Air Pollution and Pituitary Adenoma Pathogenesis: Unraveling Environmental Impacts on Neuroendocrine Function and Tumorigenesis
Abstract
1. Introduction
2. Hypothalamic–Pituitary–Adrenal (HPA) Axis and Pituitary Pathophysiology
3. Inflammatory Effects on Pituitary Adenoma Microenvironment and the HPA
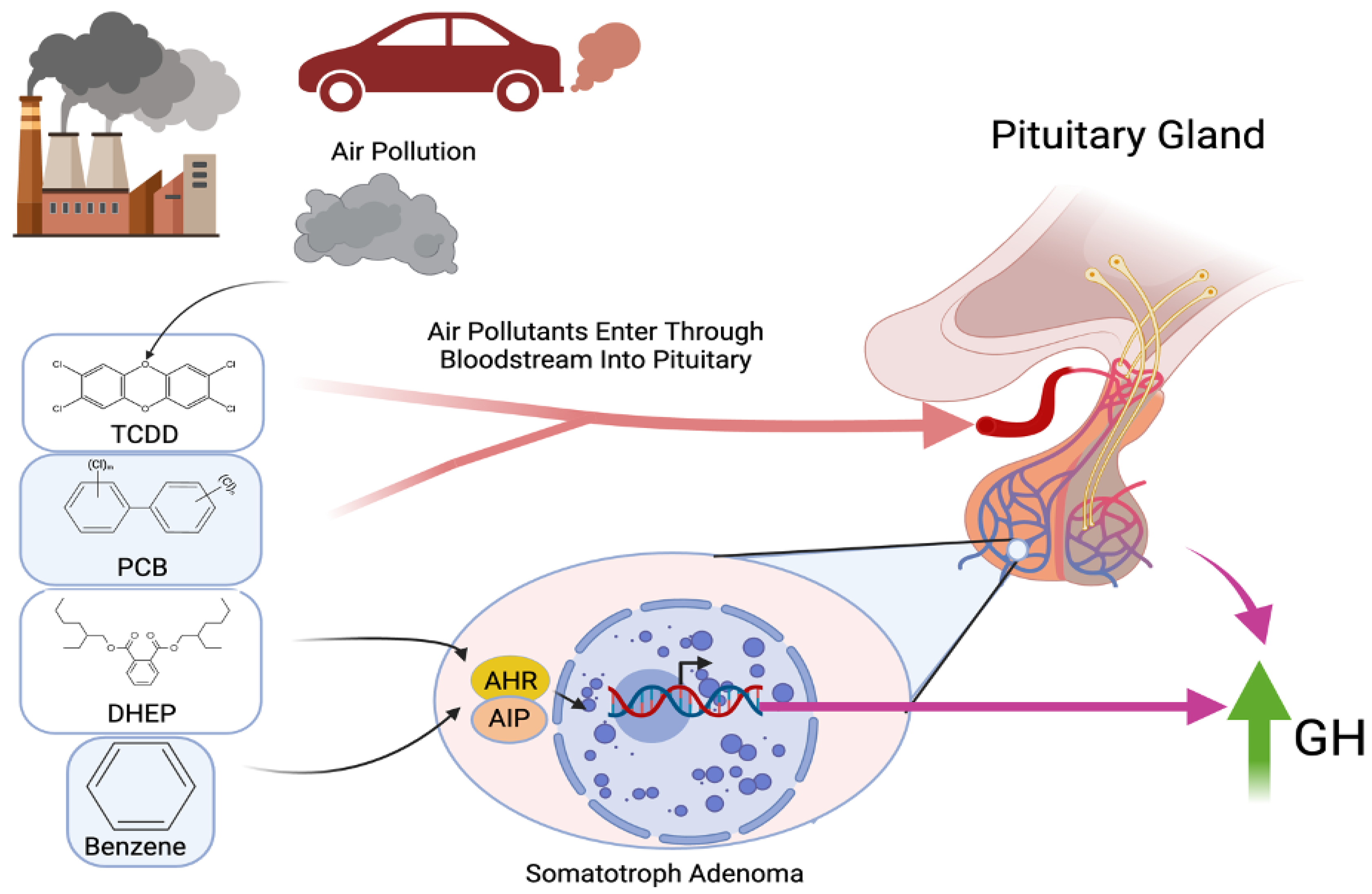
4. Air Pollution and Pituitary Tumors
5. Public Health Implications, Challenges, and Future Directions
Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AHR | Aryl hydrocarbon receptor |
| AIP | Aryl hydrocarbon receptor-interacting protein |
| ADH | Antidiuretic hormone |
| BBB | Blood–brain barrier |
| BZ | Benzene |
| CNS | Central nervous system |
| CRH | Corticotropin-releasing hormone |
| DEHP | Di(2-ethylhexyl) phthalate |
| ET-1 | Endothelin-1 |
| FSH | Follicle-stimulating hormone |
| GH | Growth hormone |
| GH3 | GH-producing pituitary adenoma cell line |
| GNAS | Guanine Nucleotide-Binding Protein, Alpha Subunit |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HPA | Hypothalamic–pituitary–adrenal |
| IL | Interleukin (e.g., IL-1β, IL-6) |
| iNOS | Inducible nitric oxide synthase |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription Pathway |
| LH | Luteinizing hormone |
| MAC1-NOX2 | Macrophage Antigen 1–NADPH Oxidase 2 Pathway |
| MAPK | Mitogen-Activated Protein Kinase |
| MEN1 | Multiple Endocrine Neoplasia Type 1 |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells |
| PCBs | Polychlorinated biphenyls |
| ROS | Reactive oxygen species |
| SSA | Somatostatin analog |
| SSTR2 | Somatostatin receptor 2 |
| TME | Tumor microenvironment |
| TNF-α | Tumor Necrosis Factor-alpha |
| MtT/E-2 | Mammosomatotrope Cell Line |
References
- Alhussaini, A.R.; Aljabri, M.R.; Al-Harbi, Z.T.; Abdulrahman Almohammadi, G.; Al-Harbi, T.M.; Bashir, S. Air Pollution and Its Adverse Effects on the Central Nervous System. Cureus 2023, 15, e38927. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.-C.; Dao, K.; Roque, P. Neurotoxicants Are in the Air: Convergence of Human, Animal, and In Vitro Studies on the Effects of Air Pollution on the Brain. BioMed Res. Int. 2014, 2014, 736385. [Google Scholar] [CrossRef]
- Block, M.L.; Elder, A.; Auten, R.L.; Bilbo, S.D.; Chen, H.; Chen, J.-C.; Cory-Slechta, D.A.; Costa, D.; Diaz-Sanchez, D.; Dorman, D.C.; et al. The Outdoor Air Pollution and Brain Health Workshop. Neurotoxicology 2012, 33, 972–984. [Google Scholar] [CrossRef]
- Block, M.L.; Calderón-Garcidueñas, L. Air Pollution: Mechanisms of Neuroinflammation and CNS Disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Sirica, A. The Pathobiology of Neoplasia; Springer: New York, NY, USA, 2014; ISBN 97814684543. [Google Scholar]
- US Environmental Protection Agency. Proposed Guidelines for Carcinogen Risk Assessment, April 1996; Bibliogov: Washington, DC, USA, 2013; ISBN 9781289209452.
- Tl, T.; Steward, P.A. Risk of Astrocytic Brain Tumors Associated with Occupational Chemical Exposures. Scand. J. Work Environ. Health 1987, 13, 417–423. [Google Scholar]
- Ljubimova, J.Y.; Braubach, O.; Patil, R.; Chiechi, A.; Tang, J.; Galstyan, A.; Shatalova, E.S.; Kleinman, M.T.; Black, K.L.; Holler, E. Coarse Particulate Matter (PM2.5-10) in Los Angeles Basin Air Induces Expression of Inflammation and Cancer Biomarkers in Rat Brains. Sci. Rep. 2018, 8, 5708. [Google Scholar] [CrossRef]
- Israel, L.L.; Braubach, O.; Shatalova, E.S.; Chepurna, O.; Sharma, S.; Klymyshyn, D.; Galstyan, A.; Chiechi, A.; Cox, A.; Herman, D.; et al. Exposure to Environmental Airborne Particulate Matter Caused Wide-Ranged Transcriptional Changes and Accelerated Alzheimer’s-Related Pathology: A Mouse Study. Neurobiol. Dis. 2023, 187, 106307. [Google Scholar] [CrossRef]
- Lenders, N.F.; Earls, P.E.; Inder, W.J.; McCormack, A.I. The Evolution in Pituitary Tumour Classification: A Clinical Perspective. Endocr. Oncol. 2023, 3, e220079. [Google Scholar] [CrossRef]
- Poulsen, A.H.; Hvidtfeldt, U.A.; Sørensen, M.; Puett, R.; Ketzel, M.; Brandt, J.; Geels, C.; Christensen, J.H.; Raaschou-Nielsen, O. Intracranial Tumors of the Central Nervous System and Air Pollution—A Nationwide Case-Control Study from Denmark. Environ. Health 2020, 19, 81. [Google Scholar] [CrossRef]
- Keswani, A.; Akselrod, H.; Anenberg, S.C. Health and Clinical Impacts of Air Pollution and Linkages with Climate Change. NEJM Evid. 2022, 1, EVIDra2200068. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.M. Air Pollution, Stress, and Allostatic Load: Linking Systemic and Central Nervous System Impacts. J. Alzheimers Dis. 2019, 69, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Lemos, M.C.; Thakker, R.V. Hypothalamic-Pituitary Axis and Endocrine Homeostasis: Physiology and Pathophysiology. In Springer Handbook of Endocrinology; Springer Nature: Cham, Switzerland, 2024; pp. 1–32. [Google Scholar]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Endocrine Society Diagnosis and Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Tapoi, D.A.; Popa, M.-L.; Tanase, C.; Derewicz, D.; Gheorghișan-Gălățeanu, A.-A. Role of Tumor Microenvironment in Pituitary Neuroendocrine Tumors: New Approaches in Classification, Diagnosis and Therapy. Cancers 2023, 15, 5301. [Google Scholar] [CrossRef]
- Han, C.; Lin, S.; Lu, X.; Xue, L.; Wu, Z.B. Tumor-Associated Macrophages: New Horizons for Pituitary Adenoma Researches. Front. Endocrinol. 2021, 12, 785050. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, C.; Wang, W.; Lu, D.; Li, J.; Li, L.; Li, Y.; Qiao, Y.; Song, H.; Deng, X. Hypoxia Promotes Proliferation of Pituitary Adenomas by HIF-1α/ALKBH5 Signaling In Vitro. Int. J. Clin. Exp. Pathol. 2020, 13, 1030–1034. [Google Scholar]
- Asa, S.L.; Ezzat, S. The pathogenesis of pituitary tumors. Annu. Rev. Pathol. 2009, 4, 97–126. [Google Scholar] [CrossRef] [PubMed]
- Hibberts, N.A.; Simpson, D.J.; Bicknell, J.E.; Broome, J.C.; Hoban, P.R.; Clayton, R.N.; Farrell, W.E. Analysis of cyclin D1 (CCND1) allelic imbalance and overexpression in sporadic human pituitary tumors. Clin. Cancer Res. 1999, 5, 2133–2139. [Google Scholar] [PubMed]
- Jaffrain-Rea, M.L.; Rotondi, S.; Alesse, E. New Insights in the Pathogenesis of Pituitary Tumours. In Hot Topics in Endocrine and Endocrine-Related Diseases; Fedele, M., Ed.; InTechOpen: London, UK, 2013; pp. 28–84. [Google Scholar] [CrossRef]
- Lange, M.; Pagotto, U.; Hopfner, U.; Ehrenreich, H.; Oeckler, R.; Sinowatz, F.; Stalla, G.K. Endothelin Expression in Normal Human Anterior Pituitaries and Pituitary Adenomas. J. Clin. Endocrinol. Metab. 1994, 79, 1864–1870. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, X.; Desiderio, D.M. Pituitary Adenoma Nitroproteomics: Current Status and Perspectives. Oxid. Med. Cell. Longev. 2013, 2013, 580710. [Google Scholar] [CrossRef]
- Ageeva, T.; Rizvanov, A.; Mukhamedshina, Y. NF-κB and JAK/STAT Signaling Pathways as Crucial Regulators of Neuroinflammation and Astrocyte Modulation in Spinal Cord Injury. Cells 2024, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, S.; Harper, C.V.; Semprini, S.; Wilding, M.; Adamson, A.D.; Spiller, D.G.; Nelson, G.; Mullins, J.J.; White, M.R.H.; Davis, J.R.E. Tumor Necrosis Factor-Alpha Activates the Human Prolactin Gene Promoter via Nuclear Factor-kappaB Signaling. Endocrinology 2006, 147, 773–781. [Google Scholar] [CrossRef]
- Arzt, E. Gp130 Cytokine Signaling in the Pituitary Gland: A Paradigm for Cytokine-Neuro-Endocrine Pathways. J. Clin. Investig. 2001, 108, 1729–1733. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, R.; Cortese, A.; Pichiecchio, A.; Berzolari, F.G.; Borrelli, P.; Mallucci, G.; Bollati, V.; Romani, A.; Nosari, G.; Villa, S.; et al. Air Pollution Is Associated to the Multiple Sclerosis Inflammatory Activity as Measured by Brain MRI. Mult. Scler. J. 2018, 24, 1578–1584. [Google Scholar] [CrossRef]
- Türk Börü, Ü.; Bölük, C.; Taşdemir, M.; Gezer, T.; Serim, V.A. Air Pollution, a Possible Risk Factor for Multiple Sclerosis. Acta Neurol. Scand. 2020, 141, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-N.; Xu, Z.; Wu, G.-C.; Mao, Y.-M.; Liu, L.-N.; Wu, Q.; Dan, Y.-L.; Tao, S.-S.; Zhang, Q.; Sam, N.B.; et al. Emerging Role of Air Pollution in Autoimmune Diseases. Autoimmun. Rev. 2019, 18, 607–614. [Google Scholar] [CrossRef]
- Cortese, A.; Lova, L.; Comoli, P.; Volpe, E.; Villa, S.; Mallucci, G.; La Salvia, S.; Romani, A.; Franciotta, D.; Bollati, V.; et al. Air Pollution as a Contributor to the Inflammatory Activity of Multiple Sclerosis. J. Neuroinflamm. 2020, 17, 334. [Google Scholar] [CrossRef]
- Iannaccone, A.; Cicchella, G. Histological Changes in the Anterior Pituitary Gland of Rats Intoxicated with Benzene. J. Endocrinol. 1958, 17, 444–448. [Google Scholar] [CrossRef]
- Fortunati, N.; Guaraldi, F.; Zunino, V.; Penner, F.; D’Angelo, V.; Zenga, F.; Pecori Giraldi, F.; Catalano, M.G.; Arvat, E. Effects of Environmental Pollutants on Signaling Pathways in Rat Pituitary GH3 Adenoma Cells. Environ. Res. 2017, 158, 660–668. [Google Scholar] [CrossRef]
- Pesatori, A.C.; Baccarelli, A.; Consonni, D.; Lania, A.; Beck-Peccoz, P.; Bertazzi, P.A.; Spada, A. Aryl Hydrocarbon Receptor-Interacting Protein and Pituitary Adenomas: A Population-Based Study on Subjects Exposed to Dioxin after the Seveso, Italy, Accident. Eur. J. Endocrinol. 2008, 159, 699–703. [Google Scholar] [CrossRef]
- Cannavò, S.; Ferraù, F.; Ragonese, M.; Curtò, L.; Torre, M.L.; Magistri, M.; Marchese, A.; Alibrandi, A.; Trimarchi, F. Increased Prevalence of Acromegaly in a Highly Polluted Area. Eur. J. Endocrinol. 2010, 163, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Tapella, L.; Sesta, A.; Cassarino, M.F.; Zunino, V.; Catalano, M.G.; Pecori Giraldi, F. Benzene and 2-Ethyl-Phthalate Induce Proliferation in Normal Rat Pituitary Cells. Pituitary 2017, 20, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Fujimoto, N.; Yin, H.; Ito, A. Growth Stimulation of a Rat Pituitary Cell Line MtT/E-2 by Environmental Estrogens In Vitro and In Vivo. Endocr. J. 1999, 46, 513–520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujimoto, N. Effects of Environmental Estrogenic Compounds on Growth of a Transplanted Estrogen Responsive Pituitary Tumor Cell Line in Rats. Food Chem. Toxicol. 2003, 41, 1711–1717. [Google Scholar] [CrossRef]
- Viñas, R.; Watson, C.S. Mixtures of Xenoestrogens Disrupt Estradiol-Induced Non-Genomic Signaling and Downstream Functions in Pituitary Cells. Environ. Health 2013, 12, 26. [Google Scholar] [CrossRef]
- Cossette, L.J.; Gaumond, I.; Martinoli, M.-G. Combined Effect of Xenoestrogens and Growth Factors in Two Estrogen-Responsive Cell Lines. Endocrine 2002, 18, 303–308. [Google Scholar] [CrossRef]
- Ronchetti, S.A.; Miler, E.A.; Duvilanski, B.H.; Cabilla, J.P. Cadmium Mimics Estrogen-Driven Cell Proliferation and Prolactin Secretion from Anterior Pituitary Cells. PLoS ONE 2013, 8, e81101. [Google Scholar] [CrossRef]
- Schraufnagel, D.E.; Balmes, J.R.; Cowl, C.T.; De Matteis, S.; Jung, S.H.; Mortimer, K.; Perez-Padilla, R.; Rice, M.B.; Riojas-Rodriguez, H.; Sood, A.; et al. Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The Damaging Effects of Air Pollution. Chest 2019, 155, 409–416. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Kodavanti, U.P.; Jackson, T.W.; Henriquez, A.R.; Snow, S.J.; Alewel, D.I.; Costa, D.L. Air Pollutant impacts on the brain and neuroendocrine system with implications for peripheral organs: A perspective. Inhal. Toxicol. 2023, 35, 109–126. [Google Scholar] [CrossRef]
- Chen, H.; Oliver, B.G.; Pant, A.; Olivera, A.; Poronnik, P.; Pollock, C.A.; Saad, S. Effects of air pollution on human health—Mechanistic evidence suggested by in vitro and in vivo modelling. Environ. Res. 2022, 212 Pt C, 113378. [Google Scholar] [CrossRef]
- Dominski, F.H.; Lorenzetti Branco, J.H.; Buonanno, G.; Stabile, L.; Gameiro da Silva, M.; Andrade, A. Effects of air pollution on health: A mapping review of systematic reviews and meta-analyses. Environ. Res. 2021, 201, 111487. [Google Scholar] [CrossRef] [PubMed]
- Shaddick, G.; Thomas, M.L.; Mudu, P.; Ruggeri, G.; Gumy, S. Half the World’s Population Are Exposed to Increasing Air Pollution. Npj Clim. Atmos. Sci. 2020, 3, 23. [Google Scholar] [CrossRef]
- Gomez, J.; Allen, R.J.; Turnock, S.T.; Horowitz, L.W.; Tsigaridis, K.; Bauer, S.E.; Olivié, D.; Thomson, E.S.; Ginoux, P. The Projected Future Degradation in Air Quality Is Caused by More Abundant Natural Aerosols in a Warmer World. Commun. Earth Environ. 2023, 4, 22. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, G.; Zhang, L.; Chen, K. Particulate matter pollution and hospital outpatient visits for endocrine, digestive, urological, and dermatological diseases in Nanjing, China. Environ. Pollut. 2020, 261, 114205. [Google Scholar] [CrossRef]
- Boogaard, H.; Crouse, D.L.; Tanner, E.; Mantus, E.; van Erp, A.M.; Vedal, S.; Samet, J. Assessing Adverse Health Effects of Long-Term Exposure to Low Levels of Ambient Air Pollution: The HEI Experience and What’s Next? Environ. Sci. Technol. 2024, 58, 12767–12783. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Benzene; U.S. Department of Health and Human Services: Washington, DC, USA, 2007.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Di(2-Ethylhexyl)Phthalate (DEHP); U.S. Department of Health and Human Services: Washington, DC, USA, 2002.
- Safe, S. Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol. 1994, 24, 87–149. [Google Scholar] [CrossRef]
- Smith, M.T. Advances in understanding benzene health effects and susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef]
- Health Effects Institute (HEI). State of Global Air 2020: Special Report on Global Exposure to Air Pollution and Its Health Impacts; Health Effects Institute: Boston, MA, USA, 2020. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyke, A.E.; Menaker, S.A.; Nunez, A.; Black, K.L.; Ljubimov, V.A. Air Pollution and Pituitary Adenoma Pathogenesis: Unraveling Environmental Impacts on Neuroendocrine Function and Tumorigenesis. J. Xenobiot. 2025, 15, 71. https://doi.org/10.3390/jox15030071
Boyke AE, Menaker SA, Nunez A, Black KL, Ljubimov VA. Air Pollution and Pituitary Adenoma Pathogenesis: Unraveling Environmental Impacts on Neuroendocrine Function and Tumorigenesis. Journal of Xenobiotics. 2025; 15(3):71. https://doi.org/10.3390/jox15030071
Chicago/Turabian StyleBoyke, Andre E., Simon A. Menaker, Alberto Nunez, Keith L. Black, and Vladimir A. Ljubimov. 2025. "Air Pollution and Pituitary Adenoma Pathogenesis: Unraveling Environmental Impacts on Neuroendocrine Function and Tumorigenesis" Journal of Xenobiotics 15, no. 3: 71. https://doi.org/10.3390/jox15030071
APA StyleBoyke, A. E., Menaker, S. A., Nunez, A., Black, K. L., & Ljubimov, V. A. (2025). Air Pollution and Pituitary Adenoma Pathogenesis: Unraveling Environmental Impacts on Neuroendocrine Function and Tumorigenesis. Journal of Xenobiotics, 15(3), 71. https://doi.org/10.3390/jox15030071






