Evaluation of the Effects of Monosodium Glutamate Overconsumption on the Functions of the Liver, Kidney, and Heart of Male Rats: The Involvement of Dyslipidemia, Oxidative Stress, and Inflammatory Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Design, Sampling and Tissues Preparation
2.3. Serum Biochemical Measurements
2.4. Assessment of Oxidative Stress and Antioxidant Markers in the Liver, Kidney and Heart Tissues
2.5. Assessment of Inflammatory Markers in the Liver, Kidney and Heart Tissues
2.6. Histopathological Investigations of Liver and Kidney
2.7. Statistical Analysis
3. Results
3.1. Monosodium Glutamate Induces Changes in the Biochemical Markers of Liver, Kidney, and Heart Functions
3.2. Monosodium Glutamate Induces a State of Dyslipidemia
3.3. Monosodium Glutamate Induces Hyperglycemia and Electrolytes Imbalance
3.4. Monosodium Glutamate Induces Changes in the Activities of iNOS, CPK, and α-KGD
3.5. Monosodium Glutamate Induces Hepatic, Renal, and Cardiac Oxidative Stress
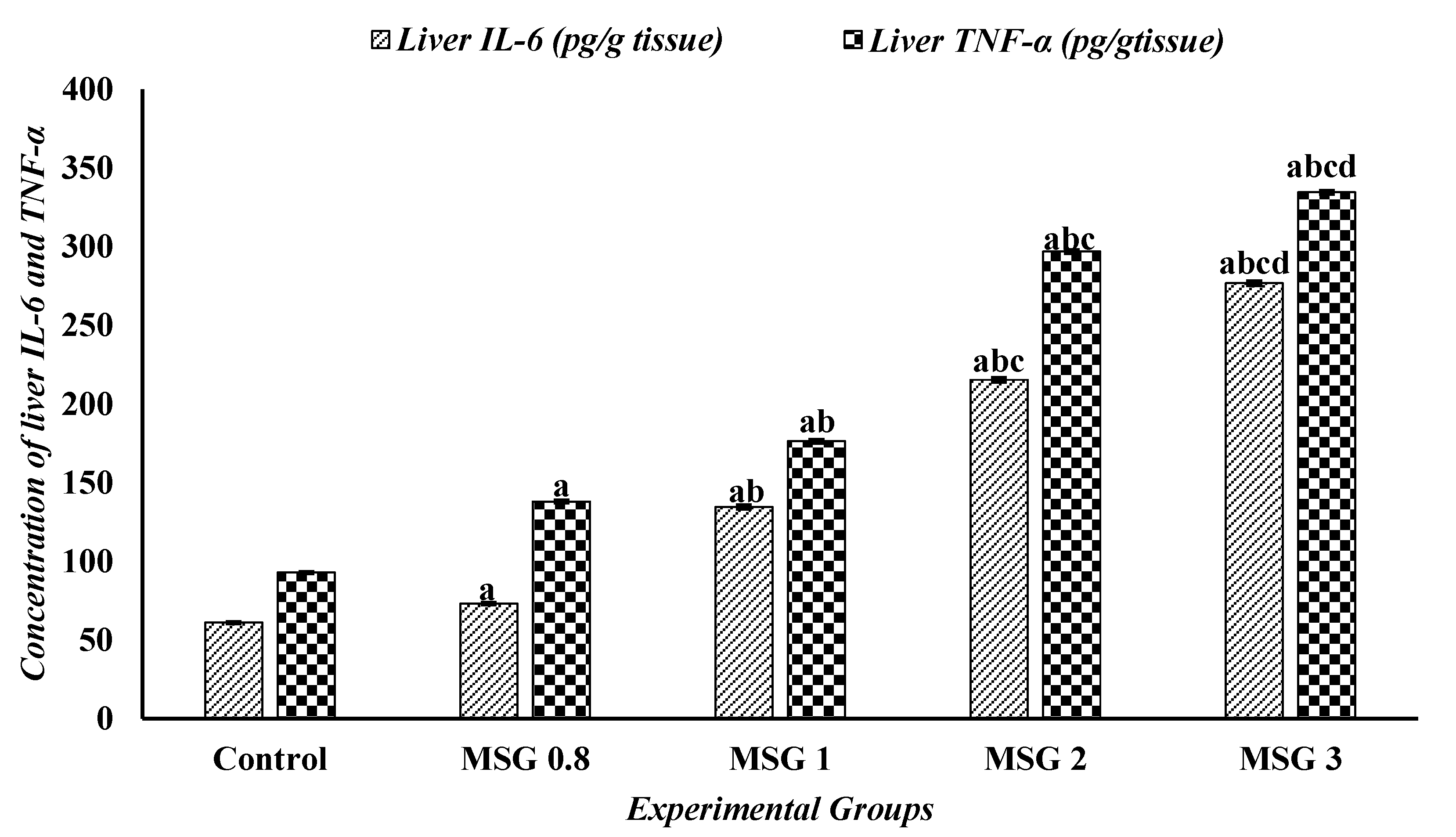
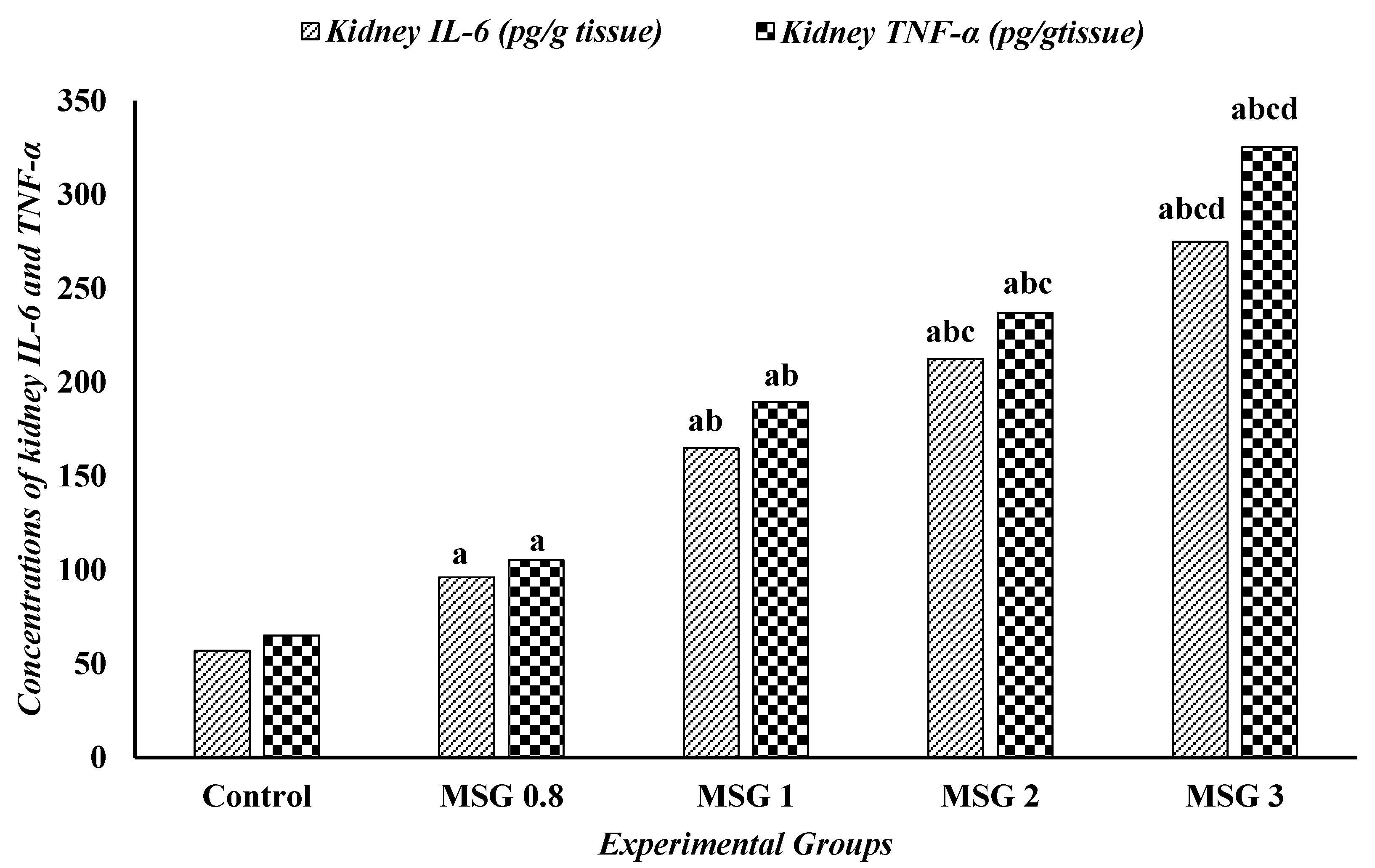
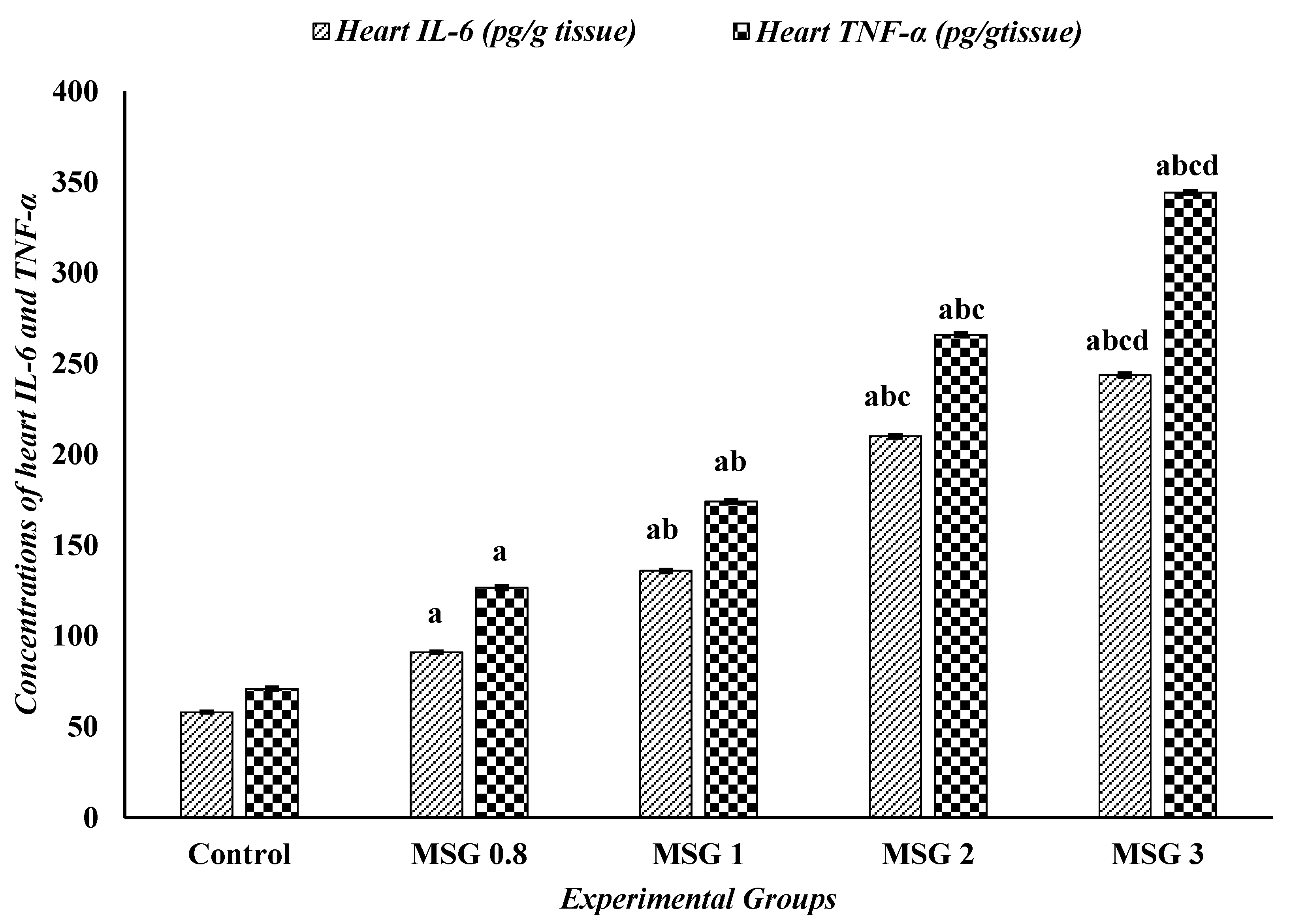
3.6. Effect of Monosodium Glutamate on Hepatic, Renal, and Cardiac Inflammatory Response
3.7. Monosodium Glutamate Elicited Histopathological Alterations in Hepatic and Renal Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Onyesife, C.O.; Chukwuma, I.F.; Okagu, I.U.; Ndefo, J.C.; Amujiri, N.A.; Ogugua, V.N. Nephroprotective effects of Piper nigrum extracts against monosodium glutamate-induced renal toxicity in rats. Sci. Afr. 2023, 19, e01453. [Google Scholar] [CrossRef]
- Tarda, A.A.K. Determining Monosodium Glutamate in Cafeteria Foods at the Eastern Mediterranean University. Master’s Thesis, Eastern Mediterranean University EMU-Doğu Akdeniz Üniversitesi (DAÜ), Famagusta, Cyprus, 2017. [Google Scholar]
- Nguyen Thuy, L.; Salanță, L.C.; Tofanǎ, M.; Socaci, S.A.; Fărcaş, A.C.; Pop, C.R. A mini review about monosodium glutamate. Bull. UASVM Food Sci. Technol. 2020, 77, 1–12. [Google Scholar]
- Yoshida, R.; Ninomiya, Y. Umami and MSG. In Umami: Taste for Health; Springer International Publishing: Cham, Switzerland, 2023; pp. 7–42. [Google Scholar]
- Abd-Elkareem, M.; Soliman, M.; Abd El-Rahman, M.A.; Abou Khalil, N.S. The protective effect of Nigella sativa seeds against monosodium glutamate-induced hepatic dysfunction in rats. Toxicol. Rep. 2022, 9, 147–153. [Google Scholar] [CrossRef]
- Islam, M.S.; Al Ferdous, K.; Amin, M.N.; Kamruzzaman, M.; Islam, M.A. Impact of Monosodium Glutamate on the Liver of Chick Embryo: Histology, Biochemical Properties, and AVBD9 Gene Expression. Int. J. Agric. Biosci. 2024, 13, 76–84. [Google Scholar]
- Bera, T.K.; Kar, S.K.; Yadav, P.K.; Mukherjee, P.; Yadav, S.; Joshi, B. Effects of monosodium glutamate on human health: A systematic review. World J. Pharm. Sci. 2017, 5, 139–144. [Google Scholar]
- Mondal, M.; Sarkar, K.; Paul, G. Fenugreek Seed Extract Mitigates MSG-Induced Uterine Dysfunction in Rats through Enhanced Antioxidative Defense Mechanisms. Int. J. Exp. Res. Rev 2024, 37, 139–148. [Google Scholar] [CrossRef]
- Geha, R.S.; Ren, C.; Beiser, A.; Patterson, R.; Greenberger, P.A.; Grammer, L.C.; Ditto, A.M.; Harris, K.E.; Shaughnessy, M.A.; Yarnold, P.R. Review of alleged reaction to monosodium glutamate and outcome of a multicenter double-blind placebo-controlled study. J. Nutr. 2000, 130, 1058S–1062S. [Google Scholar] [CrossRef]
- Igwebuike, U.M.; Ochiogu, I.S.; Ihedinihu, B.C.; Ikokide, J.E.; Idika, I.K. The effects of oral administration of monosodium glutamate (msg) on the testicular morphology and cauda epididymal sperm reserves of young and adult male rats. Vet. Arh. 2011, 81, 525–534. [Google Scholar]
- Abdulghani, M.A.; Alshehade, S.A.; Kamran, S.; Alshawsh, M.A. Effect of monosodium glutamate on serum sex hormones and uterine histology in female rats along with its molecular docking and in-silico toxicity. Heliyon 2022, 8, e10967. [Google Scholar] [CrossRef]
- Insawang, T.; Selmi, C.; Cha’on, U.; Pethlert, S.; Yongvanit, P.; Areejitranusorn, P.; Boonsiri, P.; Khampitak, T.; Tangrassameeprasert, R.; Pinitsoontorn, C. Monosodium glutamate (MSG) intake is associated with the prevalence of metabolic syndrome in a rural Thai population. Nutr. Metab. 2012, 9, 50. [Google Scholar] [CrossRef]
- Adeleke, D.A.; Olajide, P.A.; Omowumi, O.S.; Okunlola, D.D.; Taiwo, A.M.; Adetuyi, B.O. Effect of monosodium glutamate on the body system. World News Nat. Sci. 2022, 44, 1–23. [Google Scholar]
- Dief, A.E.; Kamha, E.S.; Baraka, A.M.; Elshorbagy, A.K. Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: A potential role for cyclic AMP protein kinase. Neurotoxicology 2014, 42, 76–82. [Google Scholar] [CrossRef]
- Lopes-Virella, M.; Stone, P.; Colwell, J. Serum high density lipoprotein in diabetic patients. Diabetologia 1977, 13, 285–291. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Bergmeyer, H.-U. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Fridovich, I. Superoxide dismutases: An adaptation to a paramagnetic gas. J. Biol. Chem. 1989, 264, 7761–7764. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Ferguson-Smith, A.C.; Chen, Y.-F.; Newman, M.S.; May, L.T.; Sehgal, P.B.; Ruddle, F.H. Regional localization of the interferon-β2B-cell stimulatory factor 2/hepatocyte stimulating factor gene to human chromosome 7p15-p21. Genomics 1988, 2, 203–208. [Google Scholar] [CrossRef]
- Hedayati, M.; Yazdanparast, R.; Azizi, F. Determination of human tumor necrosis factor α by a highly sensitive enzyme immunoassay. Biochem. Biophys. Res. Commun. 2001, 289, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Drury, R.A.B.; Wallington, E.A.; Cameron, S.R. Carleton’s Histological Technique; Oxford University Press: Oxford, UK, 1967. [Google Scholar]
- Altwaiq, A.M.; Ali, L.N.; Aljalab, T.Y.; Alhaj, O.A.K.A.; Aledwan, G.K.; Khouri, S.i.; Al-Sayyed, H. Identification of Monosodium Glutamate Contents as a Flavor Enhancer in Different Food Samples by HPLC Technique. Methods 2024, 19, 25–30. [Google Scholar] [CrossRef]
- Hajihasani, M.M.; Soheili, V.; Zirak, M.R.; Sahebkar, A.; Shakeri, A. Natural products as safeguards against monosodium glutamate-induced toxicity. Iran. J. Basic Med. Sci. 2020, 23, 416. [Google Scholar]
- Eid, R.A.; Al-Shraim, M.; Zaki, M.S.; Kamar, S.S.; Abdel Latif, N.S.; Negm, S.; Al-Ani, B.; Haidara, M.A. Vitamin E protects against monosodium glutamate-induced acute liver injury and hepatocyte ultrastructural alterations in rats. Ultrastruct. Pathol. 2019, 43, 199–208. [Google Scholar] [CrossRef]
- Tolulope, O.D.; Ebiwonjumi, O.; Oluwatoyin, A.L.; Dupe, A.O.; Victor, A.; Grace, A.; Johnson, A.W.; Folorunsho, A.A. Disruptive consequences of monosodium glutamate on male reproductive function: A Review. Curr. Res. Toxicol. 2024, 6, 100148. [Google Scholar]
- Ge, P.-L.; Du, S.-D.; Mao, Y.-L. Advances in preoperative assessment of liver function. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 361–370. [Google Scholar] [CrossRef]
- Salazar, J.H. Overview of urea and creatinine. Lab. Med. 2014, 45, e19–e20. [Google Scholar] [CrossRef]
- Zündorf, G.; Kahlert, S.; Bunik, V.; Reiser, G. α-Ketoglutarate dehydrogenase contributes to production of reactive oxygen species in glutamate-stimulated hippocampal neurons in situ. Neuroscience 2009, 158, 610–616. [Google Scholar] [CrossRef]
- Sharma, A. Monosodium glutamate-induced oxidative kidney damage and possible mechanisms: A mini-review. J. Biomed. Sci. 2015, 22, 93. [Google Scholar] [CrossRef]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Monosodium glutamate causes hepato-cardiac derangement in male rats. Hum. Exp. toxicology 2021, 40, S359–S369. [Google Scholar] [CrossRef]
- Yan, L.; Lan, Z.; Xu, H.-F.; Li, Y.; Fan, D.; Wei, H.; Cao, J.-M.; Xue, G. A preliminary experimental study on the cardiac toxicity of glutamate and the role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor in rats. Chin. Med. J. 2013, 126, 1323–1332. [Google Scholar]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: An overview. Toxicol. Rep. 2021, 8, 938–961. [Google Scholar] [CrossRef]
- Kayode, O.T.; Bello, J.A.; Oguntola, J.A.; Kayode, A.A.; Olukoya, D.K. The interplay between monosodium glutamate (MSG) consumption and metabolic disorders. Heliyon 2023, 9, e19675. [Google Scholar] [CrossRef]
- Harnischfeger, F.; Dando, R. Obesity-induced taste dysfunction, and its implications for dietary intake. Int. J. Obes. 2021, 45, 1644–1655. [Google Scholar] [CrossRef]
- Ibegbulem, C.O.; Chikezie, P.C.; Ukoha, A.I.; Opara, C.N. Effects of diet containing monosodium glutamate on organ weights, acute blood steroidal sex hormone levels, lipid profile and erythrocyte antioxidant enzymes activities of rats. J. Acute Dis. 2016, 5, 402–407. [Google Scholar] [CrossRef]
- Prentki, M.; Nolan, C.J. Islet β cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef]
- Macho, L.; Fickova, M.; Zorad, S. Late effects of postnatal administration of monosodium glutamate on insulin action in adult rats. Physiol. Res. 2000, 49, S79–S85. [Google Scholar]
- Ajani, E.O.; Ogunlabi, O.O.; Adegbesan, B.O.; Adeosun, O.E.; Akinwande, O. Nigerian mistletoe (Loranthus micranthusLinn) aqueous leaves extract modulates some cardiovascular disease risk factors in monosodium glutamate induced metabolic dysfunction. Afr. J. Biotechnol. 2014, 13, 3989–3998. [Google Scholar]
- Banerjee, A.; Das, D.; Paul, R.; Roy, S.; Das, U.; Saha, S.; Dey, S.; Adhikary, A.; Mukherjee, S.; Maji, B.K. Mechanistic study of attenuation of monosodium glutamate mixed high lipid diet induced systemic damage in rats by Coccinia grandis. Sci. Rep. 2020, 10, 15443. [Google Scholar] [CrossRef]
- Asejeje, F.O.; Gabriel, G.O.; Abiola, M.A. Monosodium glutamate aggravates lipopolysaccharide-induced liver injury via inflammation and oxidative stress in rats. Nutrire 2023, 48, 5. [Google Scholar] [CrossRef]
- Farombi, E.; Onyema, O. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: Modulatory role of vitamin C, vitamin E and quercetin. Hum. Exp. Toxicol. 2006, 25, 251–259. [Google Scholar] [CrossRef]
- Paul, M.S.; Abhilash, M.; Varghese, M.V.; Alex, M.; Harikumaran Nair, R. Protective effects of α-tocopherol against oxidative stress related to nephrotoxicity by monosodium glutamate in rats. Toxicol. Mech. Methods 2012, 22, 625–630. [Google Scholar] [CrossRef]
- Kassab, R.B.; Theyab, A.; Al-Ghamdy, A.O.; Algahtani, M.; Mufti, A.H.; Alsharif, K.F.; Abdella, E.M.; Habotta, O.A.; Omran, M.M.; Lokman, M.S. Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ. Sci. Pollut. Res. 2022, 29, 12208–12221. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The role of oxidative stress in cardiovascular aging and cardiovascular diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Fayed, M.R.; El-Naga, R.N.; Akool, E.-S.; El-Demerdash, E. The potential antifibrotic impact of apocynin and alpha-lipoic acid in concanavalin A-induced liver fibrosis in rats: Role of NADPH oxidases 1 and 4. Drug Discov. Ther. 2018, 12, 58–67. [Google Scholar] [CrossRef]
- Noeman, S.A.; Hamooda, H.E.; Baalash, A.A. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol. Metab. Syndr. 2011, 3, 17. [Google Scholar] [CrossRef]
- Thongsepee, N.; Martviset, P.; Chantree, P.; Sornchuer, P.; Sangpairoj, K.; Prathaphan, P.; Ruangtong, J.; Hiranyachattada, S. Daily consumption of monosodium glutamate pronounced hypertension and altered renal excretory function in normotensive and hypertensive rats. Heliyon 2022, 8, e10972. [Google Scholar] [CrossRef]
- Ahmed, R.R.; Abdul-Hamid, M.; Galaly, S.R.; Hamdalla, H.M. Monosodium glutamate-induced liver microscopic and biochemical changes in male rats, and the possible amendment of quercetin. Egypt. J. Zool. 2019, 71, 44–55. [Google Scholar] [CrossRef]
- Triantafyllou, E.; Woollard, K.J.; McPhail, M.J.; Antoniades, C.G.; Possamai, L.A. The role of monocytes and macrophages in acute and acute-on-chronic liver failure. Front. Immunol. 2018, 9, 2948. [Google Scholar] [CrossRef]
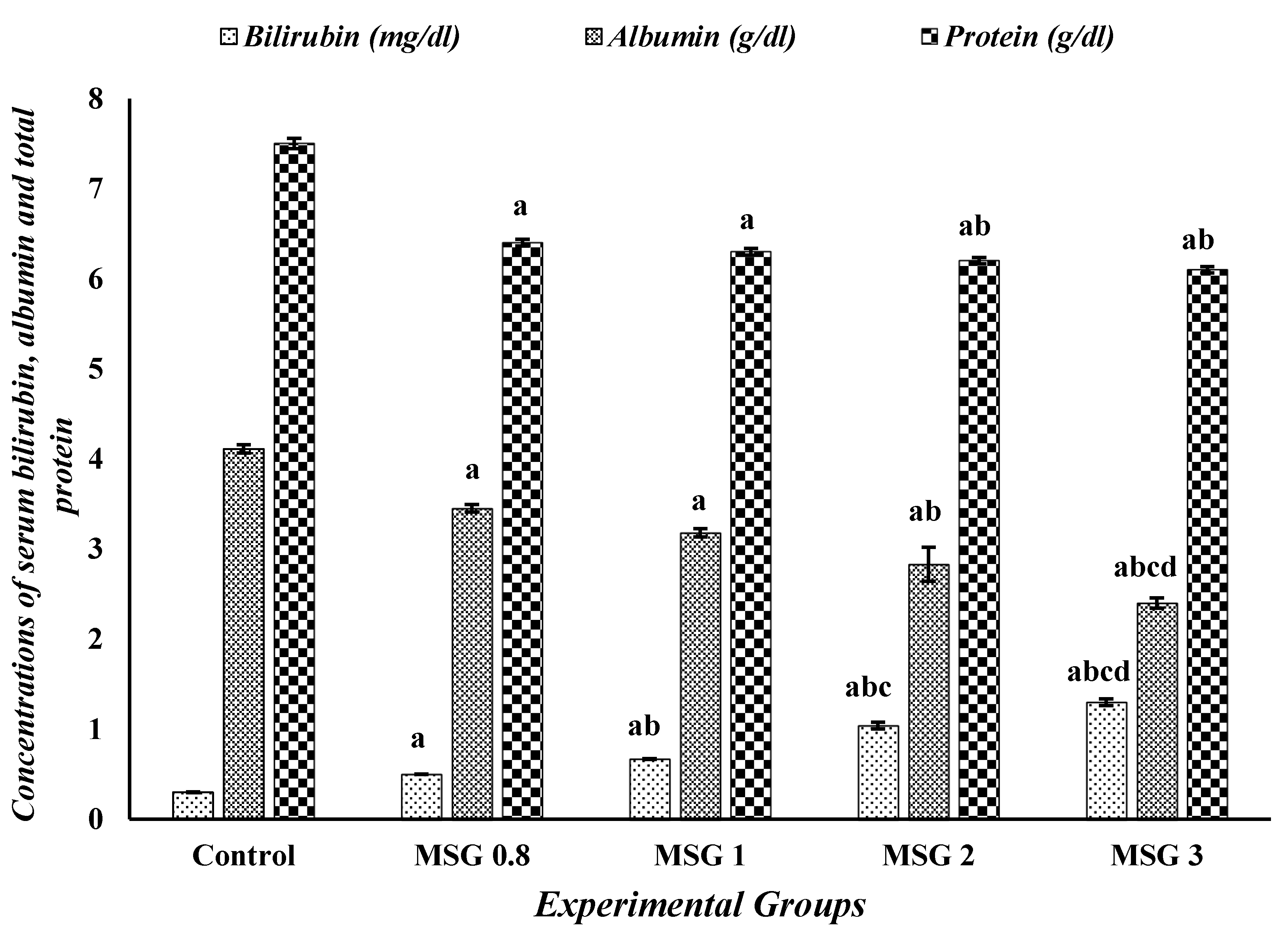
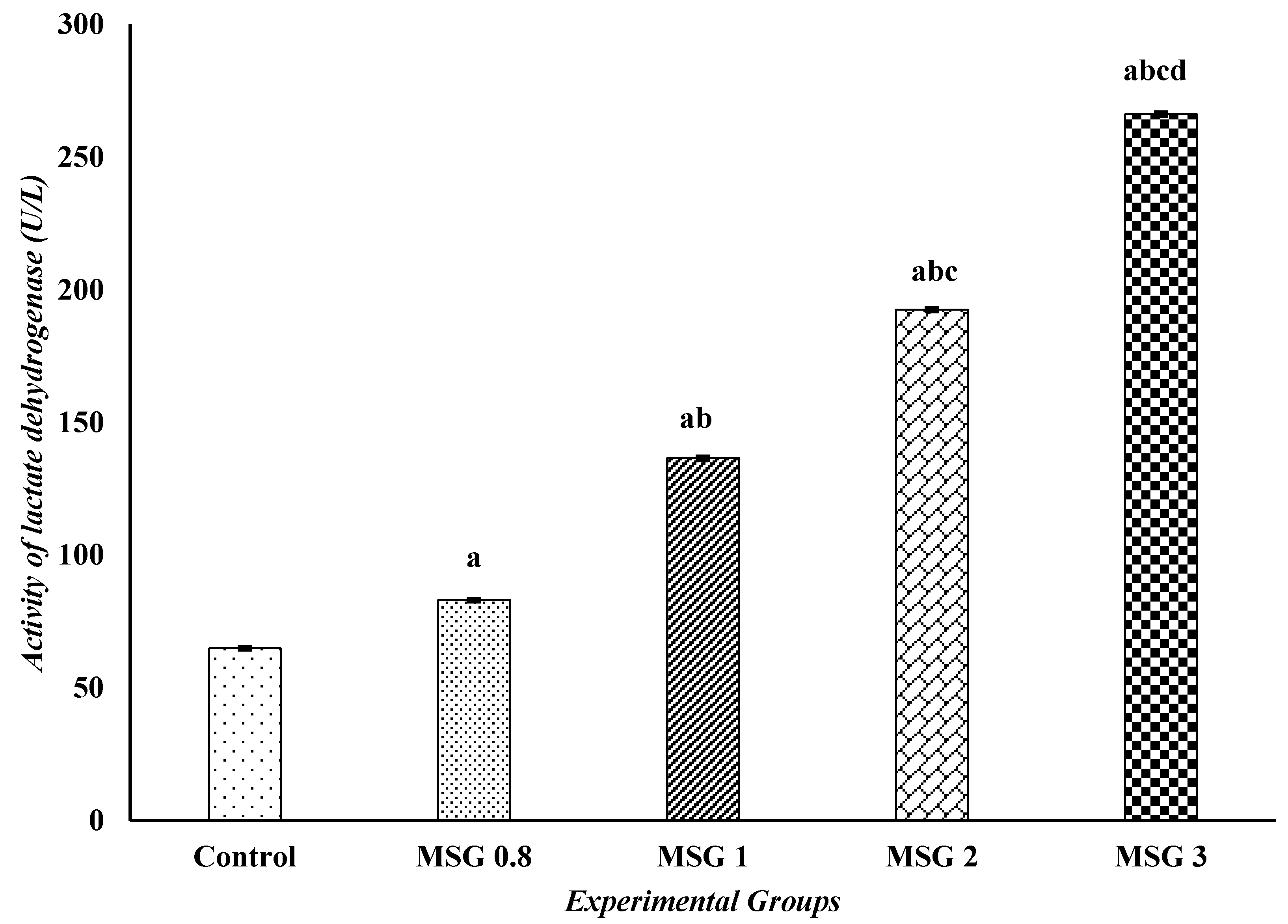
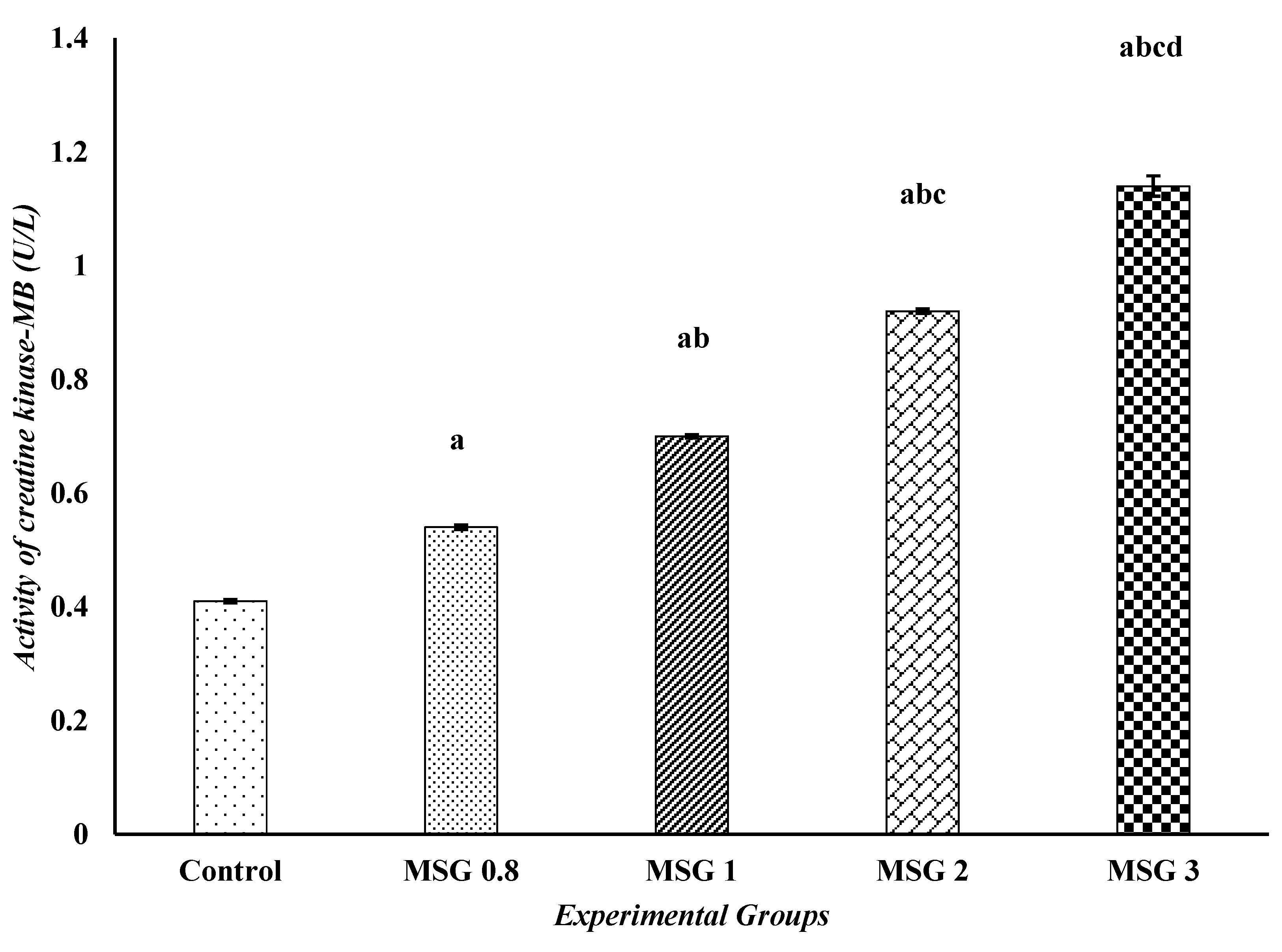

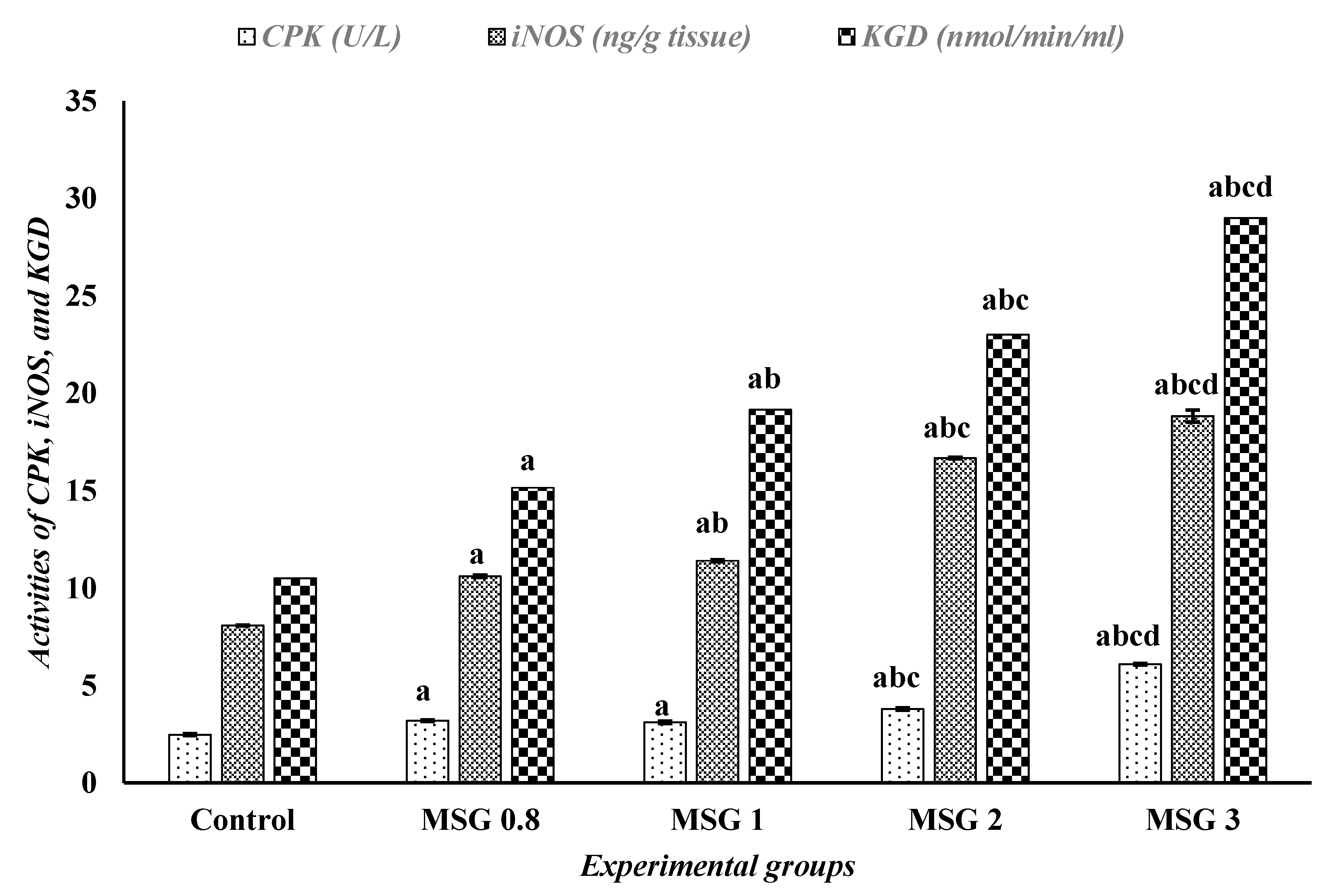


| Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | MSG 0.8 | MSG 1 | MSG 2 | MSG 3 | |
| Aspartate aminotransferase (AST) (U/L) | 64.33 ± 0.881 | 98.50 ± 0.763 a | 118.66 ± 0.494 ab | 182.50 ± 0.763 abc | 241.50 ± 0.763 abcd |
| Alanine aminotransferase (ALT) (U/L) | 36.50 ± 0.763 | 86.50 ± 0.428 a | 96.00 ± 0.577 ab | 136.50 ± 0.763 abc | 204.16 ± 0.600 abcd |
| γ-Glutamyl transferase (GGT) (U/L) | 3.65 ± 0.042 | 6.00 ± 0.036 a | 12.46 ± 0.049 ab | 18.08 ± 0.047 abc | 20.20 ± 0.073 abcd |
| Alkaline phosphatase (ALP) (U/L) | 2.80 ± 0.057 | 3.80 ± 0.036 a | 3.96 ± 0.033 a | 4.38 ± 0.047 abc | 4.58 ± 0.047 abcd |
| Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | MSG 0.8 | MSG 1 | MSG 2 | MSG 3 | |
| Urea (mg/dL) | 16.66± 0.666 | 27.50 ± 0.763 a | 32.16 ± 0.654 ab | 46.16 ± 0.477 abc | 54.66 ± 0.666 abcd |
| Creatinine (mg/dL) | 0.28 ± 0.016 | 0.46 ± 0.004 a | 0.67 ± 0.004 ab | 1.20 ± 0.036 abc | 1.78 ± 0.047 abcd |
| Uric acid (mg/dL) | 2.00 ± 0.036 | 2.51 ± 0.047 a | 3.31 ± 0.060 ab | 3.95 ± 0.042 abc | 4.65 ± 0.076 abcd |
| Calcium (mg/dL) | 10.31 ± 0.047 | 8.28 ± 0.060 a | 7.78 ± 0.047 ab | 7.38 ± 0.047 abc | 6.75 ± 0.042 abcd |
| Sodium (mmol/L) | 149.66 ± 0.557 | 136.83 ± 0.477 a | 132.66 ± 0.666 ab | 125.83 ± 0.600 abc | 118.00 ± 0.577 abcd |
| Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | MSG 0.8 | MSG 1 | MSG 2 | MSG 3 | |
| Total cholesterol (TC) (mg/dL) | 88.16± 0.600 | 128.50 ± 0.428 a | 136.00 ± 0.365 ab | 169.00 ± 0.577 abc | 204.33 ± 0.954 abcd |
| Triglycerides (TG) (mg/dL) | 58.50 ± 0.428 | 94.66 ± 0.494 a | 105.33 ± 0.557 ab | 117.00 ± 0.577 abc | 140.16 ± 0.600 abcd |
| Low density lipoprotein (LDL) (mg/dL) | 40.00 ± 0.577 | 83.16 ± 0.600 a | 85.00 ± 0.365 a | 117.33 ± 0.760 abc | 143.66 ± 1.021 abcd |
| High density lipoprotein (HDL) (mg/dL) | 40.00 ± 0.577 | 30.66 ± 0.666 a | 26.33 ± 0.494 ab | 21.00 ± 0.365 abc | 18.00 ± 0.365 abcd |
| Liver Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | MSG 0.8 | MSG 1 | MSG 2 | MSG 3 | |
| Malondialdehyde (MDA) (nmole/g. tissue) | 6.38± 0.047 | 7.55 ± 0.076 a | 18.63 ± 0.417 ab | 31.16 ± 0.477 abc | 38.83 ± 0.477 abcd |
| Nitric oxide (NO) (µmole/g. tissue) | 9.00 ± 0.365 | 22.00 ± 0.577 a | 28.50 ± 0.428 ab | 38.66 ± 1.782 abc | 42.50 ± 0.763 abc |
| Glutathione (GSH) (mmole/g. tissue) | 48.50 ± 0.428 | 39.50 ± 0.428 a | 33.00 ± 0.577 ab | 20.50 ± 0.428 abc | 17.00 ± 0.365 abcd |
| Super oxide dismutase (SOD) (U/g. tissue) | 76.50 ± 0.428 | 63.50 ± 0.763 a | 48.50 ± 0.763 ab | 43.83 ± 0.600 abc | 34.66 ± 0.666 abcd |
| Catalase (CAT) (U/g. tissue) | 54.50 ± 0.428 | 46.50 ± 0.763 a | 42.00 ± 0.577 ab | 31.00 ± 0.365 abc | 20.33 ± 0.760 abcd |
| Glutathione-S-transferase (GST) (U/g. protein) | 44.16 ± 0.600 | 32.33 ± 0.760 a | 27.50 ± 0.428 ab | 18.50 ± 0.428 abc | 11.16 ± 0.477 abcd |
| Kidney Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | MSG 0.8 | MSG 1 | MSG 2 | MSG 3 | |
| Malondialdehyde (MDA) (nmole/g. tissue) | 4.80± 0.036 | 8.01 ± 0.365 a | 19.16 ± 0.477 ab | 31.33 ± 0.557 abc | 42.83 ± 0.945 abcd |
| Nitric oxide (NO) (µmole/g. tissue) | 2.98 ± 0.087 | 15.16 ± 0.477 a | 22.33 ± 0.760 ab | 29.66 ± 0.557 abc | 35.33 ± 0.666 abcd |
| Glutathione (GSH) (mmole/g. tissue) | 48.50 ± 0.428 | 42.50 ± 0.619 a | 30.00 ± 0.365 ab | 24.33 ± 0.714 abc | 23.33 ± 0.714 abc |
| Super oxide dismutase (SOD) (U/g. tissue) | 67.33 ± 0.494 | 52.00 ± 0.577 a | 35.33 ± 0.494 ab | 25.83 ± 0.703 abc | 20.33 ± 0.494 abcd |
| Catalase (CAT) (U/g. tissue) | 37.00 ± 0.577 | 31.16 ± 0.477 a | 24.00 ± 0.856 ab | 18.00 ± 0.577 abc | 11.83 ± 0.600 abcd |
| Glutathione-S-transferase (GST) (U/g. protein) | 43.33 ± 0.494 | 30.66 ± 0.557 a | 27.33 ± 0.494 ab | 18.00 ± 0.365 abc | 12.83 ± 0.600 abcd |
| Heart Parameters | Experimental Groups | ||||
|---|---|---|---|---|---|
| Control | MSG 0.8 | MSG 1 | MSG 2 | MSG 3 | |
| Malondialdehyde (MDA) (nmole/g. tissue) | 9.00 ± 0.365 | 22.33 ± 0.802 a | 28.33 ± 0.666 ab | 36.50 ± 0.763 abc | 52.50 ± 0.763 abcd |
| Nitric oxide (NO) (µmole/g. tissue) | 8.31 ± 0.300 | 17.50 ± 0.428 a | 24.66 ± 0.421 ab | 33.50 ± 0.921 abc | 40.16 ± 0.477 abcd |
| Glutathione (GSH) (mmole/g. tissue) | 49.00 ± 0.365 | 43.50 ± 0.763 a | 28.50 ± 0.428 ab | 23.16 ± 0.600 abc | 17.16 ± 0.477 abcd |
| Super oxide dismutase (SOD) (U/g. tissue) | 75.00 ± 0.577 | 50.50 ± 0.428 a | 39.83 ± 0.477 ab | 34.00 ± 0.577 abc | 18.33 ± 0.494 abcd |
| Catalase (CAT) (U/g. tissue) | 43.50 ± 0.763 | 35.33 ± 0.666 a | 22.33 ± 0.494 ab | 19.16 ± 0.477 abc | 14.00 ± 0.577 abcd |
| Glutathione-S-transferase (GST) (U/g. protein) | 48.00 ± 0.428 | 37.16 ± 0.477 a | 22.33 ± 0.666 ab | 19.00 ± 0.577 abc | 10.00 ± 0.365 abcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdou, H.M.; El-Gendy, A.H.; Aly, R.G.; Abouzied, M.M.; Eltahir, H.M.; Al thagfan, S.S.; Eweda, S.M. Evaluation of the Effects of Monosodium Glutamate Overconsumption on the Functions of the Liver, Kidney, and Heart of Male Rats: The Involvement of Dyslipidemia, Oxidative Stress, and Inflammatory Responses. J. Xenobiot. 2025, 15, 64. https://doi.org/10.3390/jox15030064
Abdou HM, El-Gendy AH, Aly RG, Abouzied MM, Eltahir HM, Al thagfan SS, Eweda SM. Evaluation of the Effects of Monosodium Glutamate Overconsumption on the Functions of the Liver, Kidney, and Heart of Male Rats: The Involvement of Dyslipidemia, Oxidative Stress, and Inflammatory Responses. Journal of Xenobiotics. 2025; 15(3):64. https://doi.org/10.3390/jox15030064
Chicago/Turabian StyleAbdou, Heba M., Amel H. El-Gendy, Rania Gaber Aly, Mekky M. Abouzied, Heba M. Eltahir, Sultan S. Al thagfan, and Saber M. Eweda. 2025. "Evaluation of the Effects of Monosodium Glutamate Overconsumption on the Functions of the Liver, Kidney, and Heart of Male Rats: The Involvement of Dyslipidemia, Oxidative Stress, and Inflammatory Responses" Journal of Xenobiotics 15, no. 3: 64. https://doi.org/10.3390/jox15030064
APA StyleAbdou, H. M., El-Gendy, A. H., Aly, R. G., Abouzied, M. M., Eltahir, H. M., Al thagfan, S. S., & Eweda, S. M. (2025). Evaluation of the Effects of Monosodium Glutamate Overconsumption on the Functions of the Liver, Kidney, and Heart of Male Rats: The Involvement of Dyslipidemia, Oxidative Stress, and Inflammatory Responses. Journal of Xenobiotics, 15(3), 64. https://doi.org/10.3390/jox15030064









