Role of Native Arbuscular Mycorrhizal Fungi in Modulating Nutrient Subcellular Distribution in Wheat Grown in Mn-Toxic Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Soil’s Characteristics, the Plant Material, and the Experimental Design
2.2. Tissue and Subcellular Element Partitioning
2.3. Microwave-Assisted Acidic Digestion of the Wheat Tissues and Subcellular Fractions
2.4. Quantitative Analysis of Mg, Ca, P, and Mn
2.5. The Data Treatment and Statistical Analysis
3. Results
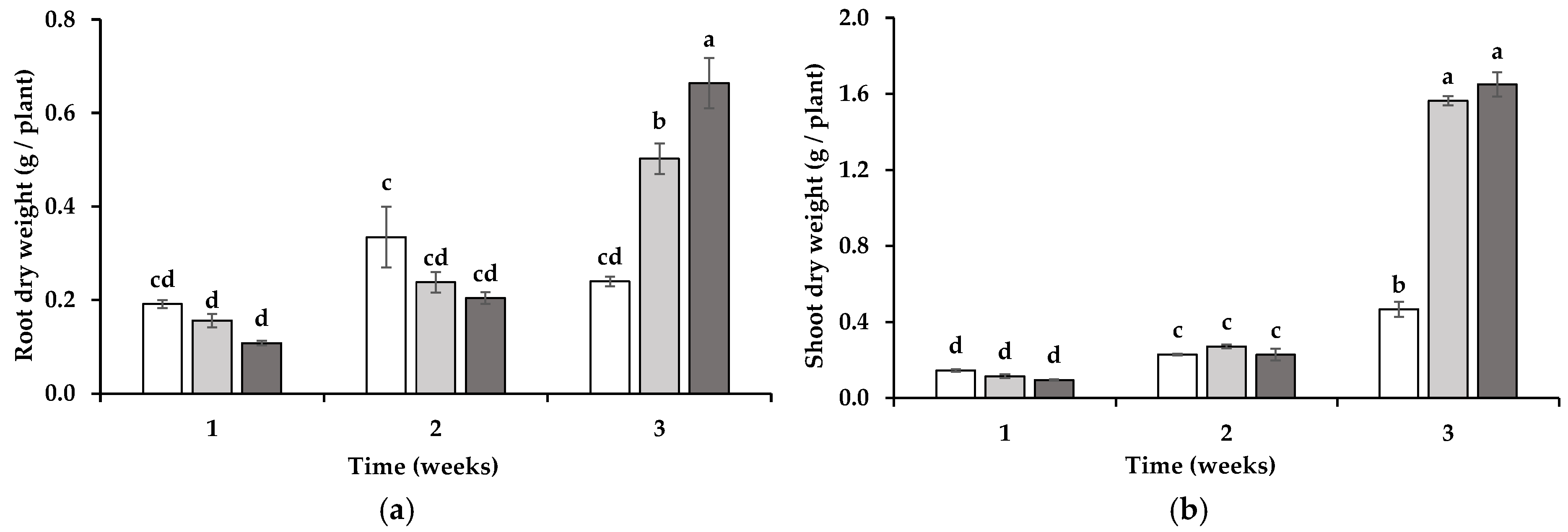
3.1. Wheat Growth
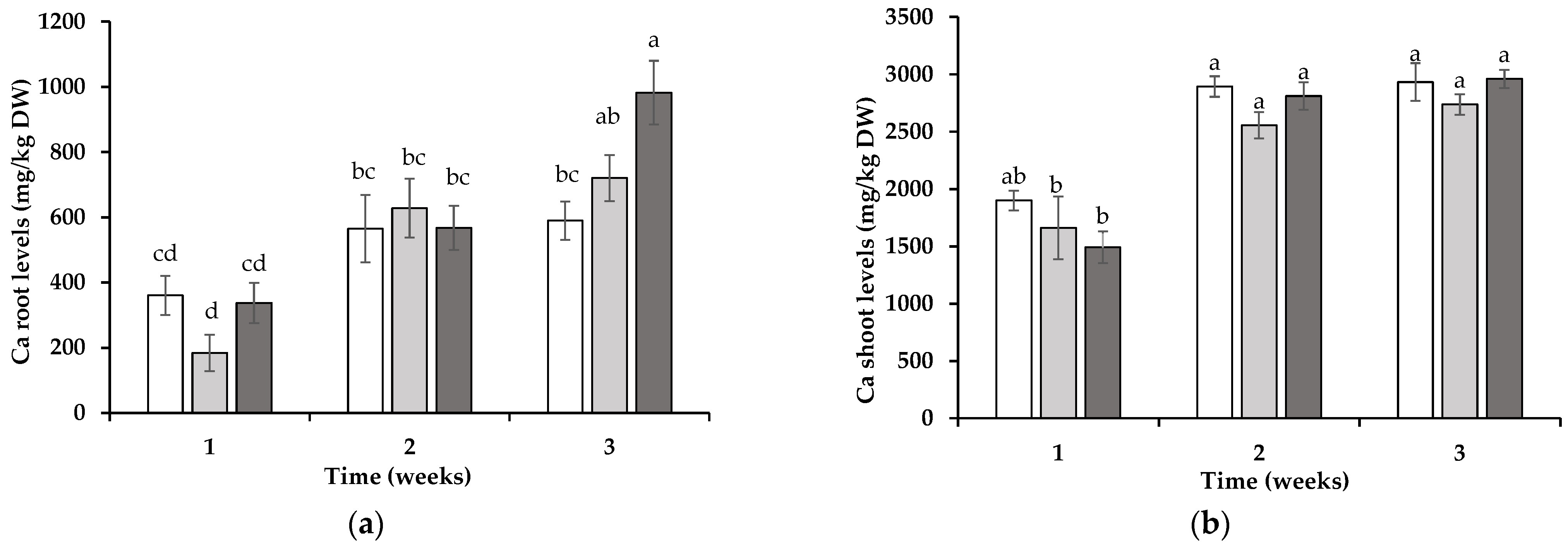
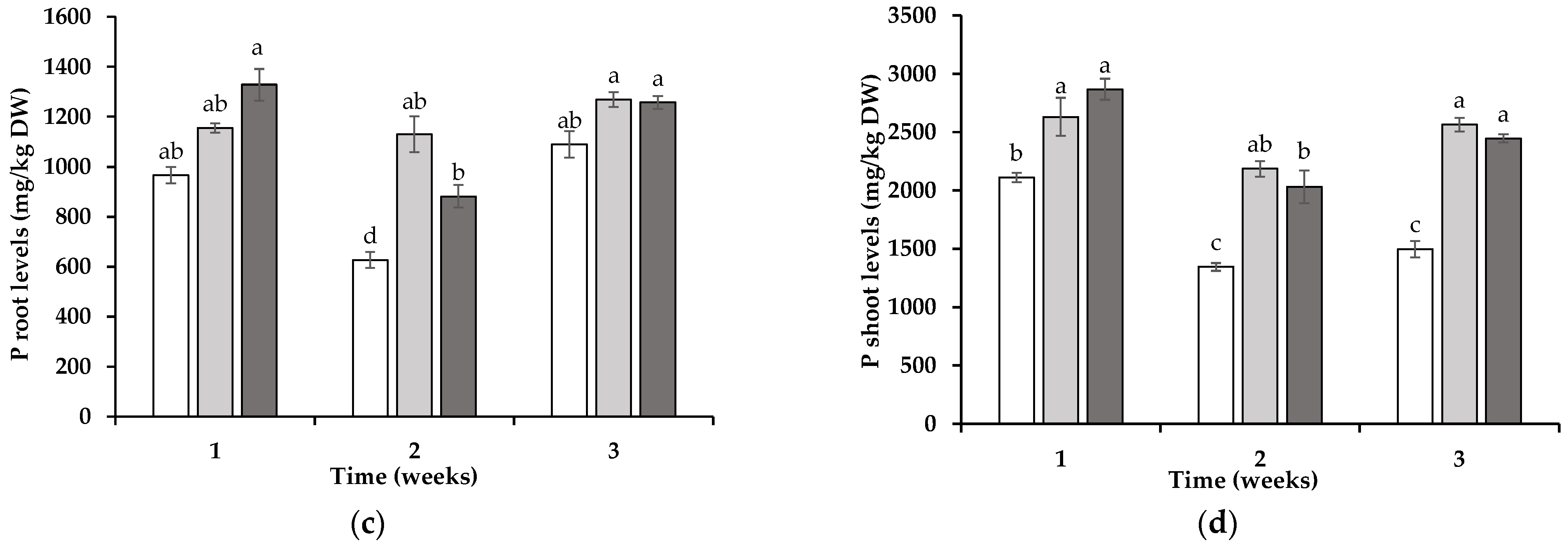
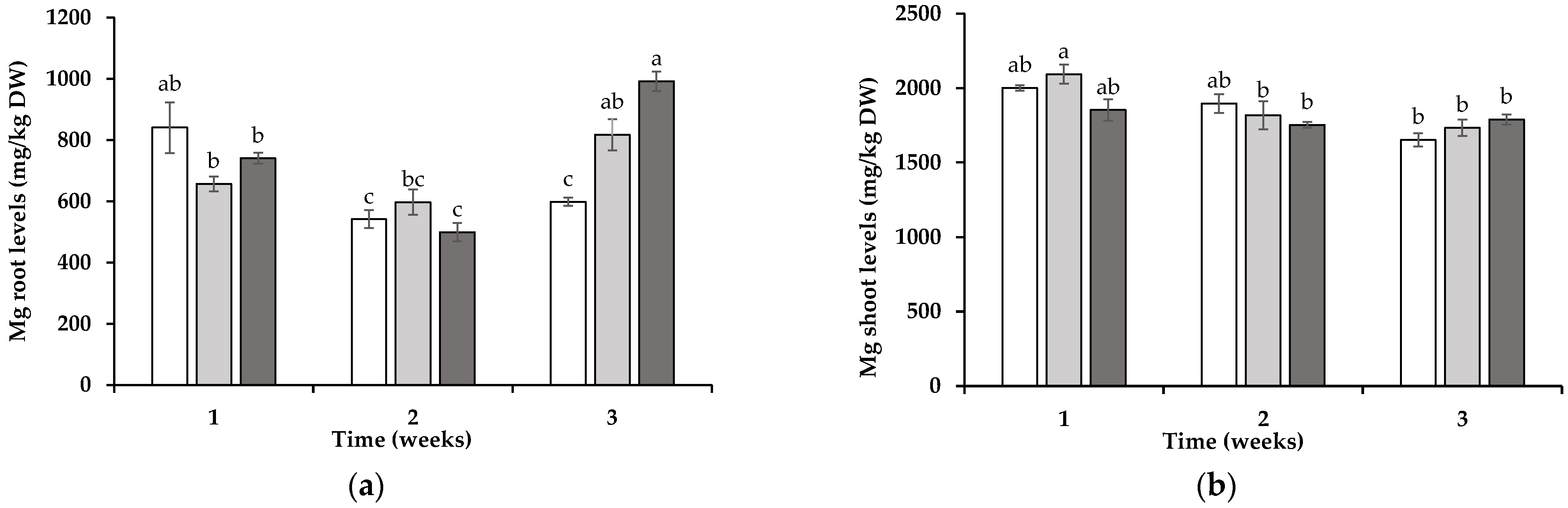
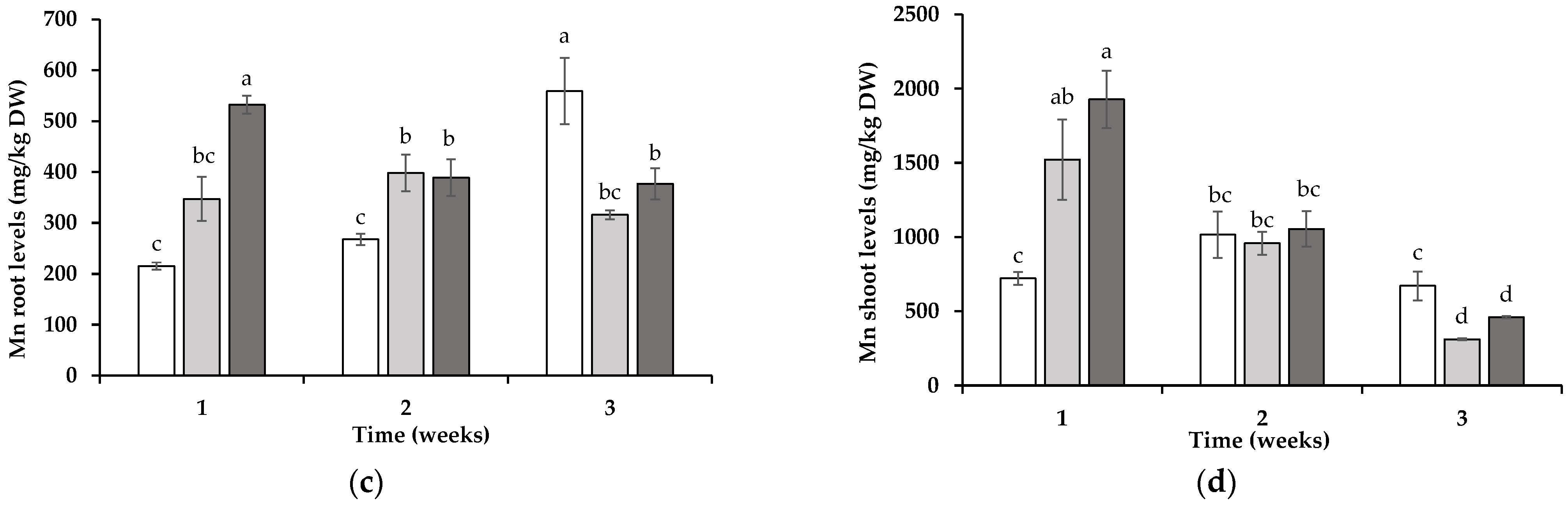
3.2. Tissue Element Levels
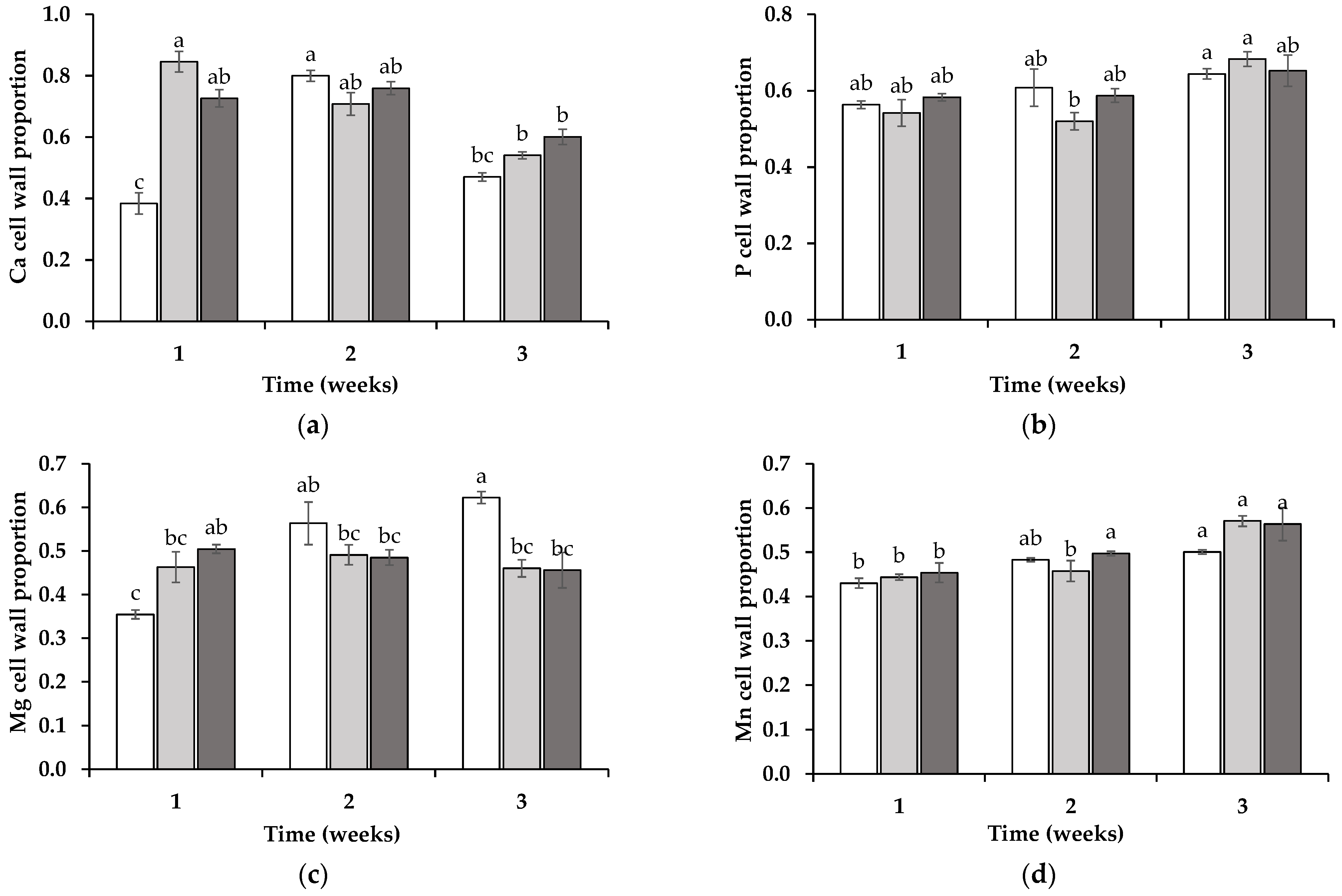
3.3. Tissue Subcellular Redistribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campillo-Cora, C.; Rodríguez-Seijo, A.; Pérez-Rodríguez, P.; Fernández-Calviño, D.; Santás-Miguel, V. Effect of Heavy Metal Pollution on Soil Microorganisms: Influence of Soil Physicochemical Properties. A Systematic Review. Eur. J. Soil Biol. 2025, 124, 103706. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and Metalloid Toxicity in Plants: An Overview on Molecular Aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. Aluminium, Iron and Silicon Subcellular Redistribution in Wheat Induced by Manganese Toxicity. Appl. Sci. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Zamanian, K.; Taghizadeh-Mehrjardi, R.; Tao, J.; Fan, L.; Raza, S.; Guggenberger, G.; Kuzyakov, Y. Acidification of European Croplands by Nitrogen Fertilization: Consequences for Carbonate Losses, and Soil Health. Sci. Total Environ. 2024, 924, 171631. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil Acidification and the Importance of Liming Agricultural Soils with Particular Reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Keisling, T.C.; Thompson, L.F.; Slabaugh, W.R. Visual Symptoms and Tissue Manganese Concentrations Associated with Manganese Toxicity in Wheat. Commun. Soil Sci. Plant Anal. 1984, 15, 537–540. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese Phytotoxicity: New Light on an Old Problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, Y.; Xu, Z.; Zhang, W.; Jiang, K. Physiological Responses of Broussonetia Papyrifera to Manganese Stress, a Candidate Plant for Phytoremediation. Ecotoxicol. Environ. Saf. 2019, 181, 18–25. [Google Scholar] [CrossRef]
- Sparrow, L.A.; Uren, N.C. The Role of Manganese Toxicity in Crop Yellowing on Seasonally Waterlogged and Strongly Acidic Soils in North-Eastern Victoria. Aust. J. Exp. Agric. 1987, 27, 303–307. [Google Scholar] [CrossRef]
- El-Jaoual, T.; Cox, D.A. Manganese Toxicity in Plants. J. Plant Nutr. 1998, 21, 353–386. [Google Scholar] [CrossRef]
- Doncheva, S.; Poschenrieder, C.; Stoyanova, Z.; Georgieva, K.; Velichkova, M.; Barceló, J. Silicon Amelioration of Manganese Toxicity in Mn-Sensitive and Mn-Tolerant Maize Varieties. Environ. Exp. Bot. 2009, 65, 189–197. [Google Scholar] [CrossRef]
- Cho, M.-J.; Park, S.-Y.; Kim, C.-G. Acidic Neutralization by Indigenous Bacteria Isolated from Abandoned Mine Areas. Appl. Sci. 2022, 12, 3324. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, D.; Li, F.; Dong, Y.; Jin, Z.; Liao, Y.; Li, X.; Peng, S.; Delgado-Baquerizo, M.; Li, X. Superiority of Native Soil Core Microbiomes in Supporting Plant Growth. Nat. Commun. 2024, 15, 6599. [Google Scholar] [CrossRef]
- Brito, I.; Carvalho, M.; Alho, L.; Goss, M.J. Managing Arbuscular Mycorrhizal Fungi for Bioprotection: Mn Toxicity. Soil Biol. Biochem. 2014, 68, 78–84. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and Soil Structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef]
- Rich, M.K.; Nouri, E.; Courty, P.E.; Reinhardt, D. Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter? Trends Plant Sci. 2017, 22, 652–660. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular Mycorrhiza and Heavy Metal Tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular Mycorrhizal Fungi-Induced Mitigation of Heavy Metal Phytotoxicity in Metal Contaminated Soils: A Critical Review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Pinto, A.P.; Teixeira, D.M.; Barrulas, P.; Brito, I.; Carvalho, M. Subcellular Element Distribution in Shoots of Wheat Grown in an Acidic Soil with Native AMF Extraradical Mycelium. Agronomy 2022, 12, 2173. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in Microbe-Assisted Reclamation of Heavy Metal Contaminated Soils over the Last Decade: A Review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Alho, L.; Carvalho, M. Toxic Levels of Manganese in an Acidic Cambisol Alters Antioxidant Enzymes Activity, Element Uptake and Subcellular Distribution in Triticum Aestivum. Ecotoxicol. Environ. Saf. 2020, 193, 110355. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. The Protective Biochemical Properties of Arbuscular Mycorrhiza Extraradical Mycelium in Acidic Soils Are Maintained throughout the Mediterranean Summer Conditions. Agronomy 2021, 11, 748. [Google Scholar] [CrossRef]
- Kabir, Z. Tillage or No-Tillage: Impact on Mycorrhizae. Can. J. Plant Sci. 2005, 85, 23–29. [Google Scholar] [CrossRef]
- Campos, C.; Nobre, T.; Goss, M.J.; Faria, J.; Barrulas, P.; Carvalho, M. Transcriptome Analysis of Wheat Roots Reveals a Differential Regulation of Stress Responses Related to Arbuscular Mycorrhizal Fungi and Soil Disturbance. Biology. 2019, 8, 93. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barrulas, P.; Pinto, A.P.; Brito, I.; Teixeira, D.M. Mycorrhizal Colonization of Wheat by Intact Extraradical Mycelium of Mn-Tolerant Native Plants Induces Different Biochemical Mechanisms of Protection. Plants 2023, 12, 2091. [Google Scholar] [CrossRef]
- Brígido, C.; van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the Biological Diversity of AM Fungi by Combination of Host Plant Succession and Integrity of Extraradical Mycelium. Soil Biol. Biochem. 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Garcia, R.; Barrulas, P.; Dias, C.; Cabrita, M.J.; Garde-Cerdán, T. Classification of Wines According to Several Factors by ICP-MS Multi-Element Analysis. Food Chem. 2019, 270, 273–280. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular Mycorrhizal Symbiosis and Abiotic Stress in Plants: A Review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- George, T.S.; French, A.S.; Brown, L.K.; Karley, A.J.; White, P.J.; Ramsay, L.; Daniell, T.J. Genotypic Variation in the Ability of Landraces and Commercial Cereal Varieties to Avoid Manganese Deficiency in Soils with Limited Manganese Availability: Is There a Role for Root-exuded Phytases? Physiol. Plant. 2014, 151, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Distribution of Carboxylates and Acid Phosphatase and Depletion of Different Phosphorus Fractions in the Rhizosphere of a Cereal and Three Grain Legumes. Plant Soil 2006, 281, 109–120. [Google Scholar] [CrossRef]
- Lambers, H.; Hayes, P.E.; Laliberté, E.; Oliveira, R.S.; Turner, B.L. Leaf Manganese Accumulation and Phosphorus-Acquisition Efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef]
- George, E.; Horst, W.J.; Neumann, E. Adaptation of plants to adverse chemical soil conditions. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 409–472. [Google Scholar] [CrossRef]
- Marschner, H. Mechanisms of Adaptation of Plants to Acid Soils. Plant Soil 1991, 134, 1–20. [Google Scholar] [CrossRef]
- Krzesłowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M.; Reyes- Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M.; Reyes-Díaz, M.; et al. Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Ferreira, D.; Barrulas, P.; Brito, I.; Pinto, A.P.; Carvalho, M. Manganese Uptake to Wheat Shoot Meristems Is Differentially Influenced by Arbuscular Mycorrhiza Fungal Communities Adapted to Acidic Soil. Soil Syst. 2022, 6, 50. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Pinto, A.P.; Barrulas, P.; Brito, I.; Teixeira, D.M. Role of Native Arbuscular Mycorrhizal Fungi in Modulating Nutrient Subcellular Distribution in Wheat Grown in Mn-Toxic Soil. J. Xenobiot. 2025, 15, 70. https://doi.org/10.3390/jox15030070
Faria JMS, Pinto AP, Barrulas P, Brito I, Teixeira DM. Role of Native Arbuscular Mycorrhizal Fungi in Modulating Nutrient Subcellular Distribution in Wheat Grown in Mn-Toxic Soil. Journal of Xenobiotics. 2025; 15(3):70. https://doi.org/10.3390/jox15030070
Chicago/Turabian StyleFaria, Jorge Miguel Silva, Ana Paula Pinto, Pedro Barrulas, Isabel Brito, and Dora M. Teixeira. 2025. "Role of Native Arbuscular Mycorrhizal Fungi in Modulating Nutrient Subcellular Distribution in Wheat Grown in Mn-Toxic Soil" Journal of Xenobiotics 15, no. 3: 70. https://doi.org/10.3390/jox15030070
APA StyleFaria, J. M. S., Pinto, A. P., Barrulas, P., Brito, I., & Teixeira, D. M. (2025). Role of Native Arbuscular Mycorrhizal Fungi in Modulating Nutrient Subcellular Distribution in Wheat Grown in Mn-Toxic Soil. Journal of Xenobiotics, 15(3), 70. https://doi.org/10.3390/jox15030070








