Contamination Profiles of Selected Pollutants in Procambarus clarkii Non-Edible Portions Highlight Their Potential Exploitation Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Campaign and Sample Preparation
2.2. Analysis of Exoskeleton Samples
2.3. Statistical Analysis
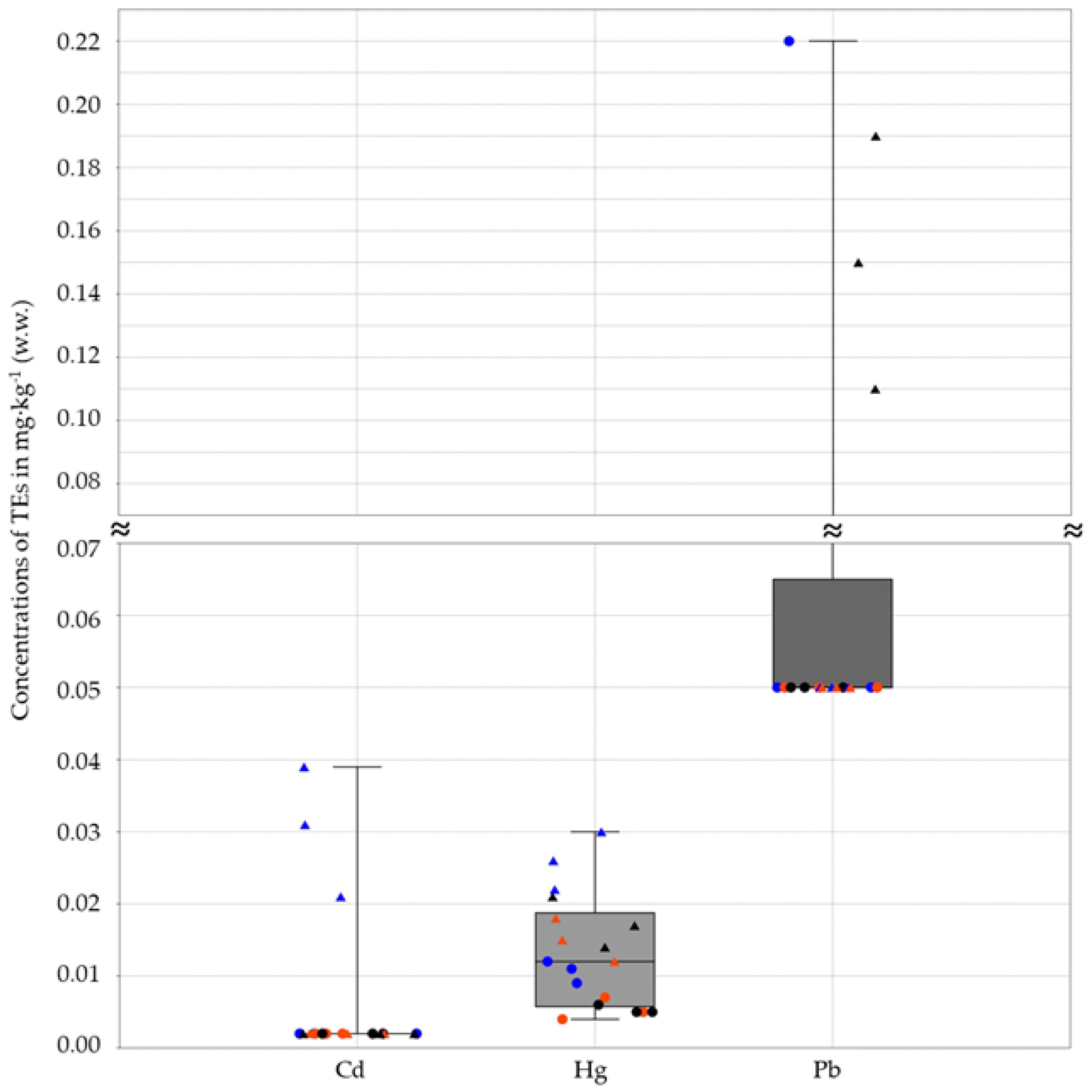
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vazzana, M.; Mauro, M.; Ceraulo, M.; Dioguardi, M.; Papale, E.; Mazzola, S.; Arizza, V.; Beltrame, F.; Inguglia, L.; Buscaino, G. Underwater high frequency noise: Biological responses in sea urchin Arbacia lixula (Linnaeus, 1758). Comp. Biochem. Physiol. Part A 2020, 242, 110650. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Lazzara, V.; Arizza, V.; Luparello, C.; Ferrantelli, V.; Cammilleri, G.; Inguglia, L.; Vazzana, M. Human drug pollution in the aquatic system: The biochemical responses of Danio rerio adults. Biology 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Okorondu, J.; Umar, N.A.; Ulor, C.O.; Onwuagba, C.G.; Diagi, B.E.; Ajiere, S.I.; Nwaogu, C. Anthropogenic Activities as Primary Drivers of Environmental Pollution and Loss of Biodiversity A Review. Int. J. Trend Sci. Res. Dev. 2022, 6, 621–643. [Google Scholar]
- Ruan, T.; Li, P.; Wang, H.; Li, T.; Jiang, G. Identification and prioritization of environmental organic pollutants: From an analytical and toxicological perspective. Chem. Rev. 2023, 123, 10584–10640. [Google Scholar] [CrossRef] [PubMed]
- Essumang, D.K.; Togoh, G.K.; Chokky, L. Pesticide residues in the water and fish (lagoon tilapia) samples from lagoons in Ghana. Bull. Chem. Soc. Ethiop. 2009, 23, 19–27. [Google Scholar] [CrossRef]
- Savoca, D.; Arculeo, M.; Arizza, V.; Pace, A.; Melfi, R.; Caracappa, S.; Caracappa, G.; Vullo, C.; Cambera, I.; Visconti, G.; et al. Impact of heavy metals in eggs and tissues of C. caretta along the Sicilian coast (Mediterranean Sea). Environments 2022, 9, 88. [Google Scholar] [CrossRef]
- Savoca, D.; Barreca, S.; Lo Coco, R.; Punginelli, D.; Orecchio, S.; Maccotta, A. Environmental aspect concerning phthalates contamination: Analytical approaches and assessment of biomonitoring in the aquatic environment. Environments 2023, 10, 99. [Google Scholar] [CrossRef]
- Savoca, D.; Pace, A.; Arizza, V.; Arculeo, M.; Melfi, R. Controlled uptake of pfoa in adult specimens of Paracentrotus lividus and evaluation of gene expression in their gonads and embryos. Environ. Sci. Pollut. Res. 2022, 30, 26094–26106. [Google Scholar] [CrossRef]
- Concha-Graña, E.; Moscoso-Pérez, C.; Fernández-González, V.; López-Mahía, P.; Gago, J.; León, V.M.; Muniategui-Lorenzo, S. Phthalates, organotin compounds and per-polyfluoroalkyl substances in semiconfined areas of the spanish coast: Occurrence, sources and risk assessment. Sci. Total Environ. 2021, 780, 146450. [Google Scholar] [CrossRef]
- Damalas, C.; Koutroubas, S. Farmers’ exposure to pesticides: Toxicity types and ways of prevention. Toxics 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Savoca, D.; Pace, A. Bioaccumulation, biodistribution, toxicology and biomonitoring of organofluorine compounds in aquatic organisms. Int. J. Mol. Sci. 2021, 22, 6276. [Google Scholar] [CrossRef]
- Pace, A.; Vaglica, A.; Maccotta, A.; Savoca, D. The origin of phthalates in algae: Biosynthesis and environmental bioaccumulation. Environments 2024, 11, 78. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Gaskins, A.J.; Meeker, J.D.; Braun, J.M.; Chavarro, J.E. Endocrine-disrupting chemicals and male reproductive health. Fertil. Steril. 2023, 120, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yao, C.; Zhou, J.; Ma, H.; Jin, J.; Song, W.; Kai, Z. Occurrence and risk assessment of pesticides, phthalates, and heavy metal residues in vegetables from hydroponic and conventional cultivation. Foods 2024, 13, 1151. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; Cardon, M.; Medlock-Kakaley, E.; Wilson, V.S.; Gray, L.E. A mixture of 15 phthalates and pesticides below individual chemical no observed adverse effect levels (NOAELs) produces reproductive tract malformations in the male rat. Environ. Int. 2021, 156, 106615. [Google Scholar] [CrossRef] [PubMed]
- Ariano, A.; Scivicco, M.; D’Ambola, M.; Velotto, S.; Andreini, R.; Bertini, S.; Zaccaroni, A.; Severino, L. Heavy metals in the muscle and hepatopancreas of red swamp crayfish (Procambarus clarkii) in Campania (Italy). Animals 2021, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Bellante, A.; Maccarone, V.; Buscaino, G.; Buffa, G.; Filiciotto, F.; Traina, A.; Del Core, M.; Mazzola, S.; Sprovieri, M. Trace element concentrations in red swamp crayfish (Procambarus clarkii) and surface sediments in Lake Preola and Gorghi Tondi natural reserve, SW Sicily. Environ. Monit. Assess. 2015, 187, 404. [Google Scholar] [CrossRef] [PubMed]
- Goretti, E.; Pallottini, M.; Ricciarini, M.I.; Selvaggi, R.; Cappelletti, D. Heavy metals bioaccumulation in selected tissues of red swamp crayfish: An easy tool for monitoring environmental contamination levels. Sci. Total Environ. 2016, 559, 339–346. [Google Scholar] [CrossRef]
- Selvaggi, R.; Pallottini, M.; Caldaroni, B.; Dörr, A.J.M.; Magara, G.; Gravina, P.; Grispoldi, L.; Cenci-Goga, B.; Goretti, E.; La Porta, G.; et al. Sex and seasonal differences in metal accumulation of selected tissues in red swamp crayfish from Lake Trasimeno (Umbria, Italy). Environ. Sci. Pollut. Res. 2023, 30, 6234–6244. [Google Scholar] [CrossRef]
- Gedik, K.; Kongchum, M.; DeLaune, R.D.; Sonnier, J.J. Distribution of Arsenic and other metals in crayfish tissues (Procambarus clarkii) under different production practices. Sci. Total Environ. 2017, 574, 322–331. [Google Scholar] [CrossRef]
- Shaaban, E.A.; Gawad, S.S.A.; El-Feky, A.; El-Sayed, A.A.M.; Mahmoud, N.H. Bioaccumulation of Cadmium and Lead in the freshwater crayfish Procambarus clarkii (Girard 1982) from the River Nile, Egypt. Al-Azhar Bull. Sci. 2017, 9, 219–233. [Google Scholar]
- Xiong, B.; Xu, T.; Li, R.; Johnson, D.; Ren, D.; Liu, H.; Xi, Y.; Huang, Y. Heavy metal accumulation and health risk assessment of crayfish collected from cultivated and uncultivated ponds in the Middle Reach of Yangtze River. Sci. Total Environ. 2020, 739, 139963. [Google Scholar] [CrossRef] [PubMed]
- Maric, D.; Stanic, M. Bioaccumulation of 18 trace metals in muscle and exoskeleton in the noble crayfish (Astacus astacus L.) in the river zeta (Montenegro). Agric. For. 2021; 67, 1–192. [Google Scholar] [CrossRef]
- El-Aziz, A. Potential carcinogenic and non-carcinogenic health risks of heavy metals ingestion from consumption of the crayfish, Procambarus clarkii in El-Rahawy Drain and El-Kanater in the River Nile, Egypt. Egypt. J. Aquat. Biol. Fish. 2022, 26, 667–686. [Google Scholar] [CrossRef]
- Manjarrés-López, D.P.; Vitale, D.; Callejas-Martos, S.; Usuriaga, M.; Picó, Y.; Pérez, S.; Montemurro, N. An effective method for the simultaneous extraction of 173 contaminants of emerging concern in freshwater invasive species and its application. Anal. Bioanal. Chem. 2023, 415, 7085–7101. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, T.G.; Anastácio, P.M.S.G.; Araujo, P.B.; Souty-Grosset, C.; Almerão, M.P. Red swamp crayfish: Biology, ecology and invasion—An overview. Nauplius 2015, 23, 1–19. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Anastácio, P.M.; Aquiloni, L.; Banha, F.; Choquer, J.; Chucholl, C.; Tricarico, E. The red swamp crayfish Procambarus clarkii in Europe: Impacts on aquatic ecosystems and human well-being. Limnologica 2016, 58, 78–93. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Gong, F.; Yang, F.; Jiang, Q.; Xu, Y.; Xia, W. A Comparison of eating safety and quality of live and dead freshwater crayfish (Procambarus clarkii) at different stages. Food Res. Int. 2022, 159, 111630. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Jia, L.; Zhang, K.; Xie, J.; Yu, E.; Tian, J.; Gong, W.; Li, Z.; Li, H.; Wang, G.; et al. Geographical origin traceability of Procambarus clarkii based on mineral elements and stable isotopes. Foods 2022, 11, 3060. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, B.; Yang, L.; Jiang, S.; Lu, J.; Lin, L. Comparison of the nutritional qualities of the pond, rice-field and wild crayfish (Procambarus clarkii) meat. Food Chem. Adv. 2023, 2, 100272. [Google Scholar] [CrossRef]
- Sanuja, R.G.; Kalutharage, N.K.; Cumaranatunga, P.R.T. Selection of the most suitable crustacean exoskeleton waste from fish processing industry to isolate chitosan. Sri Lanka J. Aquat. 2017, 22, 45–53. [Google Scholar] [CrossRef][Green Version]
- Puvvada, Y.S.; Vankayalapati, S.; Sukhavasi, S. Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. Int. Curr. Pharm. J. 2012, 1, 258–263. [Google Scholar] [CrossRef]
- Mauro, M.; Pinto, P.; Settanni, L.; Puccio, V.; Vazzana, M.; Hornsby, B.L.; Fabbrizio, A.; Di Stefano, V.; Barone, G.; Arizza, V. Chitosan film functionalized with grape seed oil—Preliminary evaluation of antimicrobial activity. Sustainability 2022, 14, 5410. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Dey, A.; Sarkar, T.; Pati, S.; Joshi, S.; Bunawan, H.; Mohammed, A.; Edinur, H.A.; Ghosh, S.; et al. Seafood discards: A potent source of enzymes and biomacromolecules with nutritional and nutraceutical significance. Front. Nutr. 2022, 9, 879929. [Google Scholar] [CrossRef] [PubMed]
- Azelee, N.I.W.; Dahiya, D.; Ayothiraman, S.; Noor, N.M.; Rasid, Z.I.A.; Ramli, A.N.M.; Ravindran, B.; Iwuchukwu, F.U.; Selvasembian, R. Sustainable valorization approaches on crustacean wastes for the extraction of chitin, bioactive compounds and their applications—A review. Int. J. Biol. Macromol. 2023, 253, 126492. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.U.; Hollah, C.; Wiesotzki, K.; Heinz, V.; Aganovic, K.; Rehman, R.U.; Petrusan, J.-I.; Zheng, L.; Zhang, J.; Sohail, S.; et al. Insect-derived chitin and chitosan: A still unexploited resource for the edible insect sector. Sustainability 2023, 15, 4864. [Google Scholar] [CrossRef]
- Camacho-Jiménez, L.; González-Ruiz, R.; Yepiz-Plascencia, G. Persistent organic pollutants (POPs) in marine crustaceans: Bioaccumulation, physiological and cellular responses. Mar. Environ. Res. 2023, 192, 106184. [Google Scholar] [CrossRef] [PubMed]
- Kuklina, I.; Kouba, A.; Buřič, M.; Horká, I.; Ďuriš, Z.; Kozák, P. Accumulation of heavy metals in crayfish and fish from selected czech reservoirs. BioMed Res. Int. 2014, 2014, 306103. [Google Scholar] [CrossRef]
- Tricarico, E.; Zanetti, M. Piano di Gestione Nazionale del Gambero Rosso della Louisiana (Procambarus clarkii). 2023. Available online: https://www.mase.gov.it/sites/default/files/archivio/allegati/biodiversita/piano_gestione_gambero_louisiana.pdf (accessed on 15 January 2024).
- Vicente, F.A.; Ventura, S.P.M.; Passos, H.; Dias, A.C.R.V.; Torres-Acosta, M.A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chem. Eng. J. 2022, 442, 135937. [Google Scholar] [CrossRef]
- Vecchioni, L.; Faraone, F.P.; Stoch, F.; Arculeo, M.; Marrone, F. Diversity and distribution of the inland water decapods of Sicily (Crustacea, Malacostraca). Diversity 2022, 14, 246. [Google Scholar] [CrossRef]
- Vecchioni, L.; Russotto, S.; Arculeo, M.; Marrone, F. On the occurrence of the invasive atlantic blue crab Callinectes sapidus Rathbun 1896 (Decapoda: Brachyura: Portunidae) in Sicilian inland waters. Nat. Hist. Sci. 2022, 9, 43–46. [Google Scholar] [CrossRef]
- Vecchioni, L.; Marrone, F.; Chirco, P.; Arizza, V.; Tricarico, E.; Arculeo, M. An update of the known distribution and status of Cherax spp. in Italy (Crustacea, Parastacidae). BioInvasions Rec. 2022, 11, 1045–1055. [Google Scholar] [CrossRef]
- Faraone, F.P.; Giacalone, G.; Canale, D.E.; D’Angelo, S.; Favaccio, G.; Garozzo, V.; Giancontieri, G.L.; Isgrò, C.; Melfi, R.; Morello, B.; et al. Tracking the invasion of the red swamp crayfish Procambarus clarkii (Girard, 1852) (Decapoda Cambaridae) in Sicily: A “Citizen Science” Approach. Biogeographia 2017, 32, 25–29. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Marrone, F. Different invasibility of permanent and temporary waterbodies in a semiarid Mediterranean Island. Inland Waters 2019, 9, 411–421. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Past: Paleontological statistics software package for educaton and data anlysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Vogan, C.L.; Powell, A.; Rowley, A.F. Shell disease in crustaceans—Just chitin recycling gone wrong? Environ. Microbiol. 2008, 10, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Refaat Mohamed Morsi, R.; Abed El-Hamied Al-Bassel, D.; El-Dash, H.; Mahmoud, M.; Zaghloul, K. Usage of crayfish chitosan composite modified film, prepared from exoskeleton of Procambarus clarkii, in treatment of water Copper toxicity. Fayoum J. Agric. Res. Dev. 2023, 37, 31–45. [Google Scholar] [CrossRef]
- Yacoubi, L.; El Zrelli, R.B.; Hsu, H.H.; Lin, Y.-J.; Savoca, D.; Gopalan, J.; Nazal, M.; Bhuyan, M.S.; Arculeo, M.; Rabaoui, L.J. Bioaccumulation of trace elements and hydrocarbons in chondrichthyans of the western arabian gulf: Environmental and human health risk assessment and implications for conservation. Sci. Total Environ. 2023, 901, 165990. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, W.; Dong, Q.; Liu, H. Exoskeleton protein repertoires in decapod crustaceans revealed distinct biomineralization evolution with molluscs. J. Proteom. 2024, 291, 105046. [Google Scholar] [CrossRef]
- Alcorlo, P.; Otero, M.; Crehuet, M.; Baltanás, A.; Montes, C. The use of the red swamp crayfish (Procambarus clarkii, girard) as indicator of the bioavailability of heavy metals in environmental monitoring in the River Guadiamar (SW, Spain). Sci. Total Environ. 2006, 366, 380–390. [Google Scholar] [CrossRef]
- Ríos, V.; Moreno, I.; Prieto, A.I.; Puerto, M.; Gutiérrez-Praena, D.; Soria-Díaz, M.E.; Cameán, A.M. Analysis of MC-LR and MC-RR in tissue from freshwater fish (Tinca tinca) and crayfish (Procambarus clarkii) in tench ponds (Cáceres, Spain) by liquid chromatography-mass spectrometry (LC-MS). Food Chem. Toxicol. 2013, 57, 170–178. [Google Scholar] [CrossRef]
- Bertrand, L.; Monferrán, M.V.; Métais, I.; Mouneyrac, C.; Amé, M.V. MTs in Palaemonetes argentinus as potential biomarkers of zinc contamination in freshwaters. Ecol. Indic. 2015, 48, 533–541. [Google Scholar] [CrossRef]
- The European Commission. Regulation No. 2023/915 of 25 April 2023 Setting Maximum Levels for Certain Contaminants in Foodstuffs and Repealing Regulation (EC) No. 1881/2006 of 19 December 2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Dinake, P.; Motswetla, O.; Kereeditse, T.T.; Kelebemang, R. Assessment of level of heavy metals in cosmetics. Toxicol. Res. Appl. 2023, 7, 239784732311566. [Google Scholar] [CrossRef]
- Fernández-Sanjuan, M.; Meyer, J.; Damásio, J.; Faria, M.; Barata, C.; Lacorte, S. Screening of perfluorinated chemicals (PFCs) in various aquatic organisms. Anal. Bioanal. Chem. 2010, 398, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Stecconi, T.; Stramenga, A.; Tavoloni, T.; Bacchiocchi, S.; Ciriaci, M.; Griffoni, F.; Palombo, P.; Sagratini, G.; Siracusa, M.; Piersanti, A. Exploring perfluoroalkyl substances (PFASs) in aquatic fauna of Lake Trasimeno (Italy): Insights from a low-anthropized area. Toxics 2024, 12, 196. [Google Scholar] [CrossRef]



| Matrix | Site | As | B | Cd | Co | Cr | Fe | Mn | Hg | Ni | Pb | Cu | Se | V | Zn | Ba |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WE (n = 3) | GB | 0.83 ± 0.11 | 2.28 ± 0.21 | < | 0.23 ± 0.08 | 0.12 ± 0.03 | 42 ± 7 | 33 ± 4 | 0.015 ± 0.003 | 0.19 ± 0.04 | < | 5.2 ± 0.7 | 0.16 ± 0.04 | < | 27.6 ± 6.0 | 26 ± 3 |
| WE (n = 3) | SLR | 0.36 ± 0.05 | 2.39 ± 0.20 | 0.030 ± 0.009 | 0.90 ± 0.14 | 0.88 ± 0.07 | 222 ± 36 | 95 ± 10 | 0.026 ± 0.004 | 1.15 ± 0.24 | < | 21.8 ± 2.8 | 0.46 ± 0.08 | 0.49 ± 0.02 | 43.1 ± 4.1 | 32 ± 4 |
| WE (n = 3) | CR | 0.64 ± 0.13 | 1.85 ± 0.31 | < | 0.41 ± 0.08 | 0.47 ± 0.15 | 237 ± 8 | 98 ± 17 | 0.017 ± 0.004 | 0.65 ± 0.09 | 0.15 ± 0.04 | 14.3 ± 1.7 | 0.32 ± 0.08 | 0.69 ± 0.07 | 36.3 ± 7.2 | 150 ± 26 |
| AbE (n = 3) | GB | 0.50 ± 0.08 | 3.72 ± 0.81 | < | 0.37 ± 0.03 | 0.36 ± 0.13 | 102 ± 4 | 41 ± 8 | 0.005 ± 0.002 | 0.16 ± 0.10 | < | 7.6 ± 1.1 | 0.14 ± 0.08 | 0.22 ± 0.004 | 8.9 ± 2.5 | 82 ± 8 |
| AbE (n = 3) | SLR | 0.27 ± 0.10 | 2.45 ± 0.26 | < | 0.97 ± 0.46 | 0.65 ± 0.17 | 411 ± 247 | 327 ± 149 | 0.011 ± 0.002 | 0.78 ± 0.26 | 0.11 ± 0.10 | 18.1 ± 2.8 | 0.24 ± 0.02 | 0.65 ± 0.30 | 10.7 ± 3.1 | 86 ± 20 |
| AbE (n = 3) | CR | 0.33 ± 0.04 | 2.93 ± 0.11 | < | 0.47 ± 0.03 | 0.40 ± 0.01 | 174 ± 6 | 94 ± 10 | 0.005 ± 0.001 | 0.50 ± 0.04 | < | 14.0 ± 1.2 | 0.23 ± 0.03 | 0.47 ± 0.01 | 8.2 ± 1.6 | 310 ± 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savoca, D.; Vazzana, M.; Arizza, V.; Maccotta, A.; Orecchio, S.; Longo, F.; Giudice, V.; D’Oca, G.; Messina, S.; Marrone, F.; et al. Contamination Profiles of Selected Pollutants in Procambarus clarkii Non-Edible Portions Highlight Their Potential Exploitation Applications. J. Xenobiot. 2024, 14, 893-906. https://doi.org/10.3390/jox14030049
Savoca D, Vazzana M, Arizza V, Maccotta A, Orecchio S, Longo F, Giudice V, D’Oca G, Messina S, Marrone F, et al. Contamination Profiles of Selected Pollutants in Procambarus clarkii Non-Edible Portions Highlight Their Potential Exploitation Applications. Journal of Xenobiotics. 2024; 14(3):893-906. https://doi.org/10.3390/jox14030049
Chicago/Turabian StyleSavoca, Dario, Mirella Vazzana, Vincenzo Arizza, Antonella Maccotta, Santino Orecchio, Francesco Longo, Vittoria Giudice, Gaetano D’Oca, Salvatore Messina, Federico Marrone, and et al. 2024. "Contamination Profiles of Selected Pollutants in Procambarus clarkii Non-Edible Portions Highlight Their Potential Exploitation Applications" Journal of Xenobiotics 14, no. 3: 893-906. https://doi.org/10.3390/jox14030049
APA StyleSavoca, D., Vazzana, M., Arizza, V., Maccotta, A., Orecchio, S., Longo, F., Giudice, V., D’Oca, G., Messina, S., Marrone, F., & Mauro, M. (2024). Contamination Profiles of Selected Pollutants in Procambarus clarkii Non-Edible Portions Highlight Their Potential Exploitation Applications. Journal of Xenobiotics, 14(3), 893-906. https://doi.org/10.3390/jox14030049








