term, Adverse Effects on Immune Gene Expression in Response to Influenza A Infection. Toxicol. Sci. 2020, 176, 312–328. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Ziliotto, M.; Chies, J.A.B. Toxicogenomics of Arsenic, Lead and Mercury: The Toxic Triad. Pollutants 2025, 5, 18. [Google Scholar] [CrossRef]
- Murdoch, C.C.; Skaar, E.P. Nutritional immunity: The battle for nutrient metals at the host-pathogen interface. Nat. Rev. Microbiol. 2022, 20, 657–670. [Google Scholar] [CrossRef]
- Sures, B. Environmental parasitology. Interactions between parasites and pollutants in the aquatic environment. Parasite 2008, 15, 434–438. [Google Scholar] [CrossRef]
- Sures, B. Host–parasite interactions in polluted environments. J. Fish. Biol. 2008, 73, 2133–2142. [Google Scholar] [CrossRef]
- Afzal, A.; Mahreen, N. Emerging insights into the impacts of heavy metals exposure on health, reproductive and productive performance of livestock. Front. Pharmacol. 2024, 15, 1375137. [Google Scholar] [CrossRef] [PubMed]
- Ziliotto, M.; Chies, J.A.B.; Ellwanger, J.H. Toxicogenomics of persistent organic pollutants: Potential impacts on biodiversity and infectious diseases. Anthropocene 2024, 48, 100450. [Google Scholar] [CrossRef]
- Rothenburger, J.L.; Himsworth, C.H.; Nemeth, N.M.; Pearl, D.L.; Jardine, C.M. Environmental Factors and Zoonotic Pathogen Ecology in Urban Exploiter Species. Ecohealth 2017, 14, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Floryszak-Wieczorek, J.; Sobieszczuk-Nowicka, E.; Mattoo, A.; Arasimowicz-Jelonek, M. Fungal and oomycete pathogens and heavy metals: An inglorious couple in the environment. IMA Fungus 2022, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, X.; Lin, J.; Wang, X.; He, D.; Zeng, R.; Meng, J.; Luo, J.; Delgado-Baquerizo, M.; Moreno-Jiménez, E.; et al. Metallic micronutrients are associated with the structure and function of the soil microbiome. Nat. Commun. 2023, 14, 8456. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Kulmann-Leal, B.; Kaminski, V.L.; Valverde-Villegas, J.M.; da Veiga, A.B.G.; Spilki, F.R.; Fearnside, P.M.; Caesar, L.; Giatti, L.L.; Wallau, G.L.; et al. Beyond diversity loss and climate change: Impacts of Amazon deforestation on infectious diseases and public health. An. Acad. Bras. Cienc. 2020, 92, e20191375. [Google Scholar] [CrossRef]
- Douine, M.; Bonifay, T.; Lambert, Y.; Mutricy, L.; Galindo, M.S.; Godin, A.; Bourhy, P.; Picardeau, M.; Saout, M.; Demar, M.; et al. Zoonoses and gold mining: A cross-sectional study to assess yellow fever immunization, Q fever, leptospirosis and leishmaniasis among the population working on illegal mining camps in French Guiana. PLoS Negl. Trop. Dis. 2022, 16, e0010326. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Fearnside, P.M.; Ziliotto, M.; Valverde-Villegas, J.M.; da Veiga, A.B.G.; Vieira, G.F.; Bach, E.; Cardoso, J.C.; Müller, N.F.D.; Lopes, G.; et al. Synthesizing the connections between environmental disturbances and zoonotic spillover. An. Acad. Bras. Cienc. 2022, 94, e20211530. [Google Scholar] [CrossRef]
- Keck, F.; Peller, T.; Alther, R.; Barouillet, C.; Blackman, R.; Capo, E.; Chonova, T.; Couton, M.; Fehlinger, L.; Kirschner, D.; et al. The global human impact on biodiversity. Nature, 2025; in press. [Google Scholar] [CrossRef]
- Mahon, M.B.; Sack, A.; Aleuy, O.A.; Barbera, C.; Brown, E.; Buelow, H.; Civitello, D.J.; Cohen, J.M.; de Wit, L.A.; Forstchen, M.; et al. A meta-analysis on global change drivers and the risk of infectious disease. Nature 2024, 629, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic spillover: Understanding basic aspects for better prevention. Genet. Mol. Biol. 2021, 44, e20200355. [Google Scholar] [CrossRef] [PubMed]
- Plowright, R.K.; Eby, P.; Hudson, P.J.; Smith, I.L.; Westcott, D.; Bryden, W.L.; Middleton, D.; Reid, P.A.; McFarlane, R.A.; Martin, G.; et al. Ecological dynamics of emerging bat virus spillover. Proc. Biol. Sci. 2015, 282, 20142124. [Google Scholar] [CrossRef] [PubMed]
- Jolles, A.E.; Beechler, B.R.; Dolan, B.P. Beyond mice and men: Environmental change, immunity and infections in wild ungulates. Parasite Immunol. 2015, 37, 255–266. [Google Scholar] [CrossRef]
- Plowright, R.K.; Parrish, C.R.; McCallum, H.; Hudson, P.J.; Ko, A.I.; Graham, A.L.; Lloyd-Smith, J.O. Pathways to zoonotic spillover. Nat. Rev. Microbiol. 2017, 15, 502–510. [Google Scholar] [CrossRef]
- Beck, M.A. Trace Minerals, Immune Function, and Viral Evolution. In Military Strategies for Sustainment of Nutrition and Immune Function in the Field; Institute of Medicine (US), Committee on Military Nutrition Research; National Academies Press: Washington DC, WA, USA, 1999. [Google Scholar]
- Trevejo, R.T.; Barr, M.C.; Robinson, R.A. Important emerging bacterial zoonotic infections affecting the immunocompromised. Vet. Res. 2005, 36, 493–506. [Google Scholar] [CrossRef]
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Ziliotto, M.; Kulmann-Leal, B.; Chies, J.A.B. Iron deficiency and soil-transmitted helminth infection: Classic and neglected connections. Parasitol. Res. 2022, 121, 3381–3392. [Google Scholar] [CrossRef]
- Griffiths, B.M.; Luther, D.C.; Pollock, H.S. Mineral Licks: An Overlooked Model System for Species Interactions. Biotropica 2025, 57, e70003. [Google Scholar] [CrossRef]
- Harada, M. The Global Lessons of Minamata Disease: An Introduction to Minamata Studies. In Taking Life and Death Seriously-Bioethics from Japan; Takahashi, T., Ed.; Emerald Group Publishing Limited: Leeds, UK, 2005; Volume 8, pp. 299–335. [Google Scholar] [CrossRef]
- Balogh, S.J.; Tsui, T.; Blum, J.D.; Matsuyama, A.; Woerndle, G.E.; Yano, S.; Tada, A. Tracking the Fate of Mercury in the Fish and Bottom Sediments of Minamata Bay, Japan, Using Stable Mercury Isotopes. Environ. Sci. Technol. 2015, 49, 5399–5406. [Google Scholar] [CrossRef]
- Neuwirth, L.S.; Whigham, K. “Only Time Will Tell”: The Underexplored Impacts of Lead Poisoning and COVID-19 on Pre-Existing ACEs in New York. Youth 2023, 3, 1212–1224. [Google Scholar] [CrossRef]
- De Alencar, L.L.; Henriques, G.S.; Cozzolino, S.M.F. Ferro. In Biodisponibilidade de Nutrientes, 7th ed.; Cozzolino, S.M.F. (Org.). Manole: Barueri, Brazil, 2024; Chapter 29; pp. 483–504. [Google Scholar]
- Duarte, G.B.S.; Vale, S.H.L.; Reis, B.Z.; Cozzolino, S.M.F. Zinco. In Biodisponibilidade de Nutrientes, 7th ed.; Cozzolino, S.M.F. (Org.). Manole: Barueri, Brazil, 2024; Chapter 31; pp. 299–335. [Google Scholar]
- WHO—World Health Organization. Lead Poisoning. Available online: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (accessed on 24 June 2025).
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Bernardino, C.A.R.; Mahler, C.F.; Preussler, K.H.; Novo, L.A.B. State of the Art of Phytoremediation in Brazil—Review and Perspectives. Water Air Soil. Pollut. 2016, 227, 272. [Google Scholar] [CrossRef]
- Wang, J.; Aghajani Delavar, M. Techno-economic analysis of phytoremediation: A strategic rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Triple planetary crisis and emerging zoonotic diseases: Consider degrowth as a solution. Earth Critical Zone 2025, 100030. [Google Scholar] [CrossRef]
- Hickel, J.; Kallis, G.; Jackson, T.; O’Neill, D.W.; Schor, J.B.; Steinberger, J.K.; Victor, P.A.; Ürge-Vorsatz, D. Degrowth can work-here’s how science can help. Nature 2022, 612, 400–403. [Google Scholar] [CrossRef]
- Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2017, 5, 156–163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).