Innovative Approaches and Evolving Strategies in Heavy Metal Bioremediation: Current Limitations and Future Opportunities
Abstract
1. Introduction
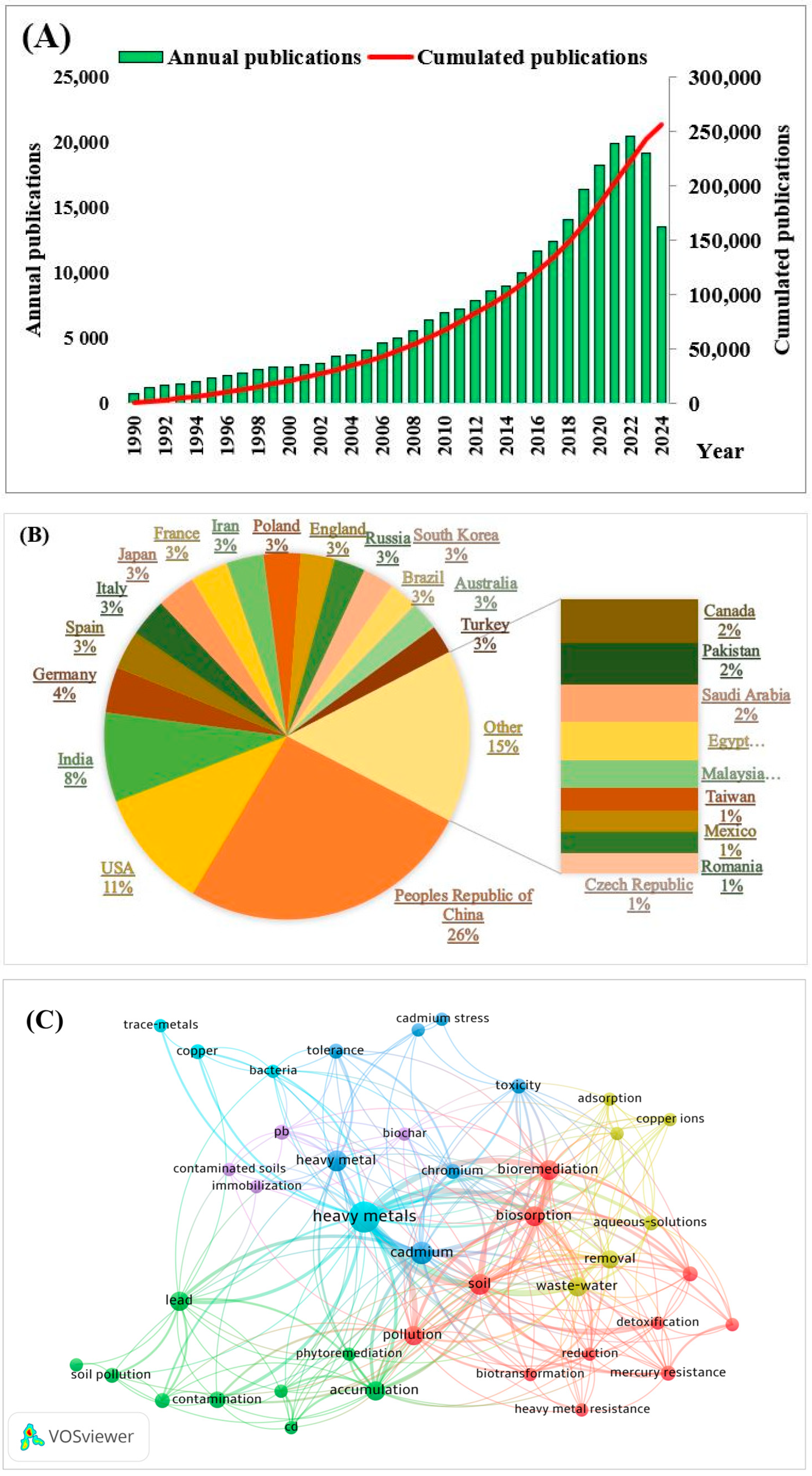
Research Publications on Heavy Metals
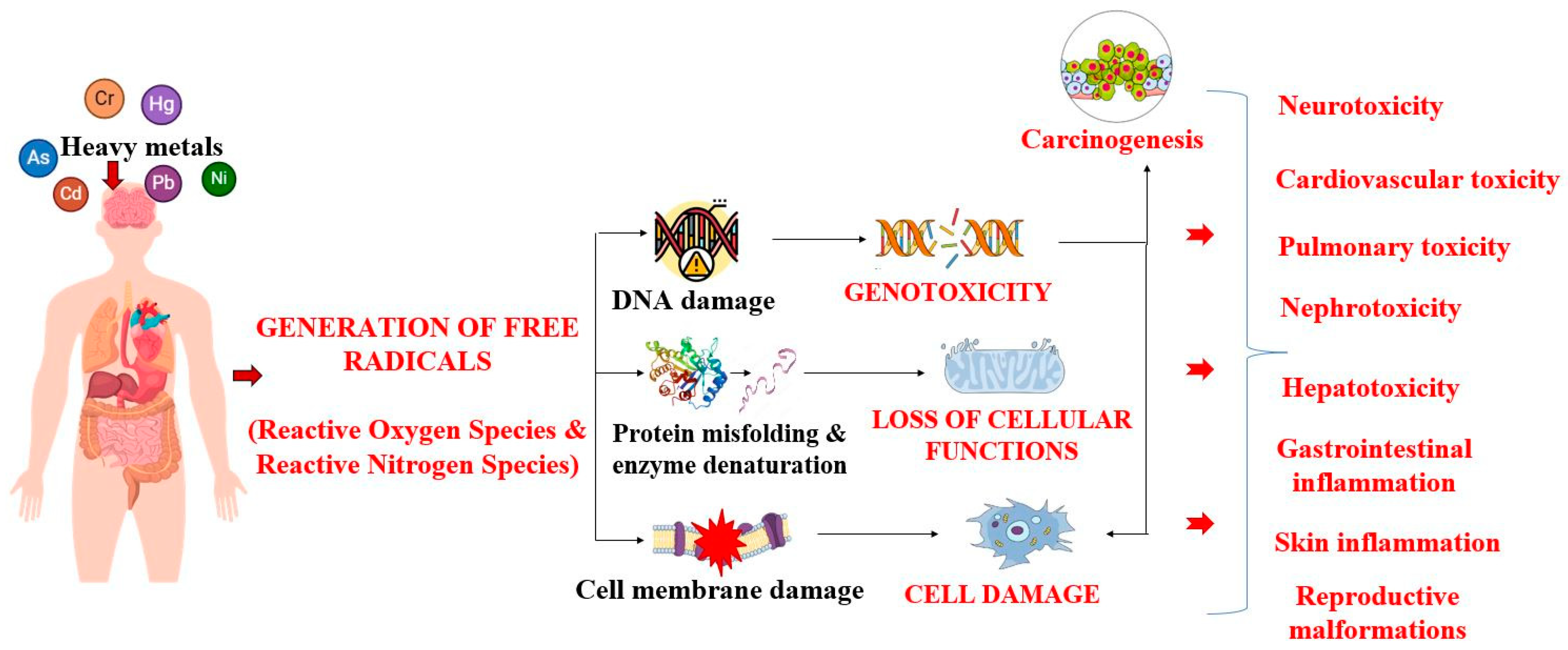
2. Heavy Metals Occurrence and Toxicity in the Environment
3. Biological Remediation Techniques Overview
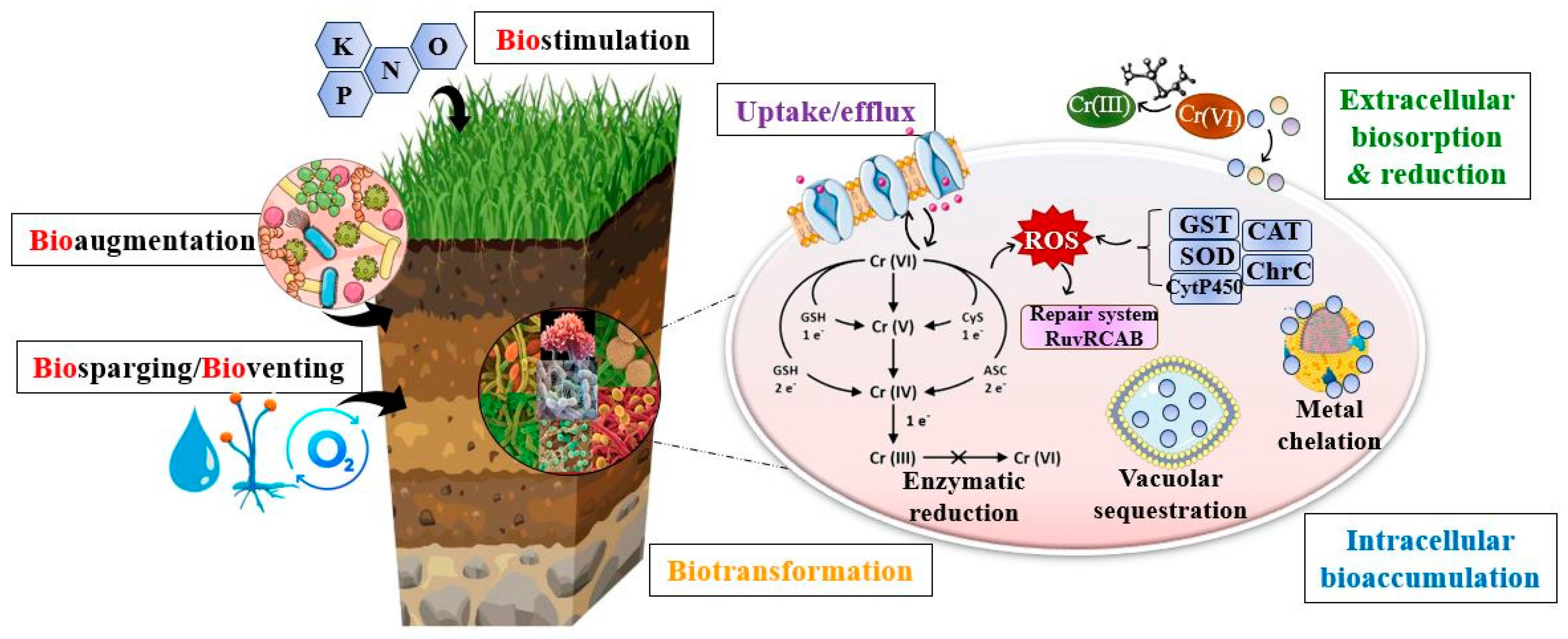
3.1. Bioremediation
3.2. Phytoremediation
4. Novel Approaches and Emerging Strategies
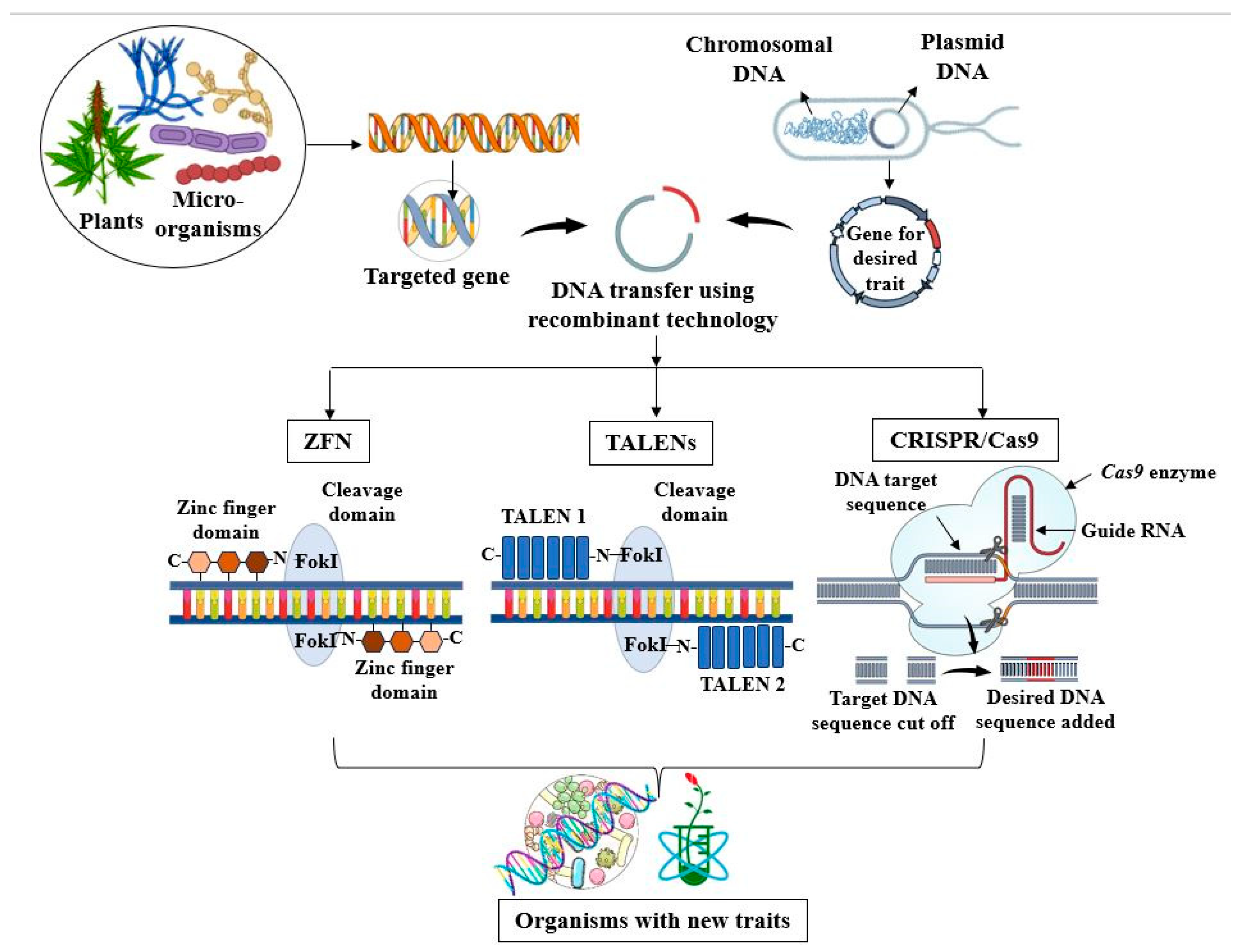
4.1. Genetic Engineering in Bioremediation
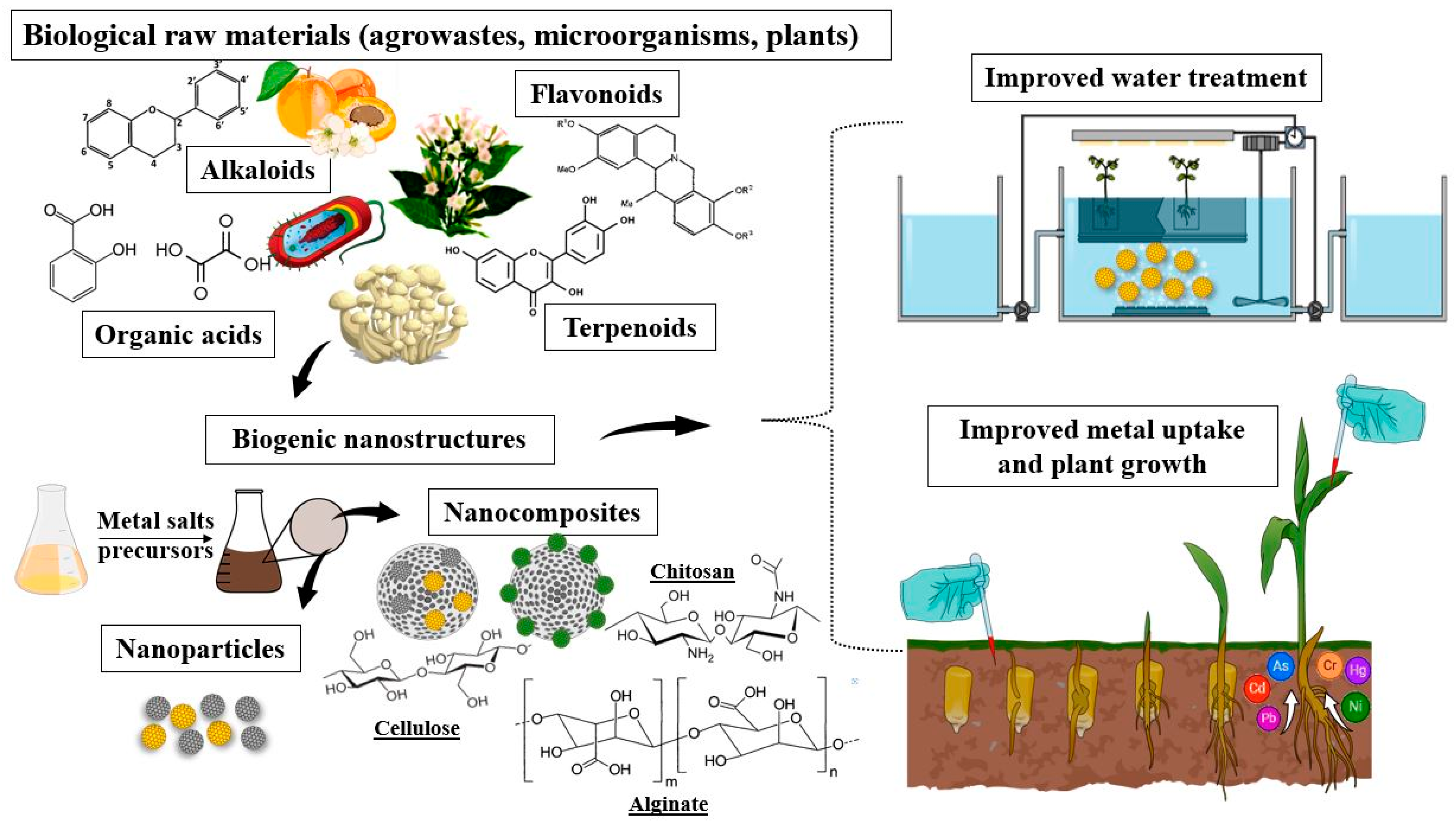
4.2. Nano-Assisted Bioremediation: Synergistic Approaches for Environmental Restoration
5. Complexities and Constraints in Bioremediation: Addressing Key Scientific and Practical Challenges
5.1. From Lab Controlled Experiments to Biosphere: Scaling up Bioremediation for Environmental Restoration to Real-World Implementation
- Initial investment and operational costs—Bioremediation involves lower setup and operational costs, especially for in situ applications (e.g., natural attenuation and bioaugmentation). It utilizes natural or engineered biological systems, reducing the dependency on expensive chemicals and high-energy processes. Depending on the type of bioremediation, costs may vary. For example, phytoremediation, although slower, may require up to 50% less cost compared with conventional treatments, while bioaugmentation requires more investments in process control, strain maintenance, and monitoring [210,211]. Physicochemical methods require high initial costs for equipment, chemicals, and infrastructure, as well as ongoing operational costs due to energy-intensive processes (e.g., electrochemical treatment or advanced oxidation). These methods generate secondary waste, requiring additional treatment and disposal procedures, thus increasing costs [212].
- Long-term sustainability and maintenance costs—Bioremediation tends to be more durable, with minimal long-term maintenance, but some methods such as phytoremediation require months to years to reach the desired level of removal, which can lead to increased monitoring costs. Physico-chemical methods provide rapid removal of heavy metals, but often require repeated applications for sustained results. These methods can degrade the structure of the ecosystem, requiring additional remediation costs [213].
- Cost of waste management—Bioremediation generates less hazardous waste compared to conventional methods, and in some cases the by-products obtained (e.g., biomass or biochar) can be reused for economic value (e.g., fertilizers and biofuels). Physico-chemical methods produce large volumes of toxic sludge or secondary pollutants, which require significant costs for handling, transportation, and disposal [214].
- Cost-effectiveness on a large scale—Bioremediation has the potential to be more economically feasible for large-scale contamination scenarios in integrated practices, both for long-term treatments as well as environmental restoration. Physico-chemical methods, although suitable for highly contaminated sites where rapid treatment is needed, require high costs that make them disadvantageous for long-term remediation [215].
5.2. Management of Bioremediation Waste
5.3. Public Perception and Acceptance from Scientific Innovation to Societal Expectations—Challenges and Pathways Forward
5.4. Future Trends in Bioremediation
- Genetically modified microorganisms (GEMs)—Through advances in biology and genetic engineering, microorganisms with greatly improved metal uptake, resistance, and degradation capabilities have been developed. Novel technologies such as CRISPR and gene editing offer the possibility of optimizing microbial metabolism to create highly specific and efficient mechanisms for bioremediation.
- Nanotechnology—The integration of nanomaterials, such as bio-nanocomposites and biogenic nanoparticles that can stabilize and immobilize metals, is another approach to stimulate microbial activity and control the bioavailability and mobility of metals for efficient remediation;
- Microbial consortia with complementary metabolic pathways can contribute to improved sequestration and degradation of heavy metals;
- Enhancing phytoremediation and rhizoremediation by using genetically modified plants and exploiting plant-microbe interactions represents a sustainable solution, especially for large-scale and in situ applications;
- Electro-bioremediation via integrating electrochemical systems with microbial cells is another approach to improve metal recovery while generating bioenergy, thus leading to a more cost-effective and sustainable remediation process;
- AI (Artificial Intelligence) and Big Data in bioremediation enable AI-based modeling and machine learning to optimize selection programs for microbial strains or plants, predict remediation efficiency, and design large-scale bioremediation strategies with high accuracy and optimized conditions.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Xu, W.; Li, J.; Song, Y.; Hua, M.; Li, W.; Wen, Y.; Li, T.; He, X. Assessing the Fractionation and Bioavailability of Heavy Metals in Soil–Rice System and the Associated Health Risk. Environ. Geochem. Health 2022, 44, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sultana, K.W.; Ndhlala, A.R.; Mondal, M.; Chandra, I. Heavy Metal Pollution in the Environment and Its Impact on Health: Exploring Green Technology for Remediation. Environ. Health Insights 2023, 17, 11786302231201259. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, J.A.; Groover, K.D. Natural and Man-Made Hexavalent Chromium, Cr(VI), in Groundwater near a Mapped Plume, Hinkley, California—Study Progress as of May 2017, and a Summative-Scale Approach to Estimate Background Cr(VI) Concentrations; U.S. Department of the Interior & U.S. Geological Survey: Reston, VA, USA, 2018.
- Ahmad, S.A.; Khan, M.H.; Haque, M. Arsenic Contamination in Groundwater in Bangladesh: Implications and Challenges for Healthcare Policy. Risk Manag. Health Policy 2018, 11, 251–261. [Google Scholar] [CrossRef]
- Mosa, A.; Duffin, J. The Interwoven History of Mercury Poisoning in Ontario and Japan. Can. Med. Assoc. J. 2017, 189, 213–215. [Google Scholar] [CrossRef]
- Michael, C.M. Aftermath of the Bunker Hill Closure. In Leaded: The Poisoning of Idaho’s Silver Valley; Oregon State University Press: Corvallis, OR, USA, 2016; pp. 197–210. [Google Scholar]
- Ali, H.; Khan, E. What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’–proposal of a comprehensive definition. Toxicol. Environ. Chem. 2017, 100, 6–19. [Google Scholar] [CrossRef]
- Maret, W. The Metals in the Biological Periodic System of the Elements: Concepts and Conjectures. Int. J. Mol. Sci. 2016, 17, 66. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Kaspari, M. The Invisible Hand of the Periodic Table: How Micronutrients Shape Ecology. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 199–219. [Google Scholar] [CrossRef]
- Ngu, Y.J.; Skalny, A.V.; Tinkov, A.A.; Tsai, C.-S.; Chang, C.-C.; Chuang, Y.-K.; Nikolenko, V.N.; Zotkin, D.A.; Chiu, C.-F.; Chang, J.-S. Association Between Essential and Non-Essential Metals, Body Composition, and Metabolic Syndrome in Adults. Biol. Trace Elem. Res. 2022, 200, 4903–4915. [Google Scholar] [CrossRef]
- Nucera, S.; Serra, M.; Caminiti, R.; Ruga, S.; Passacatini, L.C.; Macrì, R.; Scarano, F.; Maiuolo, J.; Bulotta, R.; Mollace, R.; et al. Non-Essential Heavy Metal Effects in Cardiovascular Diseases: An Overview of Systematic Reviews. Front. Cardiovasc. Med. 2024, 11, 1332339. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Chang, F.; Duan, L.; Zhang, Y. Distribution and Health-Ecological Risk Assessment of Heavy Metals: An Endemic Disease Case Study in Southwestern China. Environ. Sci. Pollut. Res. 2022, 29, 4260–4275. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Z.; Lin, J.; Zhao, H.; Yu, H.; Ma, B.; Hu, L.; Shi, J.; Chen, X.; Liu, M.; et al. Heavy Metal Contamination Collapses Trophic Interactions in the Soil Microbial Food Web via Bottom-up Regulation. Soil Biol. Biochem. 2023, 184, 109058. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.; Du, D. The Soil PH and Heavy Metals Revealed Their Impact on Soil Microbial Community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef] [PubMed]
- Kiran; Bharti, R.; Sharma, R. Effect of Heavy Metals: An Overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.K.; Verma, R.L.; Chopade, R.P.; Pandit, P.; Nagar, V.; Aseri, V.K.; Choudhary, S.; Awasthi, G.K.; Awasthi, K.S.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; Dorta, D.J., De Oliveira, D.P., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Xu, W.; Jin, Y.; Zeng, G. Introduction of Heavy Metals Contamination in the Water and Soil: A Review on Source, Toxicity and Remediation Methods. Green Chem. Lett. Rev. 2024, 17, 2404235. [Google Scholar] [CrossRef]
- Han, R.; Zhou, B.; Huang, Y.; Lu, X.; Li, S.; Li, N. Bibliometric Overview of Research Trends on Heavy Metal Health Risks and Impacts in 1989–2018. J. Clean. Prod. 2020, 276, 123249. [Google Scholar] [CrossRef]
- Renner, S.; Wellmer, F.W. Volatility Drivers on the Metal Market and Exposure of Producing Countries. Miner. Econ. 2020, 33, 311–340. [Google Scholar] [CrossRef]
- Yang, S.; Sun, L.; Sun, Y.; Song, K.; Qin, Q.; Zhu, Z.; Xue, Y. Towards an Integrated Health Risk Assessment Framework of Soil Heavy Metals Pollution: Theoretical Basis, Conceptual Model, and Perspectives. Environ. Pollut. 2023, 316, 120596. [Google Scholar] [CrossRef]
- Szynkowska, M.I.; Pawlaczyk, A.; Maćkiewicz, E. Bioaccumulation and Biomagnification of Trace Elements in the Environment. In Recent Advances in Trace Elements; Chojnacka, K., Saeid, A., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 251–276. [Google Scholar] [CrossRef]
- Sable, H.; Kumar, V.; Singh, V.; Rustagi, S.; Chahal, S.; Chaudhary, V. Strategically Engineering Advanced Nanomaterials for Heavy-Metal Remediation from Wastewater. Coord. Chem. Rev. 2024, 518, 216079. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of Heavy Metals through Terrestrial Food Webs: A Review. Environ. Monit. Assess. 2015, 187, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Sonone, S.S.; Jadhav, S.; Kumar, R. Water Contamination by Heavy Metals and Their Toxic Effect on Aquaculture and Human Health through Food Chain. Lett. Appl. NanoBioSci. 2020, 10, 2148–2166. [Google Scholar] [CrossRef]
- Adams, W.; Blust, R.; Dwyer, R.; Mount, D.; Nordheim, E.; Rodriguez, P.H.; Spry, D. Bioavailability Assessment of Metals in Freshwater Environments: A Historical Review. Environ. Toxicol. Chem. 2020, 39, 48–59. [Google Scholar] [CrossRef]
- Shahab, A.; Hui, Z.; Rad, S.; Xiao, H.; Siddique, J.; Huang, L.L.; Ullah, H.; Rashid, A.; Taha, M.R.; Zada, N. A Comprehensive Review on Pollution Status and Associated Health Risk Assessment of Human Exposure to Selected Heavy Metals in Road Dust across Different Cities of the World. Environ. Geochem. Health 2023, 45, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy Metals: Toxicity and Human Health Effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar] [CrossRef]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Agents Classified by the IARC Monographs, Volumes 1–136. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 13 January 2025).
- Sunanda; Misra, M.; Ghosh Sachan, S. Nanobioremediation of Heavy Metals: Perspectives and Challenges. J. Basic Microbiol. 2022, 62, 428–443. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Unitated States Environmental Proteiction Agency (US-EPA). National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Inorganics (accessed on 13 January 2025).
- Burmistrz, P.; Kogut, K.; Marczak, M.; Dziok, T.; Górecki, J. Mercury in Polish Coking Bituminous Coals. Energy Fuels 2018, 32, 5677–5683. [Google Scholar] [CrossRef]
- Nachman, K.E.; Ginsberg, G.L.; Miller, M.D.; Murray, C.J.; Nigra, A.E.; Pendergrast, C.B. Mitigating Dietary Arsenic Exposure: Current Status in the United States and Recommendations for an Improved Path Forward. Sci. Total Environ. 2017, 581–582, 221–236. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, T.; Wang, Y.; Zhang, X.; Zhang, H.; Lin, J.; Tang, X.; Liu, X.; Chen, M.; Khan, N.U.; et al. Investigating the Neurotoxic Impacts of Arsenic and the Neuroprotective Effects of Dictyophora Polysaccharide Using SWATH-MS-Based Proteomics. Molecules 2022, 27, 1495. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.S.; Jayasumana, C.; De Silva, P.M.C.S. Arsenic and Human Health Effects: A Review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; M.M.S., C.-P.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead Toxicity: Health Hazards, Influence on Food Chain, and Sustainable Remediation Approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Vijayakumar, N.; Kanimozhi, V.; Arbaaz, S.M.; Stacey, R.G.S.; Anusha, J.; Choudhary, R.; Lvov, V.; Tovar, G.I.; et al. Bioaccumulation of Lead (Pb) and Its Effects on Human: A Review. J. Hazard. Mater. Adv. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Gulson, B.; Mizon, K.; Korsch, M.; Taylor, A. Revisiting Mobilisation of Skeletal Lead during Pregnancy Based on Monthly Sampling and Cord/Maternal Blood Lead Relationships Confirm Placental Transfer of Lead. Arch. Toxicol. 2016, 90, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A Comprehensive Review on the Sources, Essentiality and Toxicological Profile of Nickel. RSC Adv. 2022, 12, 9139–9153. [Google Scholar] [CrossRef]
- Joh, J.-S.; Kang, M.-Y.; Myong, J.-P. Dose–Response Relationship between Environmental Exposure to Nickel and Pulmonary Function in the Korean General Population Aged 40 or Older. Int. J. Environ. Res. Public Health 2021, 18, 7016. [Google Scholar] [CrossRef]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary Concept of Nickel Toxicity—An Overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef]
- Bjørklund, G.; Pivina, L.; Dadar, M.; Semenova, Y.; Chirumbolo, S.; Aaseth, J. Mercury Exposure, Epigenetic Alterations and Brain Tumorigenesis: A Possible Relationship? Curr. Med. Chem. 2020, 27, 6596–6610. [Google Scholar] [CrossRef]
- Ye, B.-J.; Kim, B.-G.; Jeon, M.-J.; Kim, S.-Y.; Kim, H.-C.; Jang, T.-W.; Chae, H.-J.; Choi, W.-J.; Ha, M.-N.; Hong, Y.-S. Evaluation of Mercury Exposure Level, Clinical Diagnosis and Treatment for Mercury Intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Niazi, N.K.; Antunes, P.M.C. Cadmium Bioavailability, Uptake, Toxicity and Detoxification in Soil-Plant System. In Reviews of Environmental Contamination and Toxicology Volume 241. Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 241, pp. 73–137. [Google Scholar] [CrossRef]
- Georgaki, M.-N.; Tsokkou, S.; Keramas, A.; Papamitsou, T.; Karachrysafi, S.; Kazakis, N. Chromium Supplementation and Type 2 Diabetes mellitus: An Extensive Systematic Review. Environ. Geochem. Health 2024, 46, 515. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, E.; Jurkowska, K.; Piwowar, A. Chromium (III) and Chromium (VI) as Important Players in the Induction of Genotoxicity—Current View. Ann. Agric. Environ. Med. 2020, 28, 1–10. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Verma, M.; Kamal, R.; Tiwari, R.; Sanjay Chivate, M.; Rai, S.N.; Kumar, A.; Singh, A.; Singh, M.P.; et al. Hexavalent-Chromium-Induced Oxidative Stress and the Protective Role of Antioxidants against Cellular Toxicity. Antioxidants 2022, 11, 2375. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Selected Aspects of the Action of Cobalt Ions in the Human Body. Cent. Eur. J. Immunol. 2015, 2, 236–242. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Kumar, R.; Thakur, A.; Puri, P.; Kumar, R.; Kanojita, R. Cobalt Toxicity/Poisoning With Analytical Aspects And Its Management. Int. J. Med. Lab. Res. 2019, 4, 29–36. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt Toxicity in Humans—A Review of the Potential Sources and Systemic Health Effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Elnabi, A.M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.W.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World: New Tricks for an Old Dog? Karcioglu, O., Arslav, B., Eds.; IntechOpen: London, UK, 2019; Volume 1, pp. 77–101. [Google Scholar]
- Ilyas, K.; Iqbal, H.; Akash, M.S.H.; Rehman, K.; Hussain, A. Heavy Metal Exposure and Metabolomics Analysis: An Emerging Frontier in Environmental Health. Environ. Sci. Pollut. Res. 2024, 31, 37963–37987. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Brodziak-Dopierała, B. Lead—Factors Affecting Its Content in Bone Tissue. Pomeranian J. Life Sci. 2020, 66, 23–29. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Zhu, G.; Nordberg, G.F.; Jin, T.; Ding, X. The Association between Cumulative Cadmium Intake and Osteoporosis and Risk of Fracture in a Chinese Population. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 435–443. [Google Scholar] [CrossRef]
- Tokumoto, M.; Lee, J.-Y.; Fujiwara, Y.; Satoh, M. Long-Term Exposure to Cadmium Causes Hepatic Iron Deficiency through the Suppression of Iron-Transport-Related Gene Expression in the Proximal Duodenum. Toxics 2023, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Pehlivanoglu, E.; Gago-Ferrero, P.; Ozturk-Ufuk, I.; Ramadan, L.; Gutiérrez-Martín, D.; Restrepo-Montes, E.; Topuz, E. Insights into the Analytical Procedures for the Detection of Emerging Contaminants from Water, Soils, and Sediments. In Emerging Contaminants; Kumari, A., Rajput, V.D., Mandzhieva, S.S., Tatiana, M., van Hullebusch, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 17–67. [Google Scholar] [CrossRef]
- Aparicio, J.D.; Raimondo, E.E.; Saez, J.M.; Costa-Gutierrez, S.B.; Alvarez, A.; Benimeli, C.S.; Polti, M.A. The current approach to soil remediation: A review of physicochemical and biological technologies, and the potential of their strategic combination. J. Environ. Chem. Eng. 2022, 10, 107141. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent Developments in Physical, Biological, Chemical, and Hybrid Treatment Techniques for Removing Emerging Contaminants from Wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look about Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent Advances in Soil Remediation Technology for Heavy Metal Contaminated Sites: A Critical Review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Mandal, R.R.; Bashir, Z.; Mandal, J.R.; Raj, D. Potential strategies for phytoremediation of heavy metals from wastewater with circular bioeconomy approach. Environ. Monit. Assess. 2024, 196, 502. [Google Scholar] [CrossRef]
- Perez-Vasquez, A.; Barciela, P.; Prieto, M.A. In Situ and Ex Situ Bioremediation of Different Persistent Soil Pollutants as Agroecology Tool. Processes 2024, 12, 2223. [Google Scholar] [CrossRef]
- Mai, X.; Tang, J.; Tang, J.; Zhu, X.; Yang, Z.; Liu, X.; Zhuang, X.; Feng, G.; Tang, L. Research Progress on the Environmental Risk Assessment and Remediation Technologies of Heavy Metal Pollution in Agricultural Soil. J. Environ. Sci. 2025, 149, 1–20. [Google Scholar] [CrossRef]
- Fulke, A.B.; Ratanpal, S.; Sonker, S. Understanding Heavy Metal Toxicity: Implications on Human Health, Marine Ecosystems and Bioremediation Strategies. Mar. Pollut. Bull. 2024, 206, 116707. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Masud, M.A.A.; Deepo, D.M.; Das, K.; Nandi, R.; Ansary, M.W.R.; Islam, A.R.M.T.; Islam, T. Biological and Green Remediation of Heavy Metal Contaminated Water and Soils: A State-of-the-Art Review. Chemosphere 2023, 332, 138861. [Google Scholar] [CrossRef] [PubMed]
- Bala, S.; Garg, D.; Thirumalesh, B.V.; Sharma, M.; Sridhar, K.; Inbaraj, B.S.; Tripathi, M. Recent Strategies for Bioremediation of Emerging Pollutants: A Review for a Green and Sustainable Environment. Toxics 2022, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kharwar, S. Cyanoremediation: An Overview. In Phytoremediation—Biological Treatment of Environmental Pollution; Madhav, S., Yadav, R.K., van Hullebusch, R., Gupta, G.P., Mishra, R., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–37. [Google Scholar]
- Verma, S.; Bhatt, P.; Verma, A.; Mudila, H.; Prasher, P.; Rene, E.R. Microbial Technologies for Heavy Metal Remediation: Effect of Process Conditions and Current Practices. Clean. Technol. Environ. Policy 2023, 25, 1485–1507. [Google Scholar] [CrossRef]
- Imron, M.F.; Setiawan, W.; Putranto, T.W.C.; Abdullah, S.R.S.; Kurniawan, S.B. Biosorption of Chromium by Live and Dead Cells of Bacillus nitratireducens Isolated from Textile Effluent. Chemosphere 2024, 359, 142389. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kumari, S.; Rath, S.; Priyadarshanee, M.; Das, S. Diversity, Structure and Regulation of Microbial Metallothionein: Metal Resistance and Possible Applications in Sequestration of Toxic Metals. Metallomics 2020, 12, 1637–1655. [Google Scholar] [CrossRef]
- Shahpiri, A.; Mohammadzadeh, A. Mercury Removal by Engineered Escherichia coli Cells Expressing Different Rice Metallothionein Isoforms. Ann. Microbiol. 2018, 68, 145–152. [Google Scholar] [CrossRef]
- De Oliveira, V.H.; Ullah, I.; Dunwell, J.M.; Tibbett, M. Bioremediation Potential of Cd by Transgenic Yeast Expressing a Metallothionein Gene from Populus trichocarpa. Ecotoxicol. Environ. Saf. 2020, 202, 110917. [Google Scholar] [CrossRef]
- Ilyas, S.; Rehman, A.; Ilyas, Q. Heavy Metals Induced Oxidative Stress in Multi-Metal Tolerant Yeast, Candida sp. PS33 and Its Capability to Uptake Heavy Metals from Wastewater. Pak. J. Zool. 2017, 49, 769–775. [Google Scholar] [CrossRef]
- Li, J.; Hao, R.; Zhang, J.; Shan, B.; Xu, X.; Li, Y.; Ye, Y.; Xu, H. Proteomics Study on Immobilization of Pb(II) by Penicillium polonicum. Fungal Biol. 2022, 126, 449–460. [Google Scholar] [CrossRef]
- Sathendra, R.E.; Praveen Kumar, R.; Baskar, G. Microbial Transformation of Heavy Metals. In Waste Bioremediation. Energy, Environment, and Sustainability; Varjani, S., Gnansounou, E., Guranathan, B., Pant, D., Zakaria, Z., Eds.; Springer: Singapore, 2017; pp. 249–263. [Google Scholar] [CrossRef]
- Karim, M.E.; Sharmin, S.A.; Moniruzzaman, M.; Fardous, Z.; Das, K.C.; Banik, S.; Salimullah, M. Biotransformation of Chromium (VI) by Bacillus sp. Isolated from Chromate Contaminated Landfill Site. Chem. Ecol. 2020, 36, 922–937. [Google Scholar] [CrossRef]
- Bell, J.; Ma, X.; McDonald, T.J.; Huang, C.-H.; Sharma, V.K. Overlooked Role of Chromium(V) and Chromium(IV) in Chromium Redox Reactions of Environmental Importance. ACS EST Water 2022, 2, 932–942. [Google Scholar] [CrossRef]
- Chen, Z.; Fu, Q.; Cao, Y.; Wen, Q.; Wu, Y. Effects of Lime Amendment on the Organic Substances Changes, Antibiotics Removal, and Heavy Metals Speciation Transformation during Swine Manure Composting. Chemosphere 2021, 262, 128342. [Google Scholar] [CrossRef]
- Shaaban, M.T.; Ibrahim, H.A.H.; Abouhend, A.S.; El-Moselhy, K.M. Removal of Heavy Metals from Aqueous Solutions Using Multi-Metals and Antibiotics Resistant Bacterium Isolated from the Red Sea, Egypt. Am. J. Microbiol. Res. 2015, 3, 93–106. [Google Scholar]
- Nouha, K.; Kumar, R.S.; Tyagi, R.D. Heavy Metals Removal from Wastewater Using Extracellular Polymeric Substances Produced by Cloacibacterium normanense in Wastewater Sludge Supplemented with Crude Glycerol and Study of Extracellular Polymeric Substances Extraction by Different Methods. Bioresour. Technol. 2016, 212, 120–129. [Google Scholar] [CrossRef]
- Sharma, B.; Shukla, P. A Comparative Analysis of Heavy Metal Bioaccumulation and Functional Gene Annotation towards Multiple Metal Resistant Potential by Ochrobactrum intermedium BPS-20 and Ochrobactrum ciceri BPS-26. Bioresour. Technol. 2021, 320, 124330. [Google Scholar] [CrossRef]
- Lenchi, N.; Ahmedi, W.N.E.H.; Llirós, M. Simultaneous Removal of Crude Oil and Heavy Metals by Highly Adapted Bacterial Strain Cutibacterium sp. NL2 Isolated from Algerian Oilfield. Int. Microbiol. 2023, 27, 615–630. [Google Scholar] [CrossRef]
- Ameen, F.A.; Hamdan, A.M.; El-Naggar, M.Y. Assessment of the Heavy Metal Bioremediation Efficiency of the Novel Marine Lactic Acid Bacterium, Lactobacillus plantarum MF042018. Sci. Rep. 2020, 10, 314. [Google Scholar] [CrossRef]
- Njoku, K.L.; Akinyede, O.R.; F Obidi, O. Microbial Remediation of Heavy Metals Contaminated Media by Bacillus megaterium and Rhizopus stolonifer. Sci. Afr. 2020, 10, e00545. [Google Scholar] [CrossRef]
- Abbasi, M.; Khan, I.; Rehman, A.; Hayat, A.; Ur Rehman, M.; Shah, T.A.; Khan, A.A.; Ul Haq, T.; Aziz, T.; Alharbi, M.; et al. Bioremediation of Heavy Metals Contaminated Soil by Using Indigenous Metallotolerant Bacterial Isolates. Appl. Ecol. Environ. Res. 2024, 22, 1623–1648. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, I.; Cárdenas-González, J.F.; Rodríguez Pérez, A.S.; Oviedo, J.T.; Martínez-Juárez, V.M. Bioremoval of Different Heavy Metals by the Resistant Fungal Strain Aspergillus niger. Bioinorg. Chem. Appl. 2018, 2018, 3457196. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Gola, D.; Dey, P.; Malik, A. Synergistic and Antagonistic Effects on Metal Bioremediation with Increasing Metal Complexity in a Hexa-Metal Environment by Aspergillus fumigatus. Int. J. Environ. Res. 2020, 14, 761–770. [Google Scholar] [CrossRef]
- Gola, D.; Dey, P.; Bhattacharya, A.; Mishra, A.; Malik, A.; Namburath, M.; Ahammad, S.Z. Multiple Heavy Metal Removal Using an Entomopathogenic Fungi Beauveria bassiana. Bioresour. Technol. 2016, 218, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Hussain, D.F.; Mutlag, N.H. Assessment the Ability of Trichoderma harzianum Fungi in Bioremediation of Some of Heavy Metals in Waste Water. IOP Conf. Ser. Earth Environ. Sci. 2021, 790, 012087. [Google Scholar] [CrossRef]
- Imam, S.S.A.; Rajpoot, I.K.; Gajjar, B.; Sachdeva, A. Comparative Study of Heavy Metal Bioremediation in Soil by Bacillus subtilis and Saccharomyces cerevisiae. Indian J. Sci. Technol. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Resende, A.H.M.; de Almeida, D.G.; De Cássia Freire Soares da Silva, R.; Rufino, R.D.; Luna, J.M.; Banat, I.M.; Sarubbo, L.A. Candida lipolytica UCP0988 Biosurfactant: Potential as a Bioremediation Agent and in Formulating a Commercial Related Product. Front. Microbiol. 2017, 8, 767. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Chen, S.; Wang, T.; Jiang, L.; Wang, M.; Wang, S.; Li, Z. Biochemical Changes of Polysaccharides and Proteins within EPS under Pb(II) Stress in Rhodotorula mucilaginosa. Ecotoxicol. Environ. Saf. 2019, 174, 484–490. [Google Scholar] [CrossRef]
- Zango, U.U.; Muhammad, I.I.; Sharma, V.; Sharma, A.K. Effective Bioremediation of Cd, Cr, and Pb in Tannery Effluent Using Aspergillus fumigatus and Aspergillus terreus: Synergistic Effects of Using the Two Strains Together. Water Air Soil Pollut. 2023, 234, 735. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ahmed, A.; Auta, H.S.; Hamid, F.S. Enhanced Bioremediation of Heavy Metal Contaminated Landfill Soil Using Filamentous Fungi Consortia: A Demonstration of Bioaugmentation Potential. Water Air Soil Pollut. 2019, 230, 215. [Google Scholar] [CrossRef]
- Allam, N. Bioremediation Efficiency of Heavy Metals and Azo Dyes by Individual or Consortium Bacterial Species Either as Free or Immobilized Cells: A Comparative Study. Egypt. J. Bot. 2017, 57, 555–564. [Google Scholar] [CrossRef]
- Nivetha, N.; Srivarshine, B.; Sowmya, B.; Rajendiran, M.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Pham, T.H.T.; Shanmugam, V.; Dragoi, E.-N. A Comprehensive Review on Bio-Stimulation and Bio-Enhancement towards Remediation of Heavy Metals Degeneration. Chemosphere 2023, 312, 137099. [Google Scholar] [CrossRef] [PubMed]
- Adams, O.G.; Fufeyin, T.P.; Okoro, E.S.; Ehinomen, I. Bioremediation, Biostimulation and Bioaugmention: A Review. Int. J. Environ. Bioremediat. Biodegrad. 2020, 3, 28–39. [Google Scholar] [CrossRef]
- Sharma, J. Advantages and Limitations of In Situ Methods of Bioremediation. Recent Adv. Biol. Med. 2019, 5, 10941. [Google Scholar] [CrossRef]
- Sakr, M.; El Agamawi, H.; Klammler, H.; Mohamed, M.M. A Review on the Use of Permeable Reactive Barriers as an Effective Technique for Groundwater Remediation. Groundw. Sustain. Dev. 2023, 21, 100914. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Gunasundari, E. Bioremediation of Heavy Metals. In Bioremediation: Applications for Environmental Protection and Management. Energy, Environment, and Sustainability; Varjani, S., Agarwal, A., Gnansounou, E., Guranathan, B., Eds.; Springer: Singapore, 2018; pp. 165–195. [Google Scholar] [CrossRef]
- Koul, B.; Taak, P. Ex Situ Soil Remediation Strategies. In Biotechnological Strategies for Effective Remediation of Polluted Soils; Koul, B., Taak, P., Eds.; Springer: Singapore, 2018; pp. 39–57. [Google Scholar] [CrossRef]
- Chaney, R.L.; Broadhurst, C.L.; Centofanti, T. Phytoremediation of Soil Trace Elements. In Trace Elements in Soils; Hooda, P.S., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 311–352. [Google Scholar] [CrossRef]
- Cakaj, A.; Drzewiecka, K.; Hanć, A.; Lisiak-Zielińska, M.; Ciszewska, L.; Drapikowska, M. Plants as Effective Bioindicators for Heavy Metal Pollution Monitoring. Environ. Res. 2024, 256, 119222. [Google Scholar] [CrossRef]
- Siromlya, T.I.; Zagurskaya, Y.V. Problems in the Study of the Accumulation and Hyperaccumulation of Chemical Elements by Plants. Biol. Bull. Rev. 2022, 12, 334–345. [Google Scholar] [CrossRef]
- Singh, R.; Jha, A.B.; Misra, A.N.; Sharma, P. Adaption Mechanisms in Plants Under Heavy Metal Stress Conditions During Phytoremediation. In Phytomanagement of Polluted Sites. Market Opportunities in Sustainable Phytoremediation; Pandey, V.C., Bauddh, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 329–360. [Google Scholar] [CrossRef]
- Welsh, C.D. Metal-Plants and Plant-Metals: Phytoextraction by Metallophytes—A Review. Ph.D. Thesis, Brandenburg University of Technology Cottbus-Senftenberg, Cottbus, Germany, 2024. [Google Scholar]
- van der Ent, A.; Rylott, E.L. Inventing Hyperaccumulator Plants: Improving Practice in Phytoextraction Research and Terminology. Int. J. Phytoremediat. 2024, 26, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Calabrese, E.J.; Veresoglou, S.D. The Microbiome Orchestrates Contaminant Low-Dose Phytostimulation. Trends Plant Sci. 2024, 24 (Suppl. S1360–S1385), 1–11. [Google Scholar] [CrossRef]
- Liang, Z.; Neményi, A.; Kovács, G.P.; Gyuricza, C. Incorporating Functional Traits into Heavy Metals Phytoremediation: The Future of Field-Based Phytoremediation. Ecol. Indic. 2024, 166, 112262. [Google Scholar] [CrossRef]
- Eben, P.; Mohri, M.; Pauleit, S.; Duthweiler, S.; Helmreich, B. Phytoextraction Potential of Herbaceous Plant Species and the Influence of Environmental Factors—A Meta-Analytical Approach. Ecol. Eng. 2024, 199, 107169. [Google Scholar] [CrossRef]
- Bakshe, P.; Jugade, R. Phytostabilization and Rhizofiltration of Toxic Heavy Metals by Heavy Metal Accumulator Plants for Sustainable Management of Contaminated Industrial Sites: A Comprehensive Review. J. Hazard. Mater. Adv. 2023, 10, 100293. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Bora, M.S.; Sarma, K.P. Phytoremediation of Heavy Metals/Metalloids by Native Herbaceous Macrophytes of Wetlands: Current Research and Perspectives. In Emerging Issues in the Water Environment During Anthropocene—A South East Asian Perspective; Kumar, M., Snow, D.D., Honda, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 261–285. [Google Scholar]
- Kumar, K.; Gupta, D.; Mosa, K.A.; Ramamoorthy, K.; Sharma, P. Arsenic Transport, Metabolism, and Possible Mitigation Strategies in Plants. In Plant-Metal Interactions; Srivastava, S., Srivastava, A., Suprasanna, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 141–168. [Google Scholar] [CrossRef]
- Almotairy, H. Mitigating Metal/Metalloid Stress in Crops: Strategies for Sustainable Agricultural Resilience. In Abiotic Stress in Crop Plants—Ecophysiological Responses and Molecular Approaches; Hasanuzzaman, M., Nahar, K., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Priya, A.K.; Muruganandam, M.; Ali, S.S.; Kornaros, M. Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics 2023, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A. Plant in Vitro Cultures for Phytoremediation of Heavy Metals. In Back to Basics: A Multidisciplinary Approach to Organic Living and Waste Management; Mandal, P., Neogi, S., Ghosh, K.K., Eds.; Jogamaya Devi College: Kolkata, India, 2022; Volume 3, pp. 24–44. [Google Scholar]
- Kristanti, R.A.; Ngu, W.J.; Yuniarto, A.; Hadibarata, T. Rhizofiltration for Removal of Inorganic and Organic Pollutants in Groundwater: A Review. Biointerface Res. Appl. Chem. 2021, 11, 12326–12347. [Google Scholar] [CrossRef]
- Adewoye, L.; Mustapha, S.; Adeniyi, A.; Tijani, J.; Amoloye, M.; Ayinde, L. Optimization of Nickel (II) And Chromium (III) Removal From Contaminated Water Using Sorghum bicolor. Niger. J. Technol. 2017, 36, 960–972. [Google Scholar] [CrossRef]
- Mahardika, G.; Rinanti, A.; Fachrul, M.F. Phytoremediation of Heavy Metal Copper (Cu2+) by Sunflower (Helianthus snnuus L). IOP Conf. Ser. Earth Environ. Sci. 2018, 106, 012120. [Google Scholar] [CrossRef]
- Abd-Elaal, A.-E.M.; Aboelkassem, A.; Gad, A.A.M.; Ahmed, S.A.S. Removal of Heavy Metals from Wastewater by Natural Growing Plants on River Nile Banks in Egypt. Water Pract. Technol. 2020, 15, 947–959. [Google Scholar] [CrossRef]
- Ghosh, A.; Manchanda, N. Phytoremediation of Heavy Metals from Water of Yamuna River by Tagetes patula, Bassica scoparia, Portulaca grandiflora. Asian Plant Res. J. 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Kumar, V.; Ferreira, L.F.R.; Sonkar, M.; Singh, J. Phytoextraction of Heavy Metals and Ultrastructural Changes of Ricinus communis L. Grown on Complex Organometallic Sludge Discharged from Alcohol Distillery. Environ. Technol. Innov. 2021, 22, 101382. [Google Scholar] [CrossRef]
- Sarkar, M.; Rahman, A.K.M.L.; Bhoumik, N.C. Remediation of Chromium and Copper on Water Hyacinth (E. crassipes) Shoot Powder. Water Resour. Ind. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Al-Khafaji, M.S.; Al-Ani, F.H.; Ibrahim, A.F. Removal of Some Heavy Metals from Industrial Wastewater by Lemmna minor. KSCE J. Civil Eng. 2018, 22, 1077–1082. [Google Scholar] [CrossRef]
- Kumari, M.; Tripathi, B.D. Efficiency of Phragmites australis and Typha latifolia for Heavy Metal Removal from Wastewater. Ecotoxicol. Environ. Saf. 2015, 112, 80–86. [Google Scholar] [CrossRef]
- Pilipović, A.; Zalesny, R.S.; Rončević, S.; Nikolić, N.; Orlović, S.; Beljin, J.; Katanić, M. Growth, Physiology, and Phytoextraction Potential of Poplar and Willow Established in Soils Amended with Heavy-Metal Contaminated, Dredged River Sediments. J. Environ. Manag. 2019, 239, 352–365. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Liu, S.; Peng, J.; Zhang, H.; Zhao, Q.; Zheng, L.; Chen, Y.; Shen, Z.; Xu, X.; et al. Enhancing the Phytoremediation of Heavy Metals by Combining Hyperaccumulator and Heavy Metal-Resistant Plant Growth-Promoting Bacteria. Front. Plant Sci. 2022, 13, 912350. [Google Scholar] [CrossRef] [PubMed]
- Jha, S. Progress, Prospects, and Challenges of Genetic Engineering in Phytoremediation. In Bioremediation of Pollutants from Genetic Engineering to Genome Engineering; Pandey, V.C., Vijai, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–123. [Google Scholar] [CrossRef]
- Rawat, M.; Chauhan, M.; Pandey, A. Extremophiles and their expanding biotechnological applications. Arch. Microbiol. 2024, 206, 247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bano, A.; Singh, S.P.; Sharma, S.; Xia, C.; Nadda, A.K.; Lam, S.S.; Tong, Y.W. Engineered Microbes as Effective Tools for the Remediation of Polyaromatic Aromatic Hydrocarbons and Heavy Metals. Chemosphere 2022, 306, 135538. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Yi, S.; Ge, F. Genetically Engineered Microbial Remediation of Soils Co-Contaminated by Heavy Metals and Polycyclic Aromatic Hydrocarbons: Advances and Ecological Risk Assessment. J. Environ. Manag. 2021, 296, 113185. [Google Scholar] [CrossRef]
- Fatima, Z.; Azam, A.; Iqbal, M.Z.; Badar, R.; Muhammad, G. A Comprehensive Review on Effective Removal of Toxic Heavy Metals from Water Using Genetically Modified Microorganisms. Desalination Water Treat. 2024, 319, 100553. [Google Scholar] [CrossRef]
- Pant, G.; Garlapati, D.; Agrawal, U.; Prasuna, R.G.; Mathimani, T.; Pugazhendhi, A. Biological Approaches Practised Using Genetically Engineered Microbes for a Sustainable Environment: A Review. J. Hazard. Mater. 2021, 405, 124631. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Asiani, K.; Arya, S.; Rensing, C.; Stekel, D.J.; Larsson, D.G.J.; Hobman, J.L. Metal Resistance and Its Association with Antibiotic Resistance. In Advances in Microbial Physiology; Poole, R.K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 70, pp. 261–313. [Google Scholar] [CrossRef]
- Yang, S.; Deng, W.; Liu, S.; Yu, X.; Mustafa, G.R.; Chen, S.; He, L.; Ao, X.; Yang, Y.; Zhou, K.; et al. Presence of Heavy Metal Resistance Genes in Escherichia coli and Salmonella Isolates and Analysis of Resistance Gene Structure in E. coli E308. J. Glob. Antimicrob. Resist. 2020, 21, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.; Hussain, S.Z.; Rehman, A. Phenotypic and Genomic Analysis of Multiple Heavy Metal–Resistant Micrococcus luteus Strain AS2 Isolated from Industrial Waste Water and Its Potential Use in Arsenic Bioremediation. Appl. Microbiol. Biotechnol. 2020, 104, 2243–2254. [Google Scholar] [CrossRef]
- Silva, N.M.d.; Reis, G.F.; Costa, F.d.F.; Grisolia, M.E.; Geraldo, M.R.; Lustosa, B.P.R.; Lima, B.J.F.d.S.; Weiss, V.A.; de Souza, E.M.; Li, R.; et al. Genome Sequencing of Cladophialophora exuberans, a Novel Candidate for Bioremediation of Hydrocarbon and Heavy Metal Polluted Habitats. Fungal Biol. 2023, 127, 1032–1042. [Google Scholar] [CrossRef]
- He, M.; Xu, Y.; Qiao, Y.; Zhang, Z.; Liang, J.; Peng, Y.; Liao, J.; Qiao, Y.; Shang, C.; Guo, Z.; et al. A Novel Yeast Strain Geotrichum sp. CS-67 Capable of Accumulating Heavy Metal Ions. Ecotoxicol. Environ. Saf. 2022, 236, 113497. [Google Scholar] [CrossRef]
- Chi, B.-B.; Lu, Y.-N.; Yin, P.-C.; Liu, H.-Y.; Chen, H.-Y.; Shan, Y. Sequencing and Comparative Genomic Analysis of a Highly Metal-Tolerant Penicillium janthinellum P1 Provide Insights Into Its Metal Tolerance. Front. Microbiol. 2021, 12, 663217. [Google Scholar] [CrossRef] [PubMed]
- Emrani, J.; Ahmed, M.; Newman, R.H.; Thomas, M.D.; Mowa, C.N.; Teleha, J. Introducing Chemistry Students to Emerging Technologies in Gene Editing, Their Applications, and Ethical Considerations. J. Chem. Educ. 2020, 97, 1931–1943. [Google Scholar] [CrossRef]
- Hui, C.; Guo, Y.; Zhang, W.; Gao, C.; Yang, X.; Chen, Y.; Li, L.; Huang, X. Surface Display of PbrR on Escherichia coli and Evaluation of the Bioavailability of Lead Associated with Engineered Cells in Mice. Sci. Rep. 2018, 8, 5685. [Google Scholar] [CrossRef]
- Huang, K.; Chen, C.; Shen, Q.; Rosen, B.P.; Zhao, F.-J. Genetically Engineering Bacillus subtilis with a Heat-Resistant Arsenite Methyltransferase for Bioremediation of Arsenic-Contaminated Organic Waste. Appl. Environ. Microbiol. 2015, 81, 6718–6724. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, G.; Fan, C.; Zhou, M.; Tang, L.; Zhu, J.; Wan, J.; Huang, D.; Chen, M.; Xu, P.; et al. Chromosomal Expression of CadR on Pseudomonas aeruginosa for the Removal of Cd(II) from Aqueous Solutions. Sci. Total Environ. 2018, 636, 1355–1361. [Google Scholar] [CrossRef]
- Bhat, A.; Sharma, R.; Desigan, K.; Lucas, M.M.; Mishra, A.; Bowers, R.M.; Woyke, T.; Epstein, B.; Tiffin, P.; Pueyo, J.J.; et al. Horizontal Gene Transfer of the Mer Operon Is Associated with Large Effects on the Transcriptome and Increased Tolerance to Mercury in Nitrogen-Fixing Bacteria. BMC Microbiol. 2024, 24, 247. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Yu, C.Y.; Kim, W.-R.; Moon, H.-S.; Lee, J.; Kim, S.H.; Chung, I.M. Assessment of Benefits and Risk of Genetically Modified Plants and Products: Current Controversies and Perspective. Sustainability 2023, 15, 1722. [Google Scholar] [CrossRef]
- Lacroix, B.; Citovsky, V. Pathways of DNA Transfer to Plants from Agrobacterium tumefaciens and Related Bacterial Species. Annu. Rev. Phytopathol. 2019, 57, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Habib, M.; Charagh, S.; Kakavand, S.N. Genetic Engineering of Plants to Tolerate Toxic Metals and Metalloids. In Handbook of Bioremediation—Physiological, Molecular and Biotechnological Interventions; Hasanuzzaman, M., Prasad, M.N.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 411–436. [Google Scholar] [CrossRef]
- Daghan, H.; Uygur, V.; Eren, A. Lead Phytoremediation Potential of Wild Type and Transgenic Tobacco Plants. ISPEC J. Agric. Sci. 2021, 5, 168–182. [Google Scholar] [CrossRef]
- Selwal, N.; Tabassum, Z.; Rahayu, F.; Yulia, N.D.; Sugiono, S.; Endarto, O.; Riajaya, P.D.; Djajadi, D.; Khamidah, A.; Wani, A.K. Therapeutic Potential and Phytoremediation Capabilities of the Tobacco Plant: Advancements through Genetic Engineering and Cultivation Techniques. Biocatal. Agric. Biotechnol. 2023, 52, 102845. [Google Scholar] [CrossRef]
- Havryliuk, O.A.; Hovorukha, V.M.; Sachko, A.V.; Gladka, G.V.; Bida, I.O.; Tashyrev, O.B. Bioremoval of Hazardous Cobalt, Nickel, Chromium, Copper and Cadmium Compounds from Contaminated Soil by Nicotiana tabacum Plants and Associated Microbiome. Biosyst. Divers. 2021, 29, 88–93. [Google Scholar] [CrossRef]
- Estrela, R.; Cate, J.H.D. Energy Biotechnology in the CRISPR-Cas9 Era. Curr. Opin. Biotechnol. 2016, 38, 79–84. [Google Scholar] [CrossRef]
- Paulo, A.M.S.; Caetano, N.S.; Castro, P.M.L.; Marques, A.P.G.C. Phytomanagement of Zn- and Cd-Contaminated Soil: Helianthus annuus Biomass Production and Metal Remediation Abilities with Plant-Growth-Promoting Microbiota Assistance. Soil Syst. 2023, 7, 69. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V.; Gupta, P.; Chandra, A. Potential Use of Solanum lycopersicum and Plant Growth Promoting Rhizobacterial (PGPR) Strains for the Phytoremediation of Endosulfan Stressed Soil. Chemosphere 2021, 279, 130589. [Google Scholar] [CrossRef]
- Trivedi, J.; Chhaya, U. Evaluation of Phytotoxic and Clastogenic Potential of Phenolic Pollutant Bisphenol A and Its Bioremediated Product in Triticum aestivum, Cicer arietinum and Allium cepa. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 777–790. [Google Scholar] [CrossRef]
- Agnihotri, A.; Seth, C.S. Transgenic Brassicaceae: A Promising Approach for Phytoremediation of Heavy Metals. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–255. [Google Scholar] [CrossRef]
- Kumar, V.; Younis, S.A.; Vikrant, K.; Kim, K.-H. Trends in Advanced Materials for Sustainable Environmental Remediation. In Advances Materials for Sustainable Environmental Remediation; Giannakoudakis, D.A., Meili, L., Ioannis, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–2. [Google Scholar]
- Deepika; Haritash, A.K. Phytoremediation Potential of Ornamental Plants for Heavy Metal Removal from Contaminated Soil: A Critical Review. Hortic. Environ. Biotechnol. 2023, 64, 709–734. [Google Scholar] [CrossRef]
- Liu, J.; Xin, X.; Zhou, Q. Phytoremediation of Contaminated Soils Using Ornamental Plants. Environ. Rev. 2018, 26, 43–54. [Google Scholar] [CrossRef]
- Voeikova, T.A.; Zhuravliova, O.A.; Debabov, V.G. Comparative Analysis of Legal Regulation of Industrial Use of Genetic-Engineering-Modified Microorganisms in the United States, European Union, and Russian Federation. Molecular Genetics. Microbiol. Virol. 2020, 35, 69–77. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- Saxena, G.; Kishor, R.; Saratale, G.D.; Bharagava, R.N. Genetically Modified Organisms (GMOs) and Their Potential in Environmental Management: Constraints, Prospects, and Challenges. In Bioremediation of Industrial Waste for Environmental Safety; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2019; Volume 2, pp. 1–19. [Google Scholar] [CrossRef]
- Naismith, L.K. Bioremediation. Tex. A&M Law Rev. 2021, 8, 607–632. [Google Scholar] [CrossRef]
- Xue, Y.; Qiu, T.; Sun, Z.; Liu, F.; Yu, B. Mercury Bioremediation by Engineered Pseudomonas putida KT2440 with Adaptationally Optimized Biosecurity Circuit. Environ. Microbiol. 2022, 24, 3022–3036. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of Nanomaterials in Water Treatment Applications: A Review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Khakbiz, M.; Shakibania, S.; Ghazanfari, L.; Zhao, S.; Tavakoli, M.; Chen, Z. Engineered Nanoflowers, Nanotrees, Nanostars, Nanodendrites, and Nanoleaves for Biomedical Applications. Nanotechnol. Rev. 2023, 12, 20220523. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Galletta, M.; Drommi, D.; Cappello, S.; Plutino, M.R. Functional Nanohybrids and Nanocomposites Development for the Removal of Environmental Pollutants and Bioremediation. Molecules 2022, 27, 4856. [Google Scholar] [CrossRef]
- Mandeep; Shukla, P. Microbial Nanotechnology for Bioremediation of Industrial Wastewater. Front. Microbiol. 2020, 11, 590631. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green Synthesized Silver Nanoparticles: Antibacterial and Anticancer Activities, Biocompatibility, and Analyses of Surface-Attached Proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef] [PubMed]
- Schwan, J.; Markert, S.; Rosenfeldt, S.; Schüler, D.; Mickoleit, F.; Schenk, A.S. Comparing the Colloidal Stabilities of Commercial and Biogenic Iron Oxide Nanoparticles That Have Potential In Vitro/In Vivo Applications. Molecules 2023, 28, 4895. [Google Scholar] [CrossRef]
- Kumari, S.; Tyagi, M.; Jagadevan, S. Mechanistic Removal of Environmental Contaminants Using Biogenic Nano-Materials. Int. J. Environ. Sci. Technol. 2019, 16, 7591–7606. [Google Scholar] [CrossRef]
- Mohite, P.T.; Kumar, A.R.; Zinjarde, S.S. Biotransformation of Hexavalent Chromium into Extracellular Chromium(III) Oxide Nanoparticles Using Schwanniomyces occidentalis. Biotechnol. Lett. 2016, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sathyavathi, S.; Manjula, A.; Rajendhran, J.; Gunasekaran, P. Extracellular Synthesis and Characterization of Nickel Oxide Nanoparticles from Microbacterium sp. MRS-1 towards Bioremediation of Nickel Electroplating Industrial Effluent. Bioresour. Technol. 2014, 165, 270–273. [Google Scholar] [CrossRef]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an Efficient Mycosystem for the Synthesis of Metal Nanoparticles: Progress and Key Aspects of Research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nguyen, H.N.; Peña-Bahamonde, J.; Robles-Hernandez, F.C.; Gimenes, L.J.; Rodrigues, D.F. The Role of Secondary Metabolites in the Production of CuO Nanoparticles by Fungi: A Physiological and Metabolic Approach. Environ. Sci. Nano 2024, 11, 4487–4500. [Google Scholar] [CrossRef]
- Aslam, F.; Mazhar, S. Nano-Bioremediation of Heavy Metals from Environment Using a Green Synthesis Approach. Int. J. Adv. Appl. Sci. 2023, 12, 7–14. [Google Scholar] [CrossRef]
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal. Toxicol. 2016, 6, 525–2161. [Google Scholar] [CrossRef]
- Azeez, L.; Adebisi, S.A.; Adetoro, R.O.; Oyedeji, A.O.; Agbaje, W.B.; Olabode, O.A. Foliar Application of Silver Nanoparticles Differentially Intervenes Remediation Statuses and Oxidative Stress Indicators in Abelmoschus esculentus Planted on Gold-Mined Soil. Int. J. Phytoremediat. 2022, 24, 384–393. [Google Scholar] [CrossRef]
- Qi, W.-Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.-F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.-G. Selenium Nanoparticles Ameliorate Brassica napus L. Cadmium Toxicity by Inhibiting the Respiratory Burst and Scavenging Reactive Oxygen Species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef]
- Ghorbani, A.; Emamverdian, A.; Pehlivan, N.; Zargar, M.; Razavi, S.M.; Chen, M. Nano-enabled agrochemicals: Mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J. Nanobiotechnol. 2024, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.J.; Dodge, A.E.; Samarajeewa, A.D.; Beaudette, L.A. Soil Exposed to Silver Nanoparticles Reveals Significant Changes in Community Structure and Altered Microbial Transcriptional Profiles. Environ. Pollut. 2020, 258, 113816. [Google Scholar] [CrossRef]
- Verma, A.; Bharadvaja, N. Plant-Mediated Synthesis and Characterization of Silver and Copper Oxide Nanoparticles: Antibacterial and Heavy Metal Removal Activity. J. Clust. Sci. 2022, 33, 1697–1712. [Google Scholar] [CrossRef]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green Synthesis of Nanoparticles from Biodegradable Waste Extracts and Their Applications: A Critical Review. Nanotechnol. Environ. Eng. 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Prema, P.; Nguyen, V.-H.; Venkatachalam, K.; Murugan, J.M.; Ali, H.M.; Salem, M.Z.M.; Ravindran, B.; Balaji, P. Hexavalent Chromium Removal from Aqueous Solutions Using Biogenic Iron Nanoparticles: Kinetics and Equilibrium Study. Environ. Res. 2022, 205, 112477. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Velmurugan, S. Green Synthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from the Leaf Extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Geetha, N.; Bhavya, G.; Abhijith, P.; Shekhar, R.; Dayananda, K.; Jogaiah, S. Insights into Nanomycoremediation: Secretomics and Mycogenic Biopolymer Nanocomposites for Heavy metal Detoxification. J. Hazard. Mater. 2020, 409, 124541. [Google Scholar] [CrossRef]
- El-Zahed, M.M.; Abou-Dobara, M.I.; El-Khodary, M.M.; Mousa, M.M.A. Antimicrobial Activity and Nanoremediation of Heavy Metals Using Biosynthesized CS/GO/ZnO Nanocomposite by Bacillus subtilis ATCC 6633 Alone or Immobilized in a Macroporous Cryogel. Microb. Cell Factories 2024, 23, 278. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Barani, M.; Sarani, M.; Lohrasbi-Nejad, A.; Mohammadi-Nejad, G.; Salekdeh, G.H. Green Synthesis of NiO NPs for Metagenome-Derived Laccase Stabilization: Detoxifying Pollutants and Wastes. Int. J. Biol. Macromol. 2024, 266, 130986. [Google Scholar] [CrossRef]
- Rocha, V.; Lago, A.; Silva, B.; Barros, Ó.; Neves, I.C.; Tavares, T. Immobilization of Biogenic Metal Nanoparticles on Sustainable Materials—Green Approach Applied to Wastewater Treatment: A Systematic Review. Environ. Sci. Nano 2024, 11, 36–60. [Google Scholar] [CrossRef]
- Vedarethinam, V. Cutting-Edge Innovations in Nanomaterial-Enhanced Membranes for Industrial Effluent Remediation. In Bioremediation of Emerging Contaminants in Water; Shah, M.P., Vasantharaj, K., Saranya, N., Eds.; ACS Publications: Washington, DC, USA, 2024; Volume 2, pp. 189–225. [Google Scholar] [CrossRef]
- Correa, T.; Presciliano, R.; Abreu, F. Why Does Not Nanotechnology Go Green? Bioprocess Simulation and Economics for Bacterial-Origin Magnetite Nanoparticles. Front. Microbiol. 2021, 12, 718232. [Google Scholar] [CrossRef]
- EL-Moslamy, S.H. Bioprocessing Strategies for Cost-Effective Large-Scale Biogenic Synthesis of Nano-MgO from Endophytic Streptomyces Coelicolor Strain E72 as an Anti-Multidrug-Resistant Pathogens Agent. Sci. Rep. 2018, 8, 3820. [Google Scholar] [CrossRef] [PubMed]
- Wehrs, M.; Tanjore, D.; Eng, T.; Lievense, J.; Pray, T.R.; Mukhopadhyay, A. Engineering Robust Production Microbes for Large-Scale Cultivation. Trends Microbiol. 2019, 27, 524–537. [Google Scholar] [CrossRef]
- Kuppan, N.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A Comprehensive Review of Sustainable Bioremediation Techniques: Eco Friendly Solutions for Waste and Pollution Management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- Ibáñez, J.; Pérez-de-Mora, A.; Santiago-Herrera, M.; Belloncle, B.; de Wilde, H.; Martel-Martín, S.; Blanco-Alcántara, D.; Barros, R. Environmental and Socio-Economic Evaluation of a Groundwater Bioremediation Technology Using Social Cost-Benefit Analysis: Application to an in-Situ Metal(Loid) Precipitation Case Study. Sci. Total Environ. 2024, 954, 176720. [Google Scholar] [CrossRef] [PubMed]
- Odum, E.P. The Mesocosm. Bioscience 1984, 34, 558–562. [Google Scholar] [CrossRef]
- Andreolli, M.; Lampis, S.; Brignoli, P.; Vallini, G. Mesocosm-based Simulations to Optimize a Bioremediation Strategy for the Effective Restoration of Wildfire-impacted Soils Contaminated with High-molecular-weight Hydrocarbons. J. Appl. Microbiol. 2021, 131, 1249–1260. [Google Scholar] [CrossRef]
- Gosai, H.B.; Panseriya, H.Z.; Patel, P.G.; Patel, A.C.; Shankar, A.; Varjani, S.; Dave, B.P. Exploring Bacterial Communities through Metagenomics during Bioremediation of Polycyclic Aromatic Hydrocarbons from Contaminated Sediments. Sci. Total Environ. 2022, 842, 156794. [Google Scholar] [CrossRef]
- Martínez Álvarez, L.M.; Ruberto, L.A.M.; Gurevich, J.M.; Mac Cormack, W.P. Environmental Factors Affecting Reproducibility of Bioremediation Field Assays in Antarctica. Cold Reg. Sci. Technol. 2020, 169, 102915. [Google Scholar] [CrossRef]
- Ojuederie, O.; Babalola, O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Raper, E.; Stephenson, T.; Anderson, D.R.; Fisher, R.; Soares, A. Industrial Wastewater Treatment through Bioaugmentation. Process Saf. Environ. Prot. 2018, 118, 178–187. [Google Scholar] [CrossRef]
- Abdoli, S.; Asgari Lajayer, B.; Dehghanian, Z.; Bagheri, N.; Vafaei, A.H.; Chamani, M.; Rani, S.; Lin, Z.; Shu, W.; Price, G.W. A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities. Water 2024, 16, 2507. [Google Scholar] [CrossRef]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation Techniques–Classification Based on Site of Application: Principles, Advantages, Limitations and Prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.-W.; Dong, C.-D. Organic Wastes Bioremediation and Its Changing Prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent Developments and Prospects of Sustainable Remediation Treatments for Major Contaminants in Soil: A Review. Sci. Total Environ. 2024, 912, 168769. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, H. Cost-Effectiveness Analysis for Soil Heavy Metal Contamination Treatments. Water Air Soil Pollut. 2018, 229, 126. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–Benefit Calculation of Phytoremediation Technology for Heavy-Metal-Contaminated Soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef]
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Franchi, E.; Petruzzelli, G.; Ferro, S. Enhancements in Phytoremediation Technology: Environmental Assessment Including Different Options of Biomass Disposal and Comparison with a Consolidated Approach. J. Environ. Manag. 2019, 237, 560–568. [Google Scholar] [CrossRef]
- Vasileiadou, A. From Organic Wastes to Bioenergy, Biofuels, and Value-Added Products for Urban Sustainability and Circular Economy: A Review. Urban Science 2024, 8, 121. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Bin Johan, R. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; Samer, M., Ed.; InTech: Houston, TX, USA, 2017; Volume 1, pp. 10–35. [Google Scholar] [CrossRef]
- Ogundiran, M.B.; Mekwunyei, N.S.; Adejumo, S.A. Compost and Biochar Assisted Phytoremediation Potentials of Moringa oleifera for Remediation of Lead Contaminated Soil. J. Environ. Chem. Eng. 2018, 6, 2206–2213. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The Application of Biochar from Waste Biomass to Improve Soil Fertility and Soil Enzyme Activity and Increase Carbon Sequestration. Energies 2022, 16, 380. [Google Scholar] [CrossRef]
- Al Chami, Z.; Amer, N.; Smets, K.; Yperman, J.; Carleer, R.; Dumontet, S.; Vangronsveld, J. Evaluation of Flash and Slow Pyrolysis Applied on Heavy Metal Contaminated Sorghum bicolor Shoots Resulting from Phytoremediation. Biomass Bioenergy 2014, 63, 268–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Xu, W.; Liao, Q.; Zhang, H.; Hao, S.; Chen, S. Pyrolysis of Various Phytoremediation Residues for Biochars: Chemical Forms and Environmental Risk of Cd in Biochar. Bioresour. Technol. 2020, 299, 122581. [Google Scholar] [CrossRef]
- Schwartz, N.R.; Paulsen, A.D.; Blaise, M.J.; Wagner, A.L.; Yelvington, P.E. Analysis of Emissions from Combusting Pyrolysis Products. Fuel 2020, 274, 117863. [Google Scholar] [CrossRef]
- Mia, S.; Uddin, M.E.; Kader, M.A.; Ahsan, A.; Mannan, M.A.; Hossain, M.M.; Solaiman, Z.M. Pyrolysis and Co-Composting of Municipal Organic Waste in Bangladesh: A Quantitative Estimate of Recyclable Nutrients, Greenhouse Gas Emissions, and Economic Benefits. Waste Manag. 2018, 75, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Li, J.; Hou, Y.; Shi, L.; Lian, C.; Shen, Z.; Chen, Y. Evaluation of Biogas Production Potential of Trace Element-Contaminated Plants via Anaerobic Digestion. Ecotoxicol. Environ. Saf. 2021, 208, 111598. [Google Scholar] [CrossRef]
- da Silva, E.B.; Mussoline, W.A.; Wilkie, A.C.; Ma, L.Q. Anaerobic Digestion to Reduce Biomass and Remove Arsenic from As-Hyperaccumulator Pteris vittata. Environ. Pollut. 2019, 250, 23–28. [Google Scholar] [CrossRef]
- Cucina, M. Integrating Anaerobic Digestion and Composting to Boost Energy and Material Recovery from Organic Wastes in the Circular Economy Framework in Europe: A Review. Bioresour. Technol. Rep. 2023, 24, 101642. [Google Scholar] [CrossRef]
- Song, U. Improvement of Soil Properties and Plant Responses by Compost Generated from Biomass of Phytoremediation Plant. Environ. Eng. Res. 2019, 25, 638–644. [Google Scholar] [CrossRef]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy Production Potential of Phytoremediation Plant Biomass: Helianthus annuus and Silybum marianum. Ind. Crops Prod. 2019, 135, 206–216. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.Y.; Cho, J.; Kwon, E.E.; Kim, J.Y. Anaerobic Digestion as an Alternative Disposal for Phytoremediated Biomass from Heavy Metal Contaminated Sites. Environ. Pollut. 2018, 243, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.; Rai, T. Engaging with Residents’ Perceived Risks and Benefits about Technologies as a Way of Resolving Remediation Dilemmas. Sci. Total Environ. 2017, 601–602, 1649–1669. [Google Scholar] [CrossRef]
- Jha, H.; Mandal, V.; Sen, K.K.; Tandey, R.; Chouhan, K.B.S.; Mehta, R. Environmental Biotechnology: Public Perception. In Emerging Trends in Environmental Biotechnology; Mondal, S., Singh, S.P., Lahir, Y.K., Eds.; CRC Press: London, UK, 2022; Volume 1, pp. 265–273. [Google Scholar]


| Elements | Water | Soil/Sludge Applied to Soil | ||
|---|---|---|---|---|
| WHO Limits (mg/L) | US-EPA Limits (mg/L) | WHO Limits (mg/kg d.w) | US-EPA Limits (mg/kg d.w) | |
| Arsenic (As) | 0.01 | 0.01 | 20 | 0.39 |
| Cadmium (Cd) | 0.003 | 0.005 | 3 | 1.4 |
| Total Chromium (Cr) | 0.05 | 0.1 | 100 | 100 |
| Cobalt (Co) | 0.005 | - | 50 | 7.5 |
| Lead (Pb) | 0.01 | 0.015 | 50 | 400 |
| Inorganic Mercury (Hg) | 0.006 | 0.002 | 0.004 | 0.2 |
| Nickel (Ni) | 0.07 | 0.1 | 100 | 75 |
| Remediation Methods | Technologies | Environment | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| Physical |
| Aquatic |
|
| [64] |
| Terrestrial |
|
| [65] | |
| Chemical |
| Aquatic |
|
| [66] |
| Terrestrial |
|
| [67] | |
| Biological | Phytoremediation
| Aquatic |
|
| [68] |
Bioremediation:
| Terrestrial |
|
| [69] |
| Microbial Strains | Heavy Metals | Removal Efficiency (%) | Initial Concentration (mg/L) | Experiment Duration | Treated Media | Reference |
|---|---|---|---|---|---|---|
| Bacteria | ||||||
| Alteromonas macleodii | Pb Ni Cd | 73.8 54 53 | 200 | 10 h | Synthetic broth | [86] |
| Cloacibacterium normanense | Ni | 85 | 48 | 2.5 h | Municipal wastewater | [87] |
| Ochrobactrum intermedium | Pb Ni | 85.34 74.87 | 100 | 3 days | Aqueous solution | [88] |
| Ochrobactrum ciceri | Pb Ni | 71.20 88.48 | 100 | 3 days | Aqueous solution | [88] |
| Cutibacterium sp. | Pb | 35.19 | 170 | 7 days | Aqueous solution | [89] |
| Lactobacillus plantarum | Cd Pb Ni Cr | 100 100 100 100 | - | 1 h | Battery-manufacturing effluent | [90] |
| Bacillus megaterium | Pb Ni Cd | 10.54 73.02 24.68 | 3200 3200 3200 | 4 days | Aqueous solution | [91] |
| Rhizopus stolonifera | Pb Ni Cd | 23.79 58.89 17.06 | 3200 3200 800 | 4 days | Aqueous solution | [91] |
| Cupriavidus necator | Pb Cr | 63.56 81.32 | 0.247 1.82 | 5 days | Soil | [92] |
| Pseudomonas putida | Pb Cr | 83.81 80.77 | 0.247 1.82 | 5 days | Soil | [92] |
| Fungi | ||||||
| Aspergillus niger | Cr Co As Pb Cd | 100 71.4 69 59 57 | 50 100 1 100 5 | 7 days | Wastewater | [93] |
| Aspergillus fumigatus | Pb Cr Ni | 99 75 100 | 30 | 3 days | Aqueous solution | [94] |
| Beauveria bassiana | Cd Cr Ni | 63.4 61.13 75 | 30 | 5 days | Aqueous solution | [95] |
| Trichoderma harzianum | Cd Pb Ni | 98.63 84.50 69.07 | 2.19 2.69 - | 28 days | Wastewater | [96] |
| Saccharomyces cerevisiae | Cd | 69.56 | 500 500 | 5 days | Contaminated soil | [97] |
| Bacillus subtilis | Cd | 75.76 | 500 50 | 5 days | Contaminated soil | [97] |
| Candida lipolytica | Pb | 30 | 1000 | 120 days | Synthetic wastewater | [98] |
| Rhodotorula mucilaginosa | Pb | 25.2 40.6 32.6 25.24 | 500 1000 2000 2500 | 3 days | Aqueous solution | [99] |
| Consortia of microorganisms | ||||||
| Aspergillus fumigatus and Aspergillus terreus | Cd Cr Pb | 93.28 89.41 97.13 | - | 6 days | Tannery effluent | [100] |
| Perenniporia subtephropora, Daldinia starbaeckii, Phanerochaete concrescens, Cerrena aurantiopora, Fusarium equiseti, Polyporales sp., Aspergillus niger, Aspergillus fumigatus, Trametes versicolor | As Cr | 62 42 | - | 100 days | Soil | [101] |
| Paecilomyces lilacinus, Antrodia serialis, Penicillium cataractum | As Cr | 48 36 | - | 100 days | Soil | [101] |
| Sphingomonas paucimobilis, Rhizobium radiobacter, Bacillus subtilis, Bacillus pumilus | Pb Cd Cr | 98.08 95.43 97.12 | 0.314 0.285 0.174 | 4 days | Industrial wastewater | [102] |
| Plant Species | Heavy Metals | Removal Efficiency (%) | Initial Concentration (mg/L) | Experiment Duration | Treated Media | Reference |
|---|---|---|---|---|---|---|
| Sorghum bicolor | Ni | 97.28 | 25 | 20 min | Aqueous solution | [128] |
| Cr | 99.8 | 5 | 30 min | |||
| Helianthus annuus | Pb | 70.88 | 10 | 4 weeks | Contaminated soil | [129] |
| Hydrangea paniculata | Pb | 50.65 | 10 | 4 weeks | Contaminated soil | [129] |
| Echinochloa pyramidalis | Cd Ni Pb | 37.99 22.25 88.74 | 5 | 6 weeks | Soil contaminated with wastewater | [130] |
| Ludwigia stolonifera | Cd Ni Pb | 48.04 32.3 84.29 | 5 | 6 weeks | Soil contaminated with wastewater | [130] |
| Tagetes patula | Cd Cr Pb | 31.61 47.56 94.99 | 0.715 0.513 1.098 | 8 weeks | Contaminated river water | [131] |
| Portulaca grandiflora | Cd Cr Pb | 55.94 18.52 92.81 | 0.715 0.513 1.098 | 8 weeks | Contaminated river water | [131] |
| Bassica scoparia | Cd Cr Pb | 100 26.12 93.72 | 0.715 0.513 1.098 | 8 weeks | Contaminated river water | [131] |
| Ricinus communis | Cr Cd Ni Pb | 34.48 99.89 48.27 53.43 | 0.002 0.019 0.014 0.018 | Not specified | Distillery sludge | [132] |
| Eichhornia crassipes | Cr | 99.98 | 10.4749 | 3 h | Tannery effluent | [133] |
| Lemmna minor | Cd Cr Ni Pb | 44.93 32.26 74.48 79.1 | 0.0227 0.5252 0.1117 0.2526 | 5 weeks | Industrial wastewater from tannery and battery industries | [134] |
| Phragmites australis | Cd Cr Ni Pb | 43.3 51.2 55.8 45.7 | 0.079 0.142 0.088 0.060 | 2 weeks | Urban sewage mixed with industrial effluent | [135] |
| Typha latifolia | Cd Cr Ni Pb | 39.7 45.6 51.1 40 | 0.079 0.142 0.088 0.060 | 2 weeks | Urban sewage mixed with industrial effluent | [135] |
| Consortia of plants | ||||||
| Phragmites australis and Typha latifolia | Cd Cr Ni Pb | 60 68.1 73.8 61 | 0.079 0.142 0.088 0.060 | 2 weeks | Urban sewage mixed with industrial effluent | [135] |
| Echinochloa pyramidalis and Ludwigia stolonifera | Cd Ni Pb | 61.52 27.8 93.22 | 5 | 6 weeks | Soil contaminated with wastewater | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firincă, C.; Zamfir, L.-G.; Constantin, M.; Răut, I.; Jecu, M.-L.; Doni, M.; Gurban, A.-M.; Șesan, T.E. Innovative Approaches and Evolving Strategies in Heavy Metal Bioremediation: Current Limitations and Future Opportunities. J. Xenobiot. 2025, 15, 63. https://doi.org/10.3390/jox15030063
Firincă C, Zamfir L-G, Constantin M, Răut I, Jecu M-L, Doni M, Gurban A-M, Șesan TE. Innovative Approaches and Evolving Strategies in Heavy Metal Bioremediation: Current Limitations and Future Opportunities. Journal of Xenobiotics. 2025; 15(3):63. https://doi.org/10.3390/jox15030063
Chicago/Turabian StyleFirincă, Cristina, Lucian-Gabriel Zamfir, Mariana Constantin, Iuliana Răut, Maria-Luiza Jecu, Mihaela Doni, Ana-Maria Gurban, and Tatiana Eugenia Șesan. 2025. "Innovative Approaches and Evolving Strategies in Heavy Metal Bioremediation: Current Limitations and Future Opportunities" Journal of Xenobiotics 15, no. 3: 63. https://doi.org/10.3390/jox15030063
APA StyleFirincă, C., Zamfir, L.-G., Constantin, M., Răut, I., Jecu, M.-L., Doni, M., Gurban, A.-M., & Șesan, T. E. (2025). Innovative Approaches and Evolving Strategies in Heavy Metal Bioremediation: Current Limitations and Future Opportunities. Journal of Xenobiotics, 15(3), 63. https://doi.org/10.3390/jox15030063









