Comprehensive Risk Assessment of Metals and Minerals in Seafood Using Bioaccessibility Correction
Abstract
1. Introduction
- (a)
- The evaluation of mineral (P, S, K, Ca, Se) and heavy metal (Cd, Pb, Ni, Cr, Fe, Zn, Co, Mn, As) concentration levels with toxic potential from the edible tissues of several seafood species commercialized in the Lower Danube River Basin area and the selection of seafood species with the highest levels of heavy metals;
- (b)
- The performance of the bioaccessibility study using the three-step in vitro digestion model;
- (c)
- Health risk assessment of exposure to heavy metals through seafood consumption;
- (d)
- The performance of a correlation analysis between the bio-accessible fraction of heavy metals in seafood and Cancer Risk in human consumers.
2. Materials and Methods
2.1. Seafood Sampling
2.2. In Vitro Gastrointestinal Digestion Model of Cooked Seafood
2.3. Heavy Metals’ and Minerals’ Bioaccessibility from Cooked Seafood Samples
2.4. Heavy Metal Analysis
2.5. Total Reflection X-Ray Spectrometry Analysis
2.6. Health Risk Analysis
2.6.1. Non-Carcinogenic Parameters
- EF = Exposure frequency (365 d/year);
- ED = Exposure duration—adult average lifespan in Romania (74.2 years) according to the European Commission [41];
- IR = Ingestion rate (22.22 g per person/d) according to the European Market Observatory for Fisheries and Aquaculture Products, 2022;
- C = Concentration of heavy metals in seafood (mg kg−1);
- BW = Average body weight of Romanian adults (78.65 kg) according to World Data;
- ATn = Average exposure time (365 d/year × ED).
- EF = Exposure frequency (365 d/year);
- ED = Exposure duration (74.2 years);
- IR = Ingestion rate (22.22 g/d);
- C = Metal concentration in seafood (mg kg−1);
- RfD = Oral reference dose for each metal (mg kg−1 d−1);
- BW = Average body weight (78.65 kg);
- ATn = Average exposure time for non-carcinogens (365 d/year × ED).
2.6.2. Carcinogenic Indicators
- C = Heavy metal concentration in edible tissue (mg kg−1);
- IR = Ingestion rate (22.22 g per person/d);
- EF = Exposure frequency (365 d/year);
- ED = Exposure duration (74.2 years);
- BW = Average body weight (78.65 kg);
- ATc = Average exposure time for carcinogens (365 d/year × ED).
2.7. Statistical Analysis
2.8. Chemicals and Reagents
3. Results
3.1. Heavy Metals in Different Seafood Species
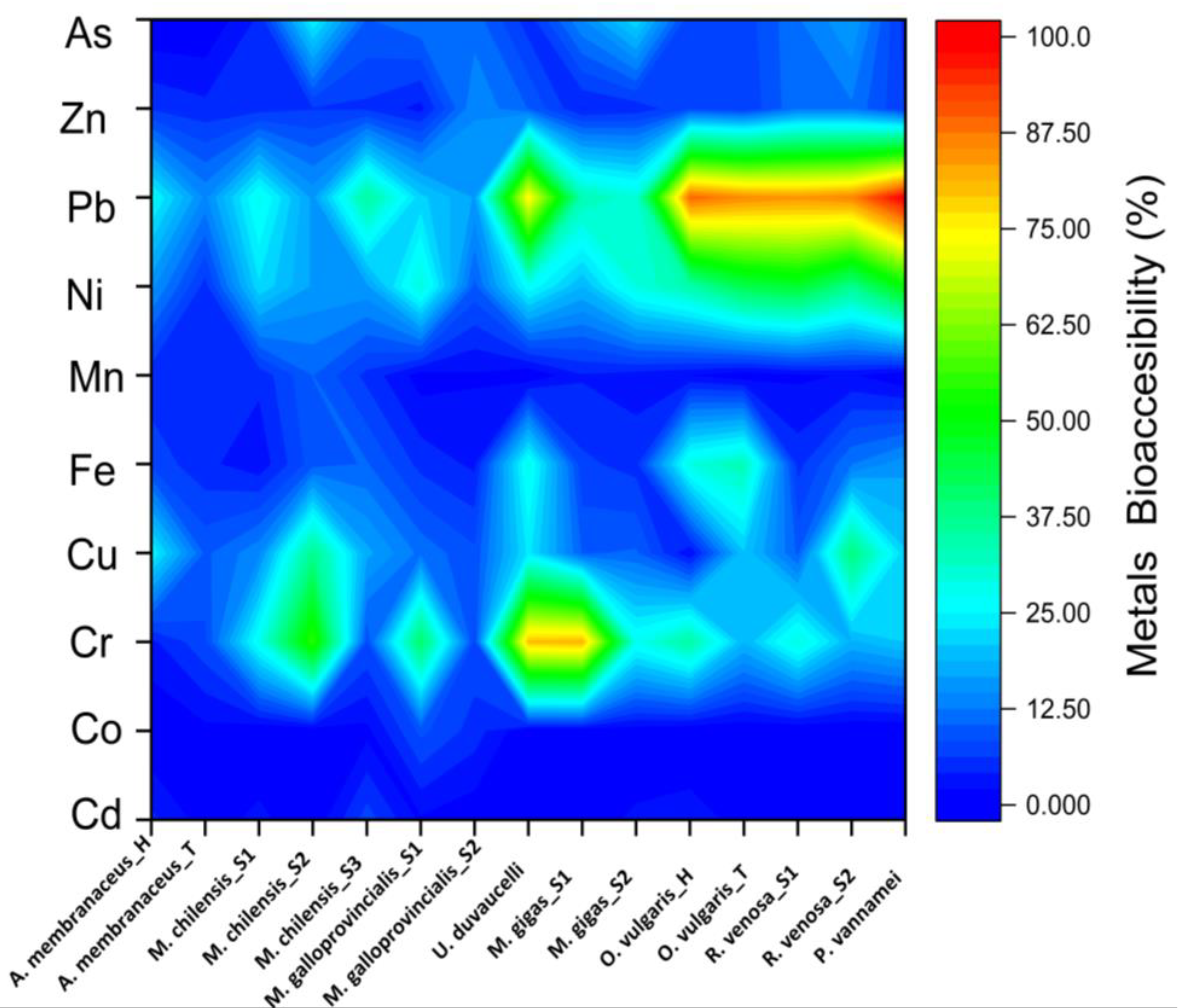
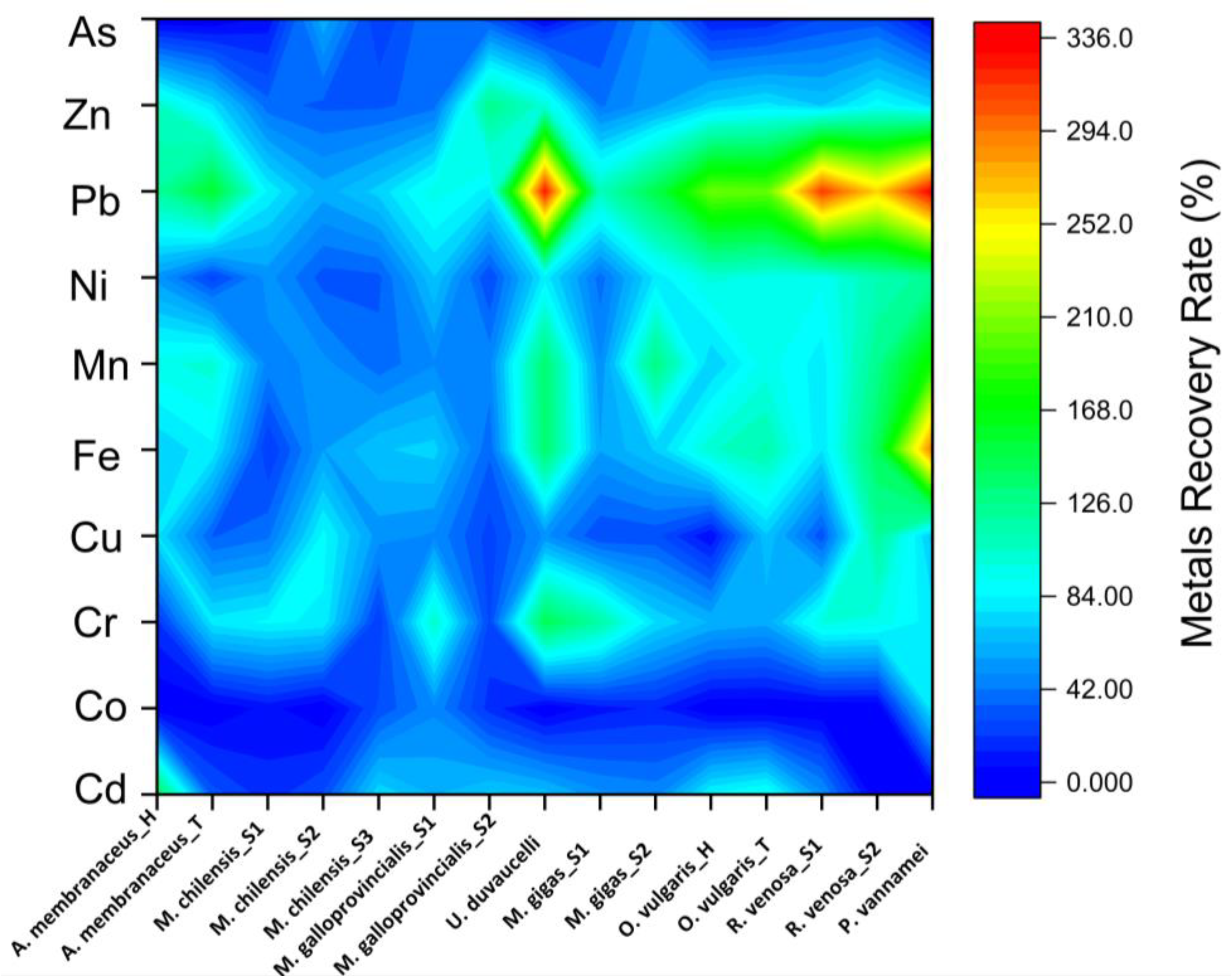
3.2. Heavy Metal Bioaccessibility and Recovery Rate of Cooked Seafood Samples
3.3. Mineral Concentrations in Seafood Samples
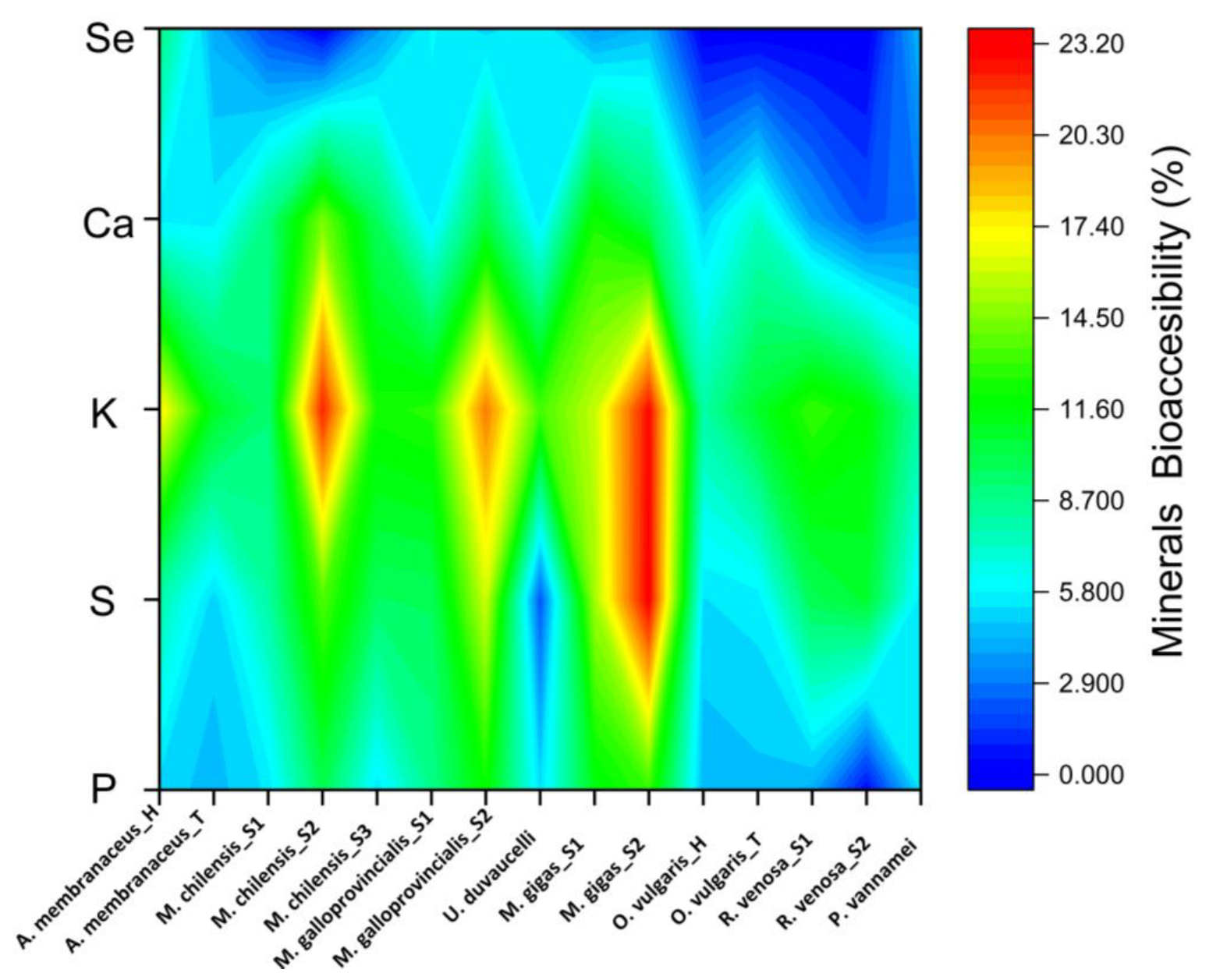
3.4. Mineral Bioaccessibility and Recovery Rate of Cooked Seafood Samples
3.5. Health Risk Analysis
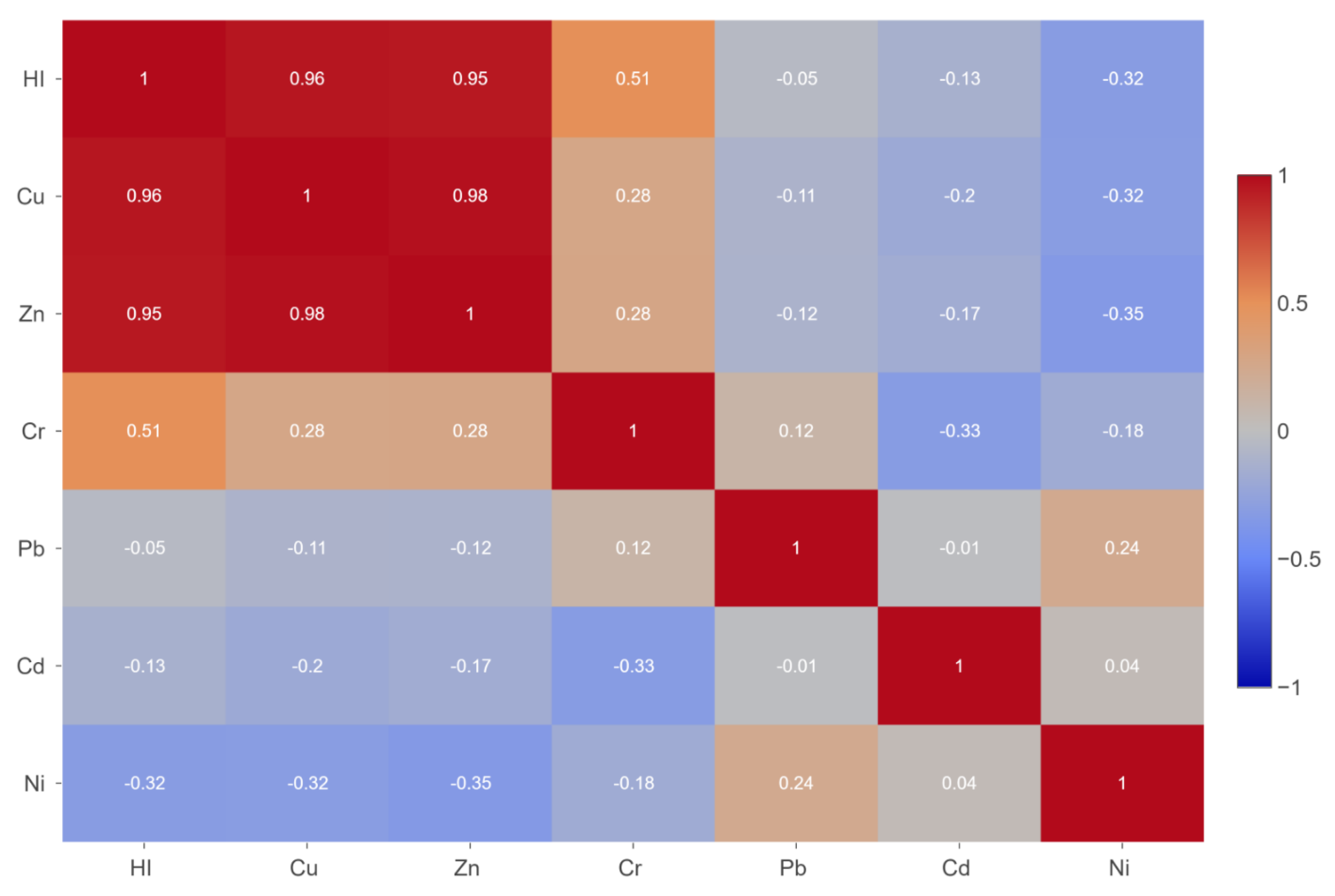
3.6. Correlation Between Analyzed Elements
4. Discussion
4.1. Heavy Metals in Different Seafood Species
4.2. Heavy Metals Bioaccessibility and Recovery Rate of Cooked Seafood Samples
4.3. Mineral Concentrations in Seafood Samples
4.4. Mineral Bioaccessibility and Recovery Rate of Cooked Seafood Samples
4.5. Health Risk Analysis
4.6. Correlation Between Analyzed Elements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrea, S.M.; Zamfir, C.; Simionov, I.A.; Mogodan, A.; Nuţă, F.M.; Rahoveanu, A.T.; Nancu, D.; Cristea, D.S.; Buhociu, F.M. A Forecasting and Prediction Methodology for Improving the Blue Economy Resilience to Climate Change in the Romanian Lower Danube Euroregion. Sustainability 2021, 13, 11563. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018 (SOFIA): Meeting the Sustainable Development Goals; The State of World Fisheries and Aquaculture (SOFIA); FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Muralisankar, T.; Mohan, K.; Udhayakumar, V.; Balamuralikrishnan, B. Potential Role of Dietary Minerals in Fish and Crustaceans. In Aquaculture Science and Engineering; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 431–461. ISBN 978-981-19081-6-3. [Google Scholar]
- Jha, A.K.; Panda, S.K.; Kishore, P.; Mathew, S.; Ravishankar, C.N. Trace-Minerals and Lipid Quality Indices in Seaweeds Growing at Okha, India: A Health Risk Assessment. Reg. Stud. Mar. Sci. 2021, 47, 101966. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Antache, A.; Calmuc, M.; Condurache, N.; Codreanu, M.; Milea, A.; Iticescu, C.; Georgescu, P.-L. Heavy metals accumulation in processed fish products and risk assessment analysis on human health. Earth Obs. 2023, 12, 290–295. [Google Scholar]
- Soltani, N.; Moore, F.; Keshavarzi, B.; Sorooshian, A.; Javid, R. Potentially Toxic Elements (PTEs) and Polycyclic Aromatic Hydrocarbons (PAHs) in Fish and Prawn in the Persian Gulf, Iran. Ecotoxicol. Environ. Saf. 2019, 173, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Li, R.; Gong, Y.; Shen, X.; Yu, L. Bioaccessibility-Corrected Health Risk of Heavy Metal Exposure via Shellfish Consumption in Coastal Region of China. Environ. Pollut. 2021, 273, 116529. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Petrea, S.-M.; Mogodan, A.; Nica, A.; Cristea, D.; Neculita, M. Effect of changes in the romanian lower sector danube river hydrological and hydrothermal regime on fish diversity. Earth Obs. 2020, 9, 106–111. [Google Scholar]
- Gedik, K. Bioaccessibility of Heavy Metals in Rapa Whelk Rapana Venosa (Valenciennes, 1846): Assessing Human Health Risk Using an in Vitro Digestion Model. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 202–213. [Google Scholar] [CrossRef]
- Popa, P.; Murariu, G.; Timofti, M.; Georgescu, L.P. Multivariate Statistical Analyses of Danube River Water Quality at Galati, Romania. Environ. Eng. Manag. J. 2018, 17, 1249–1266. [Google Scholar]
- Ahmed, N.; Mohammad, A.; Knuteson, S.L.; Samara, F. Evaluating Temporal Changes in Water Quality Due to Urbanization: A Multi-Year Observational Study in Khalid Khor, Sharjah, UAE. Front. Mar. Sci. 2025, 12, 1538897. [Google Scholar] [CrossRef]
- de Souza, D.C.D.; Viana, L.F.; Kummrow, F.; Cardoso, C.A.L.; de Lima, N.A.; Lacerda, I.A.R.; Crispim, B.d.A.; Barufatti, A.; Dias, L.A.V.; Florentino, A.C. Bioaccumulation of Metals in Fish Collected from Macapá Urban Aquatic Environments (Brazilian Amazon) and the Risks to Human Health. Toxics 2025, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Simionov, I.-A.; Cristea, V.; Petrea, S.-M.; Sirbu, E.B. Evaluation of heavy metals concentration dynamics in fish from the black sea coastal area: An overview. Environ. Eng. Manag. J. 2019, 18, 1097–1110. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud Univ.—Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- He, M.; Ke, C.-H.; Wang, W.-X. Effects of Cooking and Subcellular Distribution on the Bioaccessibility of Trace Elements in Two Marine Fish Species. J. Agric. Food Chem. 2010, 58, 3517–3523. [Google Scholar] [CrossRef]
- Reeder, R.; Schoonen, M.; Lanzirotti, A. Metal Speciation and Its Role in Bioaccessibility and Bioavailability. Rev. Mineral. Geochem. 2005, 64, 59. [Google Scholar] [CrossRef]
- Torres-Escribano, S.; Ruiz, A.; Barrios, L.; Vélez, D.; Montoro, R. Influence of Mercury Bioaccessibility on Exposure Assessment Associated with Consumption of Cooked Predatory Fish in Spain. J. Sci. Food Agric. 2011, 91, 981–986. [Google Scholar] [CrossRef]
- Torres-Escribano, S.; Denis, S.; Blanquet-Diot, S.; Calatayud, M.; Barrios, L.; Vélez, D.; Alric, M.; Montoro, R. Comparison of a Static and a Dynamic in Vitro Model to Estimate the Bioaccessibility of As, Cd, Pb and Hg from Food Reference Materials Fucus Sp. (IAEA-140/TM) and Lobster Hepatopancreas (TORT-2). Sci. Total Environ. 2011, 409, 604–611. [Google Scholar] [CrossRef]
- Cabañero, A.I.; Madrid, Y.; Cámara, C. Selenium and Mercury Bioaccessibility in Fish Samples: An in Vitro Digestion Method. Anal. Chim. Acta 2004, 526, 51–61. [Google Scholar] [CrossRef]
- Moreda–Piñeiro, J.; Alonso-Rodríguez, E.; Romarís-Hortas, V.; Moreda-Piñeiro, A.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D.; Bermejo-Barrera, P. Assessment of the Bioavailability of Toxic and Non-Toxic Arsenic Species in Seafood Samples. Food Chem. 2012, 130, 552–560. [Google Scholar] [CrossRef]
- Laird, B.D.; Chan, H.M. Bioaccessibility of Metals in Fish, Shellfish, Wild Game, and Seaweed Harvested in British Columbia, Canada. Food Chem. Toxicol. 2013, 58, 381–387. [Google Scholar] [CrossRef]
- Chen, X.; Singh, A.; Kitts, D.D. In-Vitro Bioaccessibility and Bioavailability of Heavy Metals in Mineral Clay Complex Used in Natural Health Products. Sci. Rep. 2020, 10, 8823. [Google Scholar] [CrossRef] [PubMed]
- Milea, Ș.-A.; Lazăr, N.-N.; Simionov, I.-A.; Petrea, Ș.-M.; Călmuc, M.; Călmuc, V.; Georgescu, P.-L.; Iticescu, C. Effects of Cooking Methods and Co-Ingested Foods on Mercury Bioaccessibility in Pontic Shad (Alosa Immaculata). Curr. Res. Food Sci. 2023, 7, 100599. [Google Scholar] [CrossRef] [PubMed]
- Jitar, O.; Teodosiu, C.; Nicoara, M.; Plavan, G. Study of Heavy Metal Pollution and Bioaccumulation in the Black Sea Living Environment. Environ. Eng. Manag. J. 2013, 12, 271–276. [Google Scholar] [CrossRef]
- Dey, S.; Rajak, P.; Sen, K. Bioaccumulation of Metals and Metalloids in Seafood: A Comprehensive Overview of Mobilization, Interactive Effects in Eutrophic Environments, and Implications for Public Health Risks. J. Trace Elem. Miner. 2024, 8, 100141. [Google Scholar] [CrossRef]
- Kristamtini; Widyayanti, S.; Widodo, S.; Pustika, A.B.; Purwaningsih, H.; Hanifa, A.P.; Muazam, A.; Sutardi; Badia Ginting, R.C.; Mulia, S.; et al. Potentially Toxic Elements’ (PTEs) Spatial Distribution in Agricultural Soils and Their Impact on Ecological and Health Risks. Case Stud. Chem. Environ. Eng. 2024, 10, 100936. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip Toxicol 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Gupta, R.C. Veterinary Toxicology: Basic and Clinical Principles; Elsevier Science & Technology: Kidlington, UK, 2011; ISBN 978-0-12-370467-2. [Google Scholar]
- Chen, L.; Zhou, S.; Shi, Y.; Wang, C.; Li, B.; Li, Y.; Wu, S. Heavy Metals in Food Crops, Soil, and Water in the Lihe River Watershed of the Taihu Region and Their Potential Health Risks When Ingested. Sci. Total Environ. 2018, 615, 141–149. [Google Scholar] [CrossRef]
- Liao, W.; Wang, G.; Zhao, W.; Zhang, M.; Wu, Y.; Liu, X.; Li, K. Change in Mercury Speciation in Seafood after Cooking and Gastrointestinal Digestion. J. Hazard. Mater. 2019, 375, 130–137. [Google Scholar] [CrossRef]
- Naito, S.; Matsumoto, E.; Shindoh, K.; Nishimura, T. Effects of Polishing, Cooking, and Storing on Total Arsenic and Arsenic Species Concentrations in Rice Cultivated in Japan. Food Chem. 2015, 168, 294–301. [Google Scholar] [CrossRef]
- Schmidt, L.; Bizzi, C.A.; Duarte, F.A.; Muller, E.I.; Krupp, E.; Feldmann, J.; Flores, E.M.M. Evaluation of Hg Species after Culinary Treatments of Fish. Food Control. 2015, 47, 413–419. [Google Scholar] [CrossRef]
- Almela, C.; Laparra, J.M.; Vélez, D.; Barberá, R.; Farré, R.; Montoro, R. Arsenosugars in Raw and Cooked Edible Seaweed: Characterization and Bioaccessibility. J. Agric. Food Chem. 2005, 53, 7344–7351. [Google Scholar] [CrossRef] [PubMed]
- Crews, H.M.; Clarke, P.A.; Lewis, D.J.; Owen, L.M.; Strutt, P.R.; Izquierdo, A. Investigation of Selenium Speciation in in Vitro Gastrointestinal Extracts of Cooked Cod by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry and Electrospray Mass Spectrometry. J. Anal. At. Spectrom. 1996, 11, 1177–1182. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance); European Union: Brussels, Belgium, 2023; Volume 119.
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Simionov, I.-A.; Cristea, D.S.; Petrea, S.-M.; Mogodan, A.; Nicoara, M.; Plavan, G.; Baltag, E.S.; Jijie, R.; Strungaru, S.-A. Preliminary Investigation of Lower Danube Pollution Caused by Potentially Toxic Metals. Chemosphere 2021, 264, 128496. [Google Scholar] [CrossRef]
- Lazăr, N.-N.; Simionov, I.-A.; Călmuc, M.; Călmuc, V.-A.; Iticescu, C.; Georgescu, P.-L.; Timofti, M.; Drăgan, S. Cross-Regional Elemental Comparison of Mussels Using Total Reflection X-Ray Fluorescence (TXRF). Molecules 2025, 30, 283. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Cristea, V.; Petrea, S.-M.; Mogodan, A.; Nica, A.; Strungaru, S.-A.; Ene, A.; Sarpe, D.A. Heavy metal evaluation in the lower sector of danube river. Earth Obs. 2020, 9, 11–16. [Google Scholar]
- Romania: Country Health Profile 2021|READ Online. Available online: https://read.oecd-ilibrary.org/social-issues-migration-health/romania-country-health-profile-2021_74ad9999-en (accessed on 17 November 2024).
- Djedjibegovic, J.; Marjanovic, A.; Tahirovic, D.; Caklovica, K.; Turalic, A.; Lugusic, A.; Omeragic, E.; Sober, M.; Caklovica, F. Heavy Metals in Commercial Fish and Seafood Products and Risk Assessment in Adult Population in Bosnia and Herzegovina. Sci. Rep. 2020, 10, 13238. [Google Scholar] [CrossRef]
- Risk-Based Concentration Table|Mid-Atlantic Risk Assessment|US EPA. Available online: https://archive.epa.gov/region9/superfund/web/html/index-23.html (accessed on 17 November 2024).
- Anyanwu, E.D.; Nwachukwu, E.D. Heavy Metal Content and Health Risk Assessment of a South-Eastern Nigeria River. Appl. Water Sci. 2020, 10, 210. [Google Scholar] [CrossRef]
- Health Risk Assessment of Heavy Metals in Marine Fish to the Population in Zhejiang, China|Scientific Reports. Available online: https://www.nature.com/articles/s41598-021-90665-x (accessed on 2 June 2025).
- Health Risk Assessment of Heavy Metals in Fish (Chrysichthys Nigrodigitatus from Two Lagoons in South-Western Nigeria. Available online: https://clinmedjournals.org/articles/ijtra/international-journal-of-toxicology-and-risk-assessment-ijtra-5-027.php?jid=ijtra (accessed on 2 June 2025).
- Javed, M.; Usmani, N. Accumulation of Heavy Metals and Human Health Risk Assessment via the Consumption of Freshwater Fish Mastacembelus Armatus Inhabiting, Thermal Power Plant Effluent Loaded Canal. Springerplus 2016, 5, 776. [Google Scholar] [CrossRef]
- Romero-Estévez, D.; Yánez-Jácome, G.S.; Dazzini Langdon, M.; Simbaña-Farinango, K.; Rebolledo Monsalve, E.; Durán Cobo, G.; Navarrete, H. An Overview of Cadmium, Chromium, and Lead Content in Bivalves Consumed by the Community of Santa Rosa Island (Ecuador) and Its Health Risk Assessment. Front. Environ. Sci. 2020, 8, 134. [Google Scholar] [CrossRef]
- Frontiers|The Carcinogenic and Non-Carcinogenic Health Risks of Metal(Oid)s Bioaccumulation in Leafy Vegetables: A Consumption Advisory. Available online: https://www.frontiersin.org/journals/environmental-science/articles/10.3389/fenvs.2021.742269/full (accessed on 2 June 2025).
- Ahmad, W.; Alharthy, R.D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and Heavy Metals Contamination Assessment in Soil and Water to Evaluate Human Health Risk. Sci. Rep. 2021, 11, 17006. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.-L.; Faggio, C.; Petrea, Ş.-M. Human Health Risk Assessment of Potentially Toxic Elements and Microplastics Accumulation in Products from the Danube River Basin Fish Market. Environ. Toxicol. Pharmacol. 2023, 104, 104307. [Google Scholar] [CrossRef]
- Xia, T.; Liu, X. Copper and Zinc Interaction on Water Clearance and Tissue Metal Distribution in the Freshwater Mussel, Cristaria Plicata, under Laboratory Conditions. Front. Environ. Sci. Eng. China 2011, 5, 236–242. [Google Scholar] [CrossRef]
- Publication of an Application for Registration of a Name Pursuant to Article 50(2)(a) of Regulation (EU) No 1151/2012 of the European Parliament and of the Council on Quality Schemes for Agricultural Products and Foodstuffs; European Union: Brussels, Belgium, 2024.
- Alves, R.N.; Maulvault, A.L.; Barbosa, V.L.; Fernandez-Tejedor, M.; Tediosi, A.; Kotterman, M.; van den Heuvel, F.H.M.; Robbens, J.; Fernandes, J.O.; Romme Rasmussen, R.; et al. Oral Bioaccessibility of Toxic and Essential Elements in Raw and Cooked Commercial Seafood Species Available in European Markets. Food Chem. 2018, 267, 15–27. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, W.-X. Factors Affecting the Bioaccessibility of Methylmercury in Several Marine Fish Species. J. Agric. Food Chem. 2011, 59, 7155–7162. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Zhao, W.; Wu, Y.; Rong, N.; Liu, X.; Li, K.; Wang, G. Multiple Metal(Loid)s Bioaccessibility from Cooked Seafood and Health Risk Assessment. Environ. Geochem. Health 2020, 42, 4037–4050. [Google Scholar] [CrossRef]
- Costa, F.; Mieiro, C.L.; Pereira, M.E.; Coelho, J.P. Mercury Bioaccessibility in Fish and Seafood: Effect of Method, Cooking and Trophic Level on Consumption Risk Assessment. Mar. Pollut. Bull. 2022, 179, 113736. [Google Scholar] [CrossRef]
- Fu, Y.; Yin, N.; Cai, X.; Du, H.; Wang, P.; Sultana, M.S.; Sun, G.; Cui, Y. Arsenic Speciation and Bioaccessibility in Raw and Cooked Seafood: Influence of Seafood Species and Gut Microbiota. Environ. Pollut. 2021, 280, 116958. [Google Scholar] [CrossRef]
- Xu, F.; Chen, P.; Tao, Y.; Wang, D.; Xu, Y. Effects of Steaming on the Concentration, Distribution and Bioaccessibility of Cadmium in Chlamys farreri Tissues. Food Res. Int. 2022, 162, 112126. [Google Scholar] [CrossRef]
- Wu, Z.; Feng, X.; Li, P.; Lin, C.-J.; Qiu, G.; Wang, X.; Zhao, H.; Dong, H. Comparison of In Vitro Digestion Methods for Determining Bioaccessibility of Hg in Rice of China. J. Environ. Sci. 2018, 68, 185–193. [Google Scholar] [CrossRef]
- Cheyns, K.; Waegeneers, N.; Van de Wiele, T.; Ruttens, A. Arsenic Release from Foodstuffs upon Food Preparation. J. Agric. Food Chem. 2017, 65, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Lourenço, H.M.; Brito, P.; Maulvault, A.L.; Martins, L.L.; Afonso, C. Influence of Bioaccessibility of Total Mercury, Methyl-Mercury and Selenium on the Risk/Benefit Associated to the Consumption of Raw and Cooked Blue Shark (Prionace Glauca). Environ. Res. 2015, 143, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Njinga, R.L.; Adebayo, A.S.; Olufemi, A.P. Bioaccumulation of Heavy Metals in Water and Organs of Tilapia Brevimanus and Euthynnus Alletteratus from a Coaster Water in Southwestern Nigeria. Environ. Sci. Eur. 2023, 35, 85. [Google Scholar] [CrossRef]
- Kuivenhoven, M.; Mason, K. Arsenic Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nimni, M.E.; Han, B.; Cordoba, F. Are We Getting Enough Sulfur in Our Diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef]
- Martínez-Morcillo, S.; Barrales, I.; Pérez-López, M.; Rodríguez, F.S.; Peinado, J.S.; Míguez-Santiyán, M.P. Mineral and Potentially Toxic Element Profiles in the Soil-Feed-Animal Continuum: Implications for Public, Environmental, and Livestock Health in Three Pasture-Based Sheep Farming Systems. Sci. Total Environ. 2024, 919, 170860. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary Strategies for the Treatment of Cadmium and Lead Toxicity. Nutrients 2014, 7, 552–571. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Bastías, J.M.; Balladares, P.; Acuña, S.; Quevedo, R.; Muñoz, O. Determining the Effect of Different Cooking Methods on the Nutritional Composition of Salmon (Salmo salar) and Chilean Jack Mackerel (Trachurus murphyi) Fillets. PLoS ONE 2017, 12, e0180993. [Google Scholar] [CrossRef]
- Lall, S. The Minerals. In Fish Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; Volume 3, pp. 259–308. ISBN 978-0-12-319652-1. [Google Scholar]
- Wang, M.; Zhou, J.; Pallarés, N.; Castagnini, J.M.; Carmen Collado, M.; Barba, F.J. Evaluation of Heavy Metals, Mycotoxins and Mineral Bioaccessibility through in Vitro Static Digestion Models of Rainbow Trout (Oncorhynchus mykiss) and Sole (Dover sole) Side Stream Extracts Obtained by Pressurized Liquid Extraction. Food Chem. 2023, 419, 136054. [Google Scholar] [CrossRef]
- de la Fuente, B.; Aspevik, T.; Barba, F.J.; Kousoulaki, K.; Berrada, H. Mineral Bioaccessibility and Antioxidant Capacity of Protein Hydrolysates from Salmon (Salmo salar) and Mackerel (Scomber scombrus) Backbones and Heads. Mar. Drugs 2023, 21, 294. [Google Scholar] [CrossRef] [PubMed]
- The 73th Meeting of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Khan, Z.; Elahi, A.; Bukhari, D.A.; Rehman, A. Cadmium Sources, Toxicity, Resistance and Removal by Microorganisms-A Potential Strategy for Cadmium Eradication. J. Saudi Chem. Soc. 2022, 26, 101569. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Chivukula, N.; Ramesh, K.; Singha, J.; Marigoudar, S.R.; Sharma, K.V.; Samal, A. An Integrative Data-Centric Approach to Derivation and Characterization of an Adverse Outcome Pathway Network for Cadmium-Induced Toxicity. Sci. Total Environ. 2024, 920, 170968. [Google Scholar] [CrossRef]
- Lizaola-Mayo, B.C.; Dickson, R.C.; Lam-Himlin, D.M.; Chascsa, D.M. Exogenous Copper Exposure Causing Clinical Wilson Disease in a Patient with Copper Deficiency. BMC Gastroenterol. 2021, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Nickel in Drinking-Water. Available online: https://www.who.int/publications-detail-redirect/WHO-HEP-ECH-WSH-2021.6 (accessed on 25 February 2024).
- Update of the Risk Assessment of Nickel in Food and Drinking Water | EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6268 (accessed on 25 February 2024).
- Scientific Opinion on Dietary Reference Values for Chromium. EFSA J. 2014, 12, 3845. [CrossRef]
- Nriagu, J. Zinc Toxicity in Humans. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 500–508. ISBN 978-0-444-63952-3. [Google Scholar]
- Traven, L.; Marinac-Pupavac, S.; Žurga, P.; Linšak, Ž.; Pavičić Žeželj, S.; Glad, M.; Vukić Lušić, D. Assessment of Health Risks Associated with Heavy Metal Concentration in Seafood from North-Western Croatia. Sci. Rep. 2023, 13, 16414. [Google Scholar] [CrossRef]
- Nyarko, E.; Boateng, C.M.; Asamoah, O.; Edusei, M.O.; Mahu, E. Potential Human Health Risks Associated with Ingestion of Heavy Metals through Fish Consumption in the Gulf of Guinea. Toxicol. Rep. 2023, 10, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Cegarra, L.; Aguirre, P.; Nuñez, M.T.; Gerdtzen, Z.P.; Salgado, J.C. Calcium Is a Noncompetitive Inhibitor of DMT1 on the Intestinal Iron Absorption Process: Empirical Evidence and Mathematical Modeling Analysis. Am. J. Physiol.-Cell Physiol. 2022, 323, C1791–C1806. [Google Scholar] [CrossRef]
- Rakocevic, J.; Sukovic, D.; Maric, D. Distribution and Relationships of Eleven Trace Elements in Muscle of Six Fish Species from Skadar Lake (Montenegro). Turk. J. Fish. Aquat. Sci. 2018, 18, 647–657. [Google Scholar] [CrossRef]
- Zeitoun, R.; Biswas, A. Review—Potentiometric Determination of Phosphate Using Cobalt: A Review. J. Electrochem. Soc. 2020, 167, 127507. [Google Scholar] [CrossRef]
- Toxicological Profile for Cobalt; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2024.
- Beck, K.L. Anemia: Prevention and Dietary Strategies. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 164–168. ISBN 978-0-12-384953-3. [Google Scholar]
- Hallberg, L.; Rossander-Hultén, L.; Brune, M.; Gleerup, A. Calcium and Iron Absorption: Mechanism of Action and Nutritional Importance. Eur. J. Clin. Nutr. 1992, 46, 317–327. [Google Scholar] [PubMed]
- Gaitán, D.; Flores, S.; Saavedra, P.; Miranda, C.; Olivares, M.; Arredondo, M.; López de Romaña, D.; Lönnerdal, B.; Pizarro, F. Calcium Does Not Inhibit the Absorption of 5 Milligrams of Nonheme or Heme Iron at Doses Less Than 800 Milligrams in Nonpregnant Women1,2. J. Nutr. 2011, 141, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Applegate, T.J. Effects of Dietary Copper Supplementation and Copper Source on Digesta pH, Calcium, Zinc, and Copper Complex Size in the Gastrointestinal Tract of the Broiler Chicken. Poult. Sci. 2007, 86, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship With Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Suzuki, N.; Verdugo, M.; Hatakeyama, T.; Ogra, Y. Structural Analysis of Chemically Synthesized Selenophosphate, a Donor for Selenocysteine Biosynthesis. Met. Res. 2021, 1, reg-20. [Google Scholar] [CrossRef]



| Common Name | Scientific Name | Total Length/Height * (cm) | Total Weight (g) | Origin |
|---|---|---|---|---|
| Giant cupped oyster—b (n = 10) | Magallana gigas (Thunberg, 1793) | 9.9 ± 0.3 | 74.21 ± 10.88 | Aquaculture, France |
| Purple whelk—b (n = 10) | Rapana venosa (Valenciennes, 1846) | 8.43 ± 0.40 * | 129.04 ± 18.40 | Fishing, Black Sea |
| Mediterranean mussel—b (n = 10) | Mytilus galloprovincialis (Lamarck, 1819) | 7.00 ± 0.26 | 19.71 ± 0.31 | Fishing, Black Sea |
| Whiteleg shrimp—a (n = 10) | Peneus vannamei (Boone, 1931) | 14.75 ± 1.38 | 22.11 ± 5.54 | Aquaculture, Ecuador |
| Whiteleg shrimp—b (n = 10) | Peneus vannamei (Boone, 1931) | 10.26 ± 2.25 | 24.55 ± 0.37 | Aquaculture, Ecuador |
| Common octopus—cephalopod—b (n = 10) | Octopus vulgaris (Cuvier, 1797) | 61.00 ± 1.41 | 1052 ± 0.01 | Fishing, Atlantic Ocean Eastern Central |
| Mediterranean mussel (n = 10)—b | Mytilus galloprovincialis (Lamarck, 1819) | 6.24 ± 0.79 | 11.16 ± 3.82 | Aquaculture, Italy |
| Mediterranean mussel—a (n = 10) | Mytilus galloprovincialis (Lamarck, 1819) | 7.16 ± 0.28 | 14.03 ± 2.57 | Aquaculture, Spain |
| Chilean mussel—b (n = 10) | Mytilus chilensis (Hupé, 1854) | NA ** | NA ** | Aquaculture, Chile |
| Jumbo flying squid—a cephalopod (n = 10) | Dosidicus gigas (D’Orbigny, 1835) | NA ** | NA ** | Fishing, South-East Pacific Ocean |
| Indian squid—a cephalopod (n = 10) | Uroteuthis duvaucelii (D’Orbigny, 1835) | NA ** | NA ** | Fishing, West Indian Ocean |
| Webfoot octopus—b cephalopod (n = 10) | Amphioctopus membranaceus (Quoy & Gaimard, 1832) | NA ** | NA ** | Fishing, West Indian Ocean |
| Cd | Co | Cr | Cu | Fe | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| 92.26 | 85.71 | 93.15 | 85.62 | 90.06 | 85.04 | 96.33 | 91.55 | 92.26 |
| Species | Cd | Co | Cr | Cu | Fe | Mn | Ni | Pb | Zn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| M. chilensis_C1 | 0.67 | 0.52 | 0.05 | 0.03 | 0.42 | 0.14 | 0.98 | 0.34 | 27.86 | 13.99 | 1.33 | 0.54 | 0.28 | 0.09 | 0.13 | 0.02 | 19.32 | 14.10 |
| M. chilensis_C2 | 0.10 | 0.01 | 0.02 | 0.00 | 0.23 | 0.02 | 0.72 | 0.03 | 16.95 | 0.13 | 0.85 | 0.01 | 0.22 | 0.01 | 0.14 | 0.02 | 5.05 | 0.07 |
| M. galloprovincialis_S | 0.20 | 0.03 | 0.06 | 0.01 | 0.44 | 0.10 | 1.20 | 0.20 | 29.94 | 2.24 | 1.87 | 0.25 | 0.30 | 0.10 | 0.36 | 0.06 | 20.89 | 8.53 |
| M. galloprovincialis_I | 0.14 | 0.06 | 0.12 | 0.12 | 0.34 | 0.05 | 0.60 | 0.16 | 32.02 | 8.28 | 1.93 | 0.61 | 0.67 | 0.51 | 0.49 | 0.28 | 17.59 | 22.32 |
| M. galloprovincialis_BS | 0.09 | 0.04 | 0.03 | 0.00 | 0.26 | 0.02 | 0.59 | 0.26 | 19.78 | 0.90 | 1.55 | 0.24 | 0.29 | 0.02 | 0.18 | 0.01 | 11.12 | 5.69 |
| M. gigas | 0.09 | 0.02 | 0.05 | 0.01 | 0.33 | 0.02 | 23.29 | 16.71 | 26.14 | 6.74 | 4.28 | 3.03 | 0.16 | 0.01 | 0.21 | 0.04 | 96.94 | 48.62 |
| R. venosa | 0.05 | 0.01 | 0.03 | 0.00 | 0.51 | 0.05 | 22.74 | 7.57 | 77.80 | 16.20 | 1.53 | 0.07 | 0.35 | 0.01 | 0.16 | 0.02 | 9.50 | 1.21 |
| O. vulgaris_T | 0.31 | 0.06 | 0.02 | 0.00 | 0.31 | 0.02 | 4.99 | 2.23 | 12.23 | 0.08 | 0.61 | 0.03 | 0.92 | 0.34 | 0.27 | 0.04 | 14.68 | 0.62 |
| O. vulgaris_H | 0.72 | 0.36 | 0.03 | 0.00 | 0.35 | 0.03 | 8.60 | 1.22 | 20.38 | 6.18 | 0.60 | 0.05 | 0.93 | 0.42 | 0.26 | 0.04 | 13.33 | 0.49 |
| A. membranaceus_T | 0.49 | 0.60 | 0.03 | 0.01 | 0.31 | 0.03 | 1.99 | 0.40 | 14.21 | 1.56 | 0.56 | 0.27 | 0.43 | 0.06 | 0.14 | 0.01 | 7.67 | 1.16 |
| A. membranaceus_H | 0.55 | 0.26 | 0.03 | 0.01 | 0.29 | 0.04 | 2.39 | 0.73 | 21.46 | 9.00 | 0.59 | 0.14 | 0.43 | 0.11 | 0.13 | 0.02 | 8.17 | 1.02 |
| D. gigas | 0.06 | 0.01 | 0.01 | 0.00 | 0.25 | 0.01 | 0.80 | 0.32 | 9.08 | 0.65 | 0.24 | 0.03 | 0.26 | 0.03 | 0.10 | 0.01 | 8.36 | 0.69 |
| U. duvaucelli | 0.05 | 0.00 | 0.01 | 0.00 | 0.24 | 0.02 | 1.05 | 0.04 | 12.37 | 0.26 | 0.45 | 0.04 | 0.37 | 0.03 | 3.02 | 0.17 | 5.61 | 0.31 |
| P. vannamei_P | 0.02 | 0.00 | 0.03 | 0.02 | 0.64 | 0.35 | 3.12 | 0.86 | 27.32 | 17.91 | 0.74 | 0.48 | 0.49 | 0.27 | 0.13 | 0.03 | 7.88 | 0.62 |
| P. vannamei_R | 0.01 | 0.00 | 0.02 | 0.00 | 0.29 | 0.06 | 4.24 | 0.72 | 26.60 | 12.32 | 0.76 | 0.22 | 0.34 | 0.03 | 0.13 | 0.02 | 7.25 | 0.26 |
| Species | Pb | Cd |
|---|---|---|
| Crustaceans | 0.5 | 0.5 |
| Bivalve mollusks | 1.5 | 1 |
| Cephalopods | 0.3 | 1 |
| Sample | P (mg g−1) | S (mg g−1) | K (mg g−1) | Ca (mg g−1) | Se (µg g−1) | As (µg g−1) |
|---|---|---|---|---|---|---|
| A. membranaceus_H | 0.26 ± 0.01 | 1.02 ± 0.05 | 0.10 ± 0.00 | 1.04 ± 0.03 | 0.31 ± 0.00 | 0.35 ± 0.06 |
| A. membranaceus_T | 0.32 ± 0.01 | 1.08 ± 0.05 | 0.14 ± 0.01 | 0.80 ± 0.05 | 0.36 ± 0.00 | 0.3 ± 0.18 |
| M. chilensis | 1.44 ± 0.08 | 2.88 ± 0.22 | 2.29 ± 0.12 | 0.89 ± 0.03 | 1.68 ± 0.07 | 6.39 ± 0.27 |
| M. chilensis | 2.94 ± 0.04 | 2.78 ± 0.11 | 2.88 ± 0.11 | 0.70 ± 0.04 | 0.60 ± 0.02 | 3.21 ± 0.16 |
| M. chilensis | 2.24 ± 0.02 | 3.35 ± 0.06 | 2.86 ± 0.05 | 0.86 ± 0.01 | 0.82 ± 0.03 | 3.88 ± 0.03 |
| M. galloprovincialis | 2.80 ± 0.10 | 6.56 ± 0.35 | 6.50 ± 0.01 | 0.97 ± 0.04 | 0.95 ± 0.04 | 10.2 ± 0.25 |
| M. galloprovincialis | 1.07 ± 0.02 | 3.21 ± 0.07 | 3.41 ± 0.15 | 0.82 ± 0.01 | 0.90 ± 0.02 | 5.73 ± 0.34 |
| U. duvaucelli | 0.65 ± 0.02 | 1.63 ± 0.14 | 0.51 ± 0.00 | 0.97 ± 0.00 | 0.35 ± 0.03 | 1.16 ± 0.08 |
| M. gigas | 0.51 ± 0.01 | 2.51 ± 0.04 | 2.12 ± 0.09 | 2.16 ± 0.01 | 0.21 ± 0.01 | 5.66 ± 0.21 |
| O. vulgaris_H | 1.08 ± 0.02 | 3.13 ± 0.01 | 1.31 ± 0.04 | 0.94 ± 0.02 | 0.33 ± 0.00 | 5.47 ± 0.13 |
| O. vulgaris_T | 0.99 ± 0.03 | 3.08 ± 0.23 | 1.16 ± 0.05 | 0.76 ± 0.02 | 0.31 ± 0.02 | 4.19 ± 0.28 |
| M. gigas | 0.50 ± 0.02 | 2.31 ± 0.14 | 2.59 ± 0.05 | 0.63 ± 0.01 | 0.17 ± 0.01 | 7.14 ± 0.28 |
| R. venosa | 0.59 ± 0.02 | 1.85 ± 0.04 | 2.88 ± 0.05 | 1.84 ± 0.01 | 0.13 ± 0.00 | 1.29 ± 0.23 |
| R. venosa | 0.94 ± 0.06 | 2.45 ± 0.18 | 4.47 ± 0.20 | 1.78 ± 0.11 | 0.27 ± 0.00 | 1.92 ± 0.07 |
| P. vannamei | 1.14 ± 0.01 | 2.68 ± 0.09 | 5.40 ± 0.35 | 0.90 ± 0.01 | 0.44 ± 0.00 | 0.54 ± 0.02 |
| Species | Cd | Cr | Cu | Ni | Pb | Zn | HI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EDI | THQ | EDI | THQ | EDI | THQ | EDI | THQ | EDI | THQ | EDI | THQ | ||
| A. membranaceus_H | 0.013 | 1.30 × 10−5 | 0.070 | 2.30 × 10−5 | 0.356 | 9.00 × 10−6 | 0.083 | 8.00 × 10−6 | 0.051 | 1.50 × 10−5 | 0.498 | 2.00 × 10−6 | 0.00007 |
| A. membranaceus_T | 0.024 | 2.40 × 10−5 | 0.056 | 1.90 × 10−5 | 0.448 | 1.10 × 10−5 | 0.079 | 7.00 × 10−6 | 0.035 | 1.00 × 10−5 | 0.515 | 2.00 × 10−6 | 7.25 × 10−5 |
| M. chilensis_S1 | 0.022 | 2.20 × 10−5 | 0.087 | 2.90 × 10−5 | 0.148 | 4.00 × 10−6 | 0.110 | 1.00 × 10−5 | 0.033 | 9.00 × 10−6 | 0.767 | 3.00 × 10−6 | 7.65 × 10−5 |
| M. chilensis_S2 | 0.001 | 1.00 × 10−6 | 0.313 | 1.04 × 10−4 | 0.182 | 5.00 × 10−6 | 0.097 | 9.00 × 10−6 | 0.038 | 1.10 × 10−5 | 0.711 | 2.00 × 10−6 | 0.00013 |
| M. chilensis_S3 | 0.047 | 4.70 × 10−5 | 0.068 | 2.30 × 10−5 | 0.142 | 4.00 × 10−6 | 0.087 | 8.00 × 10−6 | 0.032 | 9.00 × 10−6 | 0.721 | 2.00 × 10−6 | 9.2 × 10−5 |
| M. galloprovincialis_S1 | 0.002 | 2.00 × 10−6 | 0.104 | 3.50 × 10−5 | 0.077 | 2.00 × 10−6 | 0.106 | 1.00 × 10−5 | 0.037 | 1.10 × 10−5 | 1.117 | 4.00 × 10−6 | 6.3 × 10−5 |
| M. galloprovincialis_S2 | 0.000 | 0.00 | 0.073 | 2.40 × 10−5 | 0.076 | 2.00 × 10−6 | 0.078 | 7.00 × 10−6 | 0.032 | 9.00 × 10−6 | 1.908 | 6.00 × 10−6 | 4.9 × 10−5 |
| U. duvaucelli | BDL | BDL | 0.177 | 5.90 × 10−5 | 0.138 | 3.00 × 10−6 | 0.044 | 4.00 × 10−6 | 0.031 | 9.00 × 10−6 | 0.205 | 1.00 × 10−6 | 7.2 × 10−5 |
| M. gigas_S1 | 0.001 | 1.00 × 10−6 | 0.321 | 1.07 × 10−4 | 7.886 | 1.97 × 10−4 | 0.077 | 7.00 × 10−6 | 0.038 | 1.10 × 10−5 | 9.252 | 3.10 × 10−5 | 0.00035 |
| M. gigas_S2 | 0.004 | 4.00 × 10−6 | 0.094 | 3.10 × 10−5 | 10.157 | 2.54 × 10−4 | 0.059 | 5.00 × 10−6 | 0.030 | 9.00 × 10−6 | 12.679 | 4.20 × 10−5 | 0.0003 |
| O. vulgaris_H | 0.005 | 5.00 × 10−6 | 0.199 | 6.60 × 10−5 | 0.400 | 1.00 × 10−5 | 0.078 | 7.00 × 10−6 | 0.033 | 1.00 × 10−5 | 0.673 | 2.00 × 10−6 | 0.0001 |
| O. vulgaris_T | 0.001 | 1.00 × 10−6 | 0.087 | 2.90 × 10−5 | 0.359 | 9.00 × 10−6 | 0.067 | 6.00 × 10−6 | 0.030 | 9.00 × 10−6 | 0.509 | 2.00 × 10−6 | 5.6 × 10−5 |
| R. venosa | BDL | BDL | 0.084 | 2.80 × 10−5 | 0.684 | 1.70 × 10−5 | 0.127 | 1.20 × 10−5 | 0.035 | 1.00 × 10−5 | 0.757 | 3.00 × 10−6 | 9.4 × 10−5 |
| R. venosa | BDL | BDL | 0.068 | 2.30 × 10−5 | 2.135 | 5.30 × 10−5 | 0.103 | 9.00 × 10−6 | 0.031 | 9.00 × 10−6 | 0.733 | 2.00 × 10−6 | 5.3 × 10−5 |
| P. vannamei | BDL | BDL | 0.070 | 2.30 × 10−5 | 0.520 | 1.30 × 10−5 | 0.103 | 9.00 × 10−6 | 0.037 | 1.10 × 10−5 | 0.333 | 1.00 × 10−6 | 6.6 × 10−5 |
| Species | TR | |||
|---|---|---|---|---|
| Cd | Cr | Ni | Pb | |
| A. membranaceus_H | 4.85 × 10−9 | 3.48 × 10−8 | 1.41 × 10−7 | 4.34 × 10−10 |
| A. membranaceus_T | 9.11 × 10−9 | 2.78 × 10−8 | 1.34 × 10−7 | 2.94 × 10−10 |
| M. chilensis | 8.3 × 10−9 | 4.35 × 10−8 | 1.87 × 10−7 | 2.81 × 10−10 |
| M. chilensis | 2.42 × 10−10 | 1.56 × 10−7 | 1.65 × 10−7 | 3.25 × 10−10 |
| M. chilensis | 1.78 × 10−8 | 3.4 × 10−8 | 1.48 × 10−7 | 2.69 × 10−10 |
| M. galloprovincialis | 9.01 × 10−10 | 5.22 × 10−8 | 1.81 × 10−7 | 3.14 × 10−10 |
| M. galloprovincialis | 1.04 × 10−10 | 3.65 × 10−8 | 1.33 × 10−7 | 2.71 × 10−10 |
| U. duvaucelli | BDL | 8.84 × 10−8 | 7.46 × 10−8 | 2.6 × 10−10 |
| M. gigas | 4.18 × 10−10 | 1.6 × 10−7 | 1.31 × 10−7 | 3.23 × 10−10 |
| O. vulgaris_H | 2.01 × 10−9 | 9.93 × 10−8 | 1.32 × 10−7 | 2.83 × 10−10 |
| O. vulgaris_T | 5.36 × 10−10 | 4.36 × 10−8 | 1.14 × 10−7 | 2.55 × 10−10 |
| M. gigas | 1.36 × 10−9 | 4.72 × 10−8 | 1.01 × 10−7 | 2.58 × 10−10 |
| R. venosa | BDL | 4.18 × 10−8 | 2.16 × 10−7 | 2.99 × 10−10 |
| R. venosa | BDL | 3.42 × 10−8 | 1.75 × 10−7 | 2.67 × 10−10 |
| P. vannamei | BDL | 3.48 × 10−8 | 1.75 × 10−7 | 3.17 × 10−10 |
| Fish/Seafood Sample | Cooked/Raw | Studied Element | Metal Bioaccessibility (%) | Reference |
|---|---|---|---|---|
| Cod | Cooked | Selenium | 61 | [35] |
| Tuna | Cooked | Mercury | 6–10 | [18] |
| Tuna | Raw | Mercury | 13–19 | [18] |
| Swordfish | Raw | Mercury | 59–87 | [18] |
| Swordfish | Cooked | Mercury | 38–49 | [18] |
| Tope shark | Raw | Mercury | 59–69 | [18] |
| Tope shark | Cooked | Mercury | 34–47 | [18] |
| Butter clams | Raw | Cadmium | 107 | [22] |
| Arsenic | 108 | |||
| Mercury | 50 | |||
| Selenium | 98 | |||
| Manganese | 102 | |||
| Copper | 117 | |||
| Salmon eggs | Raw | Cadmium | 74 | [22] |
| Arsenic | 73 | |||
| Mercury | 10 | |||
| Selenium | 76 | |||
| Manganese | 96 | |||
| Copper | 106 | |||
| Chinook salmon | Raw | Cadmium | 18 | [22] |
| Arsenic | 57 | |||
| Mercury | 49 | |||
| Selenium | 52 | |||
| Manganese | 38 | |||
| Copper | 64 | |||
| Sockeye salmon | Raw | Cadmium | 61 | [22] |
| Arsenic | 68 | |||
| Mercury | 46 | |||
| Selenium | 50 | |||
| Manganese | 48 | |||
| Copper | 63 | |||
| Seaweed | Raw | Cadmium | 64 | [22] |
| Arsenic | 79 | |||
| Mercury | - | |||
| Selenium | 65 | |||
| Manganese | 86 | |||
| Copper | 59 | |||
| Rapa whelk | Raw | Chromium | 24.27–57.14 | [10] |
| Manganese | 17.47–32.43 | |||
| Copper | 70.97–83.56 | |||
| Zinc | 58.21–71.12 | |||
| Cadmium | 61.58–79.46 | |||
| Nichel | 3.26–71.48 | |||
| Seabass | Raw | Arsenic | 81.2–90.8 | [55] |
| Cooked | 73.3–86.2 | |||
| Raw | Cadmium | 84.8–93.2 | ||
| Cooked | 36.2–86.5 | |||
| Raw | Copper | 81.4–84.2 | ||
| Cooked | 54.2–71.2 | |||
| Raw | Iron | 51.3–58.0 | ||
| Cooked | 27.7–53.7 | |||
| Raw | Selenium | 60.8–61.0 | ||
| Cooked | 29.0–62.2 | |||
| Raw | Zinc | 70.4–72.3 | ||
| Cooked | 36.8–52.2 | |||
| Red seabream | Raw | Arsenic | 74.0–87.9 | [55] |
| Cooked | 3.7–86.5 | |||
| Raw | Cadmium | 73.7–89.9 | ||
| Cooked | 52.2–88.0 | |||
| Raw | Copper | 82.7–85.4 | ||
| Cooked | 58.2–71.2 | |||
| Raw | Iron | 45.4–52.0 | ||
| Cooked | 24.6–53.2 | |||
| Raw | Selenium | 48.0–63.6 | ||
| Cooked | 27.4–66.2 | |||
| Raw | Zinc | 66.6–73.1 | ||
| Cooked | 28.1–51.5 | |||
| Shellfish—C. ariakensis | Raw | Zinc | 16.24–22.52 | [8] |
| Copper | 42.88–6.38 | |||
| Cadmium | 12.59–33.22 | |||
| Arsenic | 49.52–69.23 | |||
| Chromium | 65.41–66.36 | |||
| Lead | 22.96–73.64 | |||
| Shellfish—C. farreri | Raw | Zinc | 21.95–48.80 | [8] |
| Copper | 34.63–70.13 | |||
| Cadmium | 11.42–45.41 | |||
| Arsenic | 61.49–79.31 | |||
| Chromium | 58.72–66.54 | |||
| Lead | 45.90–82.35 | |||
| Shellfish—S. constricta | Raw | Zinc | 17.26–3.7.01 | [8] |
| Copper | 32.79–49.88 | |||
| Cadmium | 12.31–29.11 | |||
| Arsenic | 28.69–61.68 | |||
| Chromium | 56.72–59.88 | |||
| Lead | 44.62–72.36 | |||
| Yellow croaker | Raw | Arsenic | 87.4–98.4 | [56] |
| Snapper | Raw | Arsenic | 98.4 | |
| Turbot | Raw | Hg | 96.8 | |
| Clam | Raw | Hg | 64.7 | |
| Turbot | Raw | Cadmium | 60.0–99.4 | |
| Turbot | Raw | Lead | 78.9–93.8 | |
| Turbot | Raw | Nichel | 75.9–94.3 | |
| Snapper | Raw | Chromium | 20.2 | |
| Clam | Raw | Chromium | 87.6 | |
| Seafood | Cooked | Mercury | 14–39 | |
| Seafood | Cooked | Arsenic | 2.7–35.2 | |
| Black scabbardfish Black scabbardfish | Raw | Mercury | 40 | [57] |
| Cooked | Mercury | 10 | ||
| Mollusks | Raw | Arsenobetaine | 100 | [58] |
| Shellfishes | Cooked | Arsenic | Gastric: 40.9, Intestinal: 52.5 | |
| Chlamys farreri-mantle | Raw | Cadmium | 11.2–49.5 | [59] |
| Chlamys farreri-mantle | Cooked | Cadmium | 9.4–27.1 | |
| Octopus | Raw | Mercury | 11 | [54] |
| Cooked | 1 | |||
| Hake | Raw | Mercury | 61 | |
| Cooked | 19 | |||
| Octopus | Arsenic | 98 | ||
| Mussel | Raw | Cadmium | 103 | |
| Cooked | 83 | |||
| Shrimp | Raw | Cadmium | 75 | |
| Cooked | 54 | |||
| Tuna | Raw | Cadmium | 41 | |
| Cooked | 53 | |||
| Shrimp | Raw | Selenium | 93 | |
| Cooked | 74 | |||
| Octopus | Raw | Copper | 93 | |
| Cooked | 100 | |||
| Octopus | Raw | Manganese | 113 | |
| Mussel | Raw | Iron | 26 | |
| Cooked | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milea, Ș.-A.; Simionov, I.-A.; Lazăr, N.-N.; Iticescu, C.; Timofti, M.; Georgescu, P.-L.; Faggio, C. Comprehensive Risk Assessment of Metals and Minerals in Seafood Using Bioaccessibility Correction. J. Xenobiot. 2025, 15, 92. https://doi.org/10.3390/jox15030092
Milea Ș-A, Simionov I-A, Lazăr N-N, Iticescu C, Timofti M, Georgescu P-L, Faggio C. Comprehensive Risk Assessment of Metals and Minerals in Seafood Using Bioaccessibility Correction. Journal of Xenobiotics. 2025; 15(3):92. https://doi.org/10.3390/jox15030092
Chicago/Turabian StyleMilea, Ștefania-Adelina, Ira-Adeline Simionov, Nina-Nicoleta Lazăr, Cătălina Iticescu, Mihaela Timofti, Puiu-Lucian Georgescu, and Caterina Faggio. 2025. "Comprehensive Risk Assessment of Metals and Minerals in Seafood Using Bioaccessibility Correction" Journal of Xenobiotics 15, no. 3: 92. https://doi.org/10.3390/jox15030092
APA StyleMilea, Ș.-A., Simionov, I.-A., Lazăr, N.-N., Iticescu, C., Timofti, M., Georgescu, P.-L., & Faggio, C. (2025). Comprehensive Risk Assessment of Metals and Minerals in Seafood Using Bioaccessibility Correction. Journal of Xenobiotics, 15(3), 92. https://doi.org/10.3390/jox15030092










