Ionic Liquid 1-Octyl-3-Methylimidazolium (M8OI) Is Mono-Oxygenated by CYP3A4 and CYP3A5 in Adult Human Liver
Abstract
1. Introduction
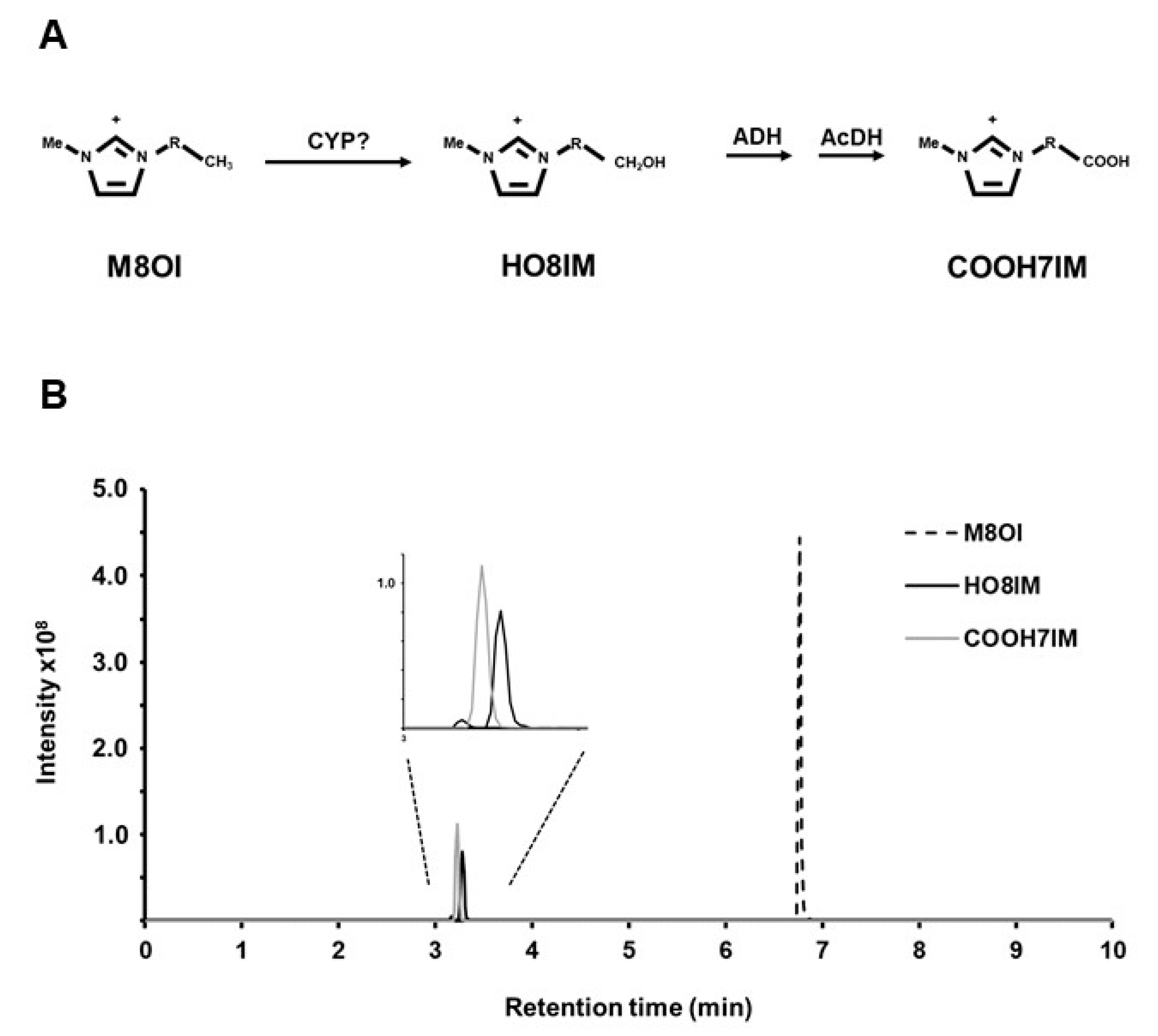
2. Materials and Methods
2.1. Materials
2.2. Human Hepatocyte Isolation and Culture
2.3. Immunohistochemistry
2.4. Gene Expression
2.5. CYP3A Activity
2.6. M8OI Incubation with Hepatocytes
2.7. M8OI Metabolism in Supersomes
2.8. M8OI and Metabolite Determinations
2.9. Thiazolyl Blue Tetrazolium Bromide Assay
2.10. Statistical Analyses
3. Results
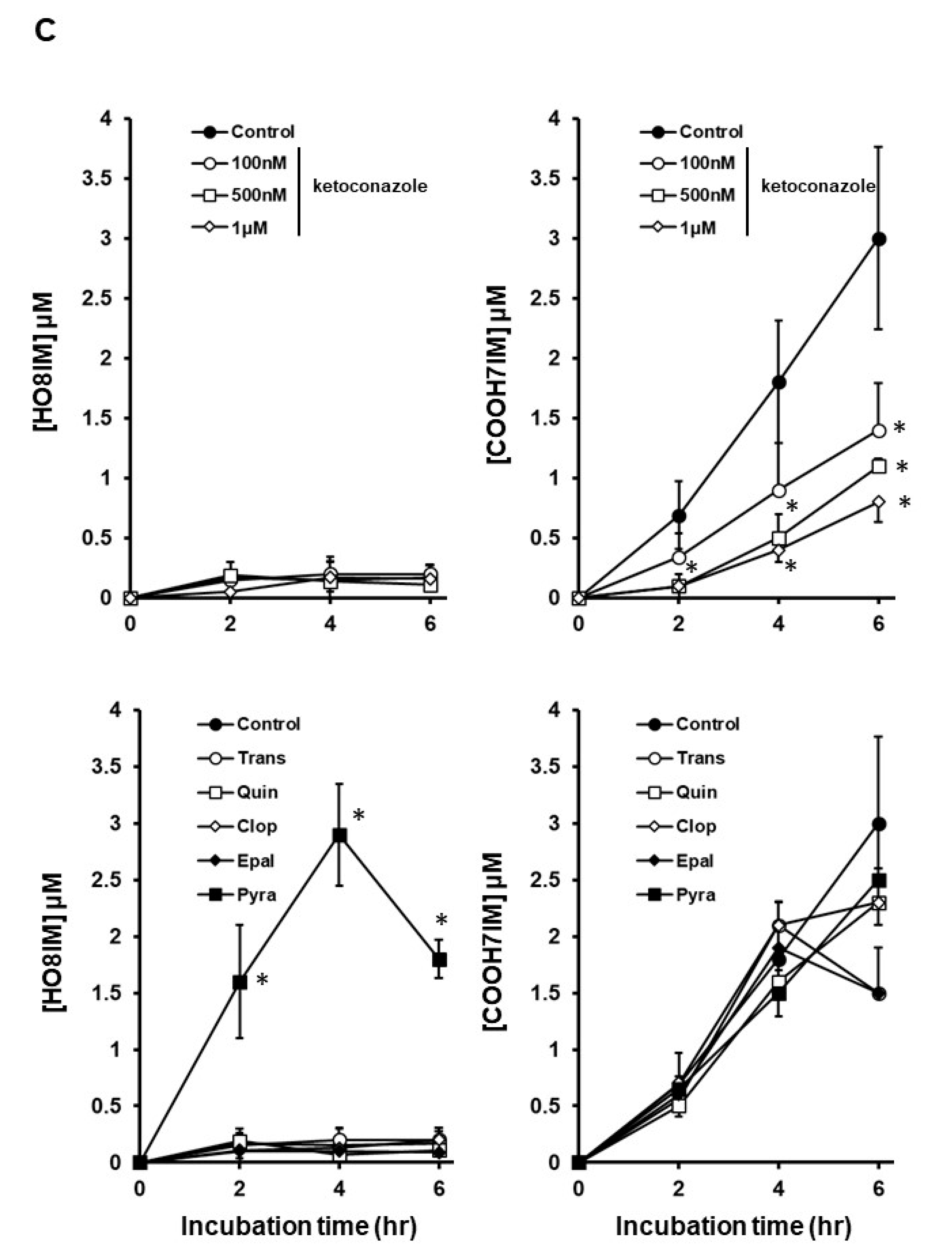
3.1. M8OI Metabolism by Human Hepatocytes Is Potently Inhibited by Ketoconazole in a Dose-Dependent Manner
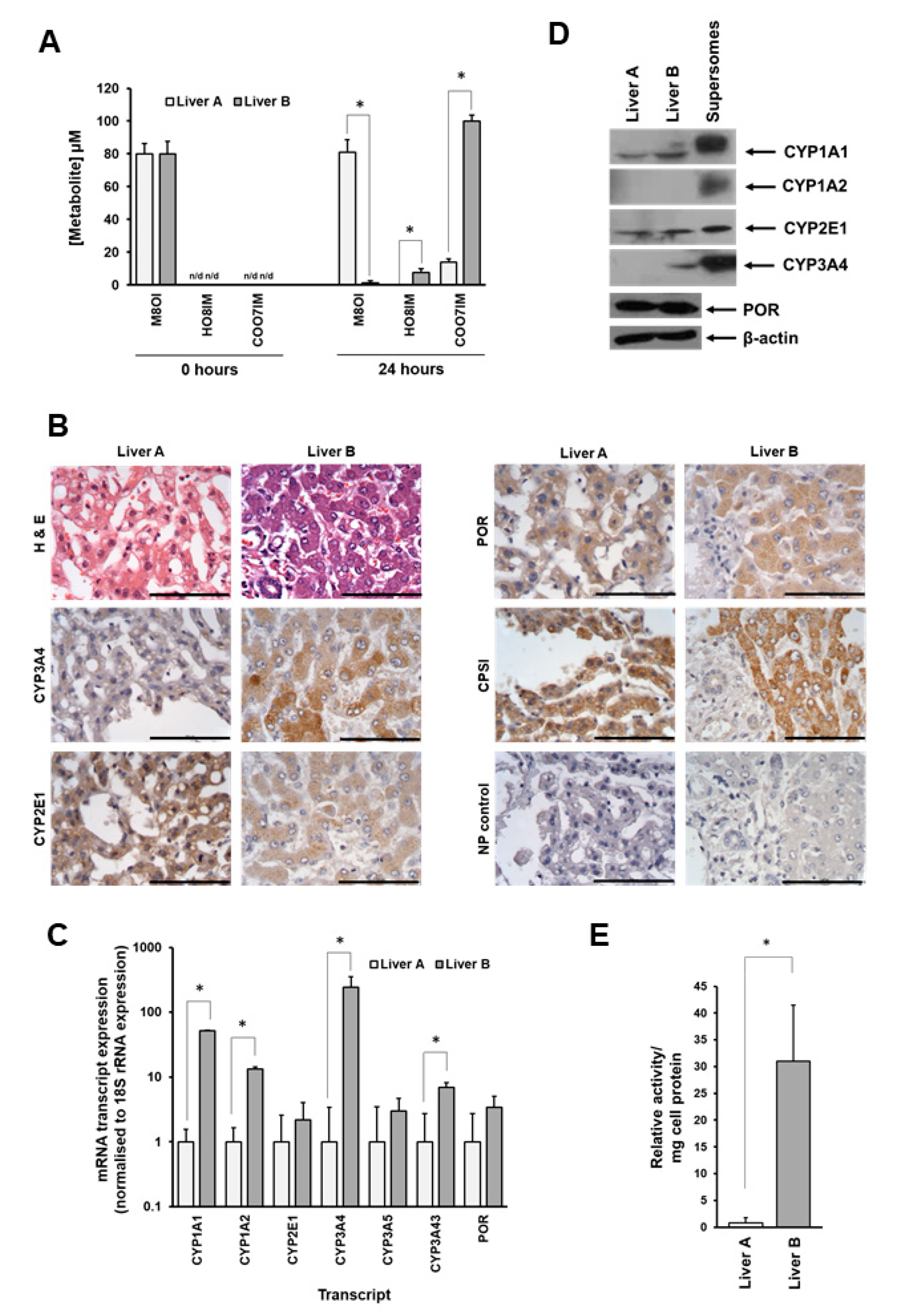
3.2. Metabolism of M8OI Is Significantly Reduced in Hepatocytes Isolated from Donor Expressing Low Levels of CYP3A4
3.3. Supersome Recombinant CYP3A4 Mono-Oxygenated M8OI to form HO8IM and Two Other Hydroxylated Metabolites
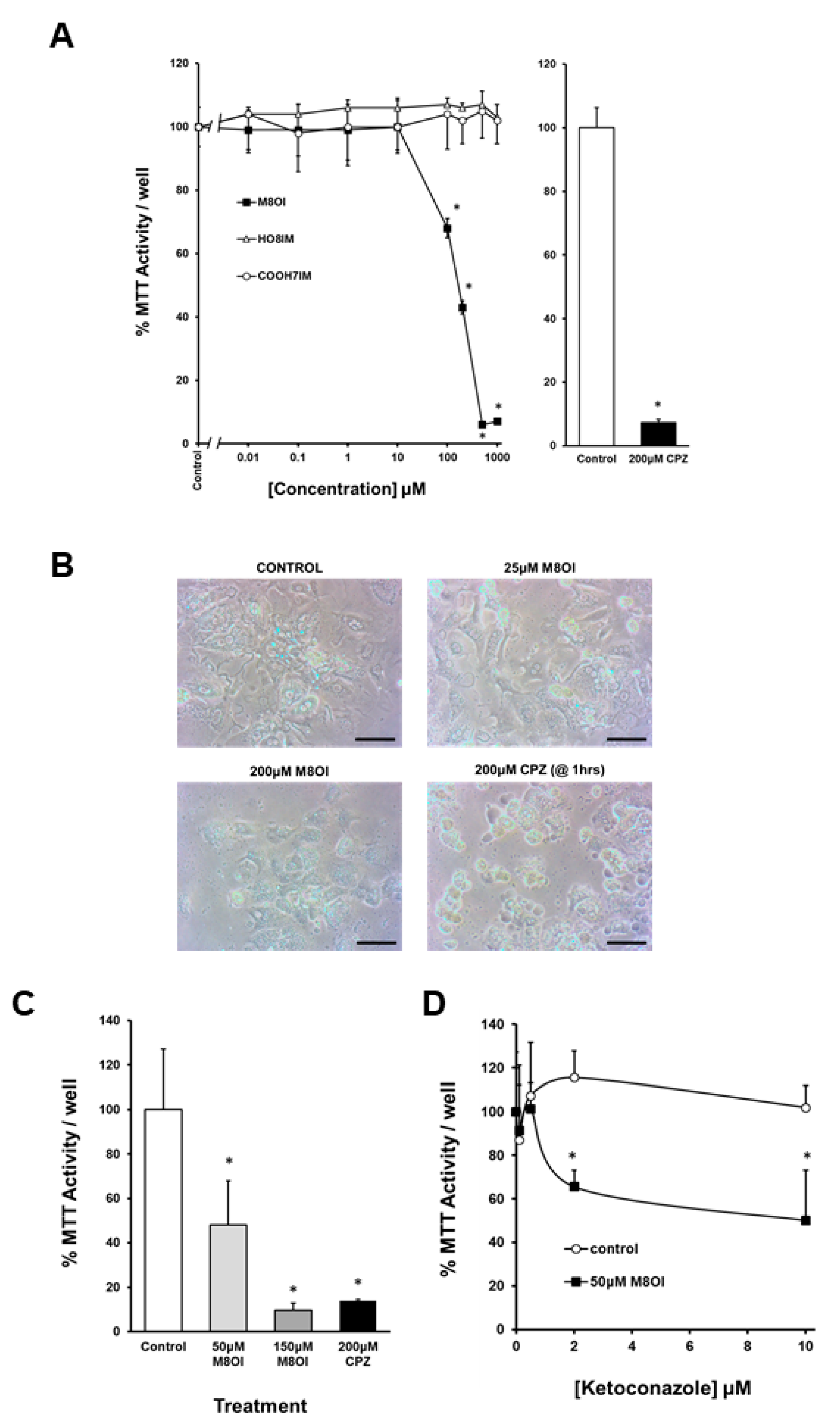
3.4. The Oxidation of M8OI Represents a Detoxification Pathway in Human Hepatocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Oskarsson, A.; Wright, M.C. Ionic Liquids: New Emerging Pollutants, Similarities with Perfluorinated Alkyl Substances (PFASs). Environ. Sci. Technol. 2019, 53, 10539–10541. [Google Scholar] [CrossRef] [PubMed]
- Leitch, A.C.; Abdelghany, T.M.; Probert, P.M.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Cooke, M.P.; Blake, L.I.; Morse, K.; Rosenmai, A.K.; et al. The toxicity of the methylimidazolium ionic liquids, with a focus on M8OI and hepatic effects. Food Chem. Toxicol. 2020, 136, 111069. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.M.; Leitch, A.C.; Nevjestić, I.; Ibrahim, I.; Miwa, S.; Wilson, C.; Heutz, S.; Wright, M.C. Emerging risk from “environmentally-friendly” solvents: Interaction of methylimidazolium ionic liquids with the mitochondrial electron transport chain is a key initiation event in their mammalian toxicity. Food Chem. Toxicol. 2020, 145, 111593. [Google Scholar] [CrossRef] [PubMed]
- Probert, P.M.; Leitch, A.C.; Dunn, M.P.; Meyer, S.K.; Palmer, J.M.; Abdelghany, T.M.; Lakey, A.F.; Cooke, M.P.; Talbot, H.; Wills, C.; et al. Identification of a xenobiotic as a potential environmental trigger in primary biliary cholangitis. J. Hepatol. 2018, 69, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Leitch, A.C.; Abdelghany, T.M.; Charlton, A.; Grigalyte, J.; Oakley, F.; Borthwick, L.A.; Reed, L.; Knox, A.; Reilly, W.J.; Agius, L.; et al. Renal injury and hepatic effects from the methylimidazolium ionic liquid M8OI in mouse. Ecotoxicol. Environ. Saf. 2020, 202, 110902. [Google Scholar] [CrossRef]
- Young, G.R.; Abdelghany, T.M.; Leitch, A.C.; Dunn, M.P.; Blain, P.G.; Lanyon, C.; Wright, M.C. Changes in the gut microbiota of mice orally exposed to methylimidazolium ionic liquids. PLoS ONE 2020, 15, e0229745. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Hedya, S.; De Santis, C.; El-Rahmand, S.S.; Gill, J.H.; Abdelkader, N.F.; Wright, M.C. Potential for cardiac toxicity with methylimidazolium ionic liquids. Ecotoxicol. Environ. Saf. 2023, 249, 114439. [Google Scholar] [CrossRef]
- Leitch, A.C.; Lakey, A.F.; Hotham, W.E.; Agius, L.; Kass, G.E.N.; Blain, P.G.; Wright, M.C. The ionic liquid 1-octyl-3-methylimidazolium (M8OI) is an activator of the human estrogen receptor alpha. Biochem. Biophys. Res. Commun. 2018, 503, 2167–2172. [Google Scholar] [CrossRef]
- Leitch, A.C.; Ibrahim, I.; Abdelghany, T.M.; Charlton, A.; Roper, C.; Vidler, D.; Palmer, J.M.; Wilson, C.; Jones, D.E.; Blain, P.G.; et al. The methylimidazolium ionic liquid M8OI is detectable in human sera and is subject to biliary excretion in perfused human liver. Toxicology 2021, 459, 152854. [Google Scholar] [CrossRef]
- Draper, A.J.; Madan, A.; Parkinson, A. Inhibition of coumarin 7-hydroxylase activity in human liver microsomes. Arch. Biochem. Biophys. 1997, 341, 47–61. [Google Scholar] [CrossRef]
- Zhang, W.; Kilicarslan, T.; Tyndale, R.F.; Sellers, E.M. Evaluation of methoxsalen, tranylcypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metab. Dispos. 2001, 29, 897–902. [Google Scholar] [PubMed]
- Taavitsainen, P.; Juvonen, R.; Pelkonen, O. In vitro inhibition of cytochrome P450 enzymes in human liver microsomes by a potent CYP2A6 inhibitor, trans-2-phenylcyclopropylamine (tranylcypromine), and its nonamine analog, cyclopropylbenzene. Drug Metab. Dispos. 2001, 29, 217–222. [Google Scholar] [PubMed]
- Walsky, R.L.; Gaman, E.A.; Obach, R.S. Examination of 209 drugs for inhibition of cytochrome P450 2C8. J. Clin. Pharmacol. 2005, 45, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Walsky, R.L.; Astuccio, A.V.; Obach, R.S. Evaluation of 227 drugs for in vitro inhibition of cytochrome P450 2B6. J. Clin. Pharmacol. 2006, 46, 1426–1438. [Google Scholar] [CrossRef]
- Walsky, R.L.; Obach, R.S. A comparison of 2-phenyl-2-(1- piperidinyl)propane (ppp), 1, 10, 100-phosphinothioylidynetrisaziridine (thioTEPA), clopidogrel, and ticlopidine as selective inactivators of human cytochrome P450 2B6. Drug Metab. Dispos. 2007, 35, 2053–2059. [Google Scholar] [CrossRef]
- Bourrie, M.; Meunier, V.; Berger, Y.; Fabre, G.J. Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human liver microsomes. Pharmacol. Exp. Ther. 1996, 277, 321–332. [Google Scholar]
- Moody, G.C.; Griffin, S.J.; Mather, A.N.; McGinnity, D.F.; Riley, R.J. Fully automated analysis of activities catalysed by the major human liver cytochrome P450 (CYP) enzymes: Assessment of human CYP inhibition potential. Xenobiotica 1999, 29, 53–75. [Google Scholar] [CrossRef]
- Jones, J.P.; Joswig-Jones, C.A.; Hebner, M.; Chu, Y.; Koop, D.R. The effects of nitrogen-heme-iron coordination on substrate affinities for cytochrome P450 2E1. Chem. Biol. Interact. 2011, 193, 50–56. [Google Scholar] [CrossRef]
- Pietruszko, R. Human liver alcohol dehydrogenase—Inhibition of methanol activity by pyrazole, 4-methylpyrazole, 4-hydroxymethylpyrazole and 4-carboxypyrazole. Biochem. Pharmacol. 1975, 24, 1603–1607. [Google Scholar] [CrossRef]
- Gibbs, M.A.; Thummel, K.E.; Shen, D.D.; Kunze, K.L. Inhibition of cytochrome P–450 3A (CYP3A) in human intestinal and liver microsomes: Comparison of Ki values and impact of CYP3A5 expression. Drug Metab. Dispos. 1999, 27, 180–187. [Google Scholar] [PubMed]
- Khojasteh, S.C.; Prabhu, S.; Kenny, J.R.; Halladay, J.S.; Lu, A.Y. Chemical inhibitors of cytochrome P450 isoforms in human liver microsomes: A re-evaluation of P450 isoform selectivity. Eur. J. Drug Metab. Pharmacokinet. 2011, 36, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yamaori, S.; Araki, N.; Shionoiri, M.; Ikehata, K.; Kamijo, S.; Ohmori, S.; Watanabe, K. A Specific Probe Substrate for Evaluation of CYP4A11 Activity in Human Tissue Microsomes and a Highly Selective CYP4A11 Inhibitor: Luciferin-4A and Epalrestat. J. Pharmacol. Exp. Ther. 2018, 366, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.L.; Paine, A.J.; Maurel, P.; Wright, M.C. Effect of the adrenal 11-beta-hydroxylase inhibitor metyrapone on human hepatic cytochrome P-450 expression: Induction of cytochrome P-450 3A4. Drug Metab. Dispos. 2000, 28, 96–101. [Google Scholar] [PubMed]
- Probert, P.M.; Ebrahimkhani, M.R.; Oakley, F.; Mann, J.; Burt, A.D.; Mann, D.A.; Wright, M.C. A reversible model for periportal fibrosis and a refined alternative to bile duct ligation. Toxicol. Res. 2014, 3, 98–109. [Google Scholar] [CrossRef]
- Fairhall, E.A.; Charles, M.A.; Probert, P.M.; Wallace, K.; Gibb, J.; Ravindan, C.; Soloman, M.; Wright, M.C. Pancreatic B-13 Cell Trans-Differentiation to Hepatocytes Is Dependent on Epigenetic-Regulated Changes in Gene Expression. PLoS ONE 2016, 11, e0150959. [Google Scholar] [CrossRef] [PubMed]
- Hedya, S.; Charlton, A.; Leitch, A.C.; Pinker, B.; Aljehani, F.; Wright, M.C.; Abdelghany, T.M. The methylimidazolium ionic liquid M8OI is a substrate for OCT1 and p-glycoprotein-1 in rat. Toxicol. In Vitro 2023, 88, 105550. [Google Scholar] [CrossRef]
- Koop, D.R.; Morgan, E.T.; Tarr, G.E.; Coon, M.J. Purification and characterization of a unique isozyme of cytochrome P-450 from liver microsomes of ethanol-treated rabbits. J. Biol. Chem. 1982, 257, 8472–8480. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450 2E1 and its roles in disease. Chem. Biol. Interact. 2020, 322, 109056. [Google Scholar] [CrossRef]
- Wright, M.C.; Issa, R.; Smart, D.E.; Trim, N.; Murray, G.I.; Primrose, J.N.; Arthur, M.J.; Iredale, J.P.; Mann, D.A. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology 2001, 121, 685–698. [Google Scholar] [CrossRef]
- Li, H.; Lampe, J.N. Neonatal cytochrome P450 CYP3A7: A comprehensive review of its role in development, disease, and xenobiotic metabolism. Arch. Biochem. Biophys. 2019, 673, 108078. [Google Scholar] [CrossRef]
- Domanski, T.L.; Finta, C.; Halpert, J.R.; Zaphiropoulos, P.G. cDNA cloning and initial characterization of CYP3A43, a novel human cytochrome P450. Mol. Pharmacol. 2001, 59, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Gaedigk, A.; Boone, E.C.; Turner, A.J.; van Schaik, R.H.N.; Chernova, D.; Wang, W.Y.; Broeckel, U.; Granfield, C.A.; Hodge, J.C.; Ly, R.C.; et al. Characterization of Reference Materials for CYP3A4 and CYP3A5: A (GeT-RM) Collaborative Project. J. Mol. Diagn. 2023, 25, 655–664. [Google Scholar] [CrossRef]
- Yu, S.; Rao, S.; Reddy, J.K. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr. Mol. Med. 2003, 3, 561–572. [Google Scholar] [CrossRef] [PubMed]
- van Dam, G.M.; Gips, C.H. Primary biliary cirrhosis (PBC) in an European country--a description of death rates in The Netherlands (1979–1992). Hepatogastroenterology 1996, 43, 906–913. [Google Scholar]
- Jeong, S.H. Current epidemiology and clinical characteristics of autoimmune liver diseases in South Korea. Clin. Mol. Hepatol. 2018, 24, 10–19. [Google Scholar] [CrossRef]
- Tanaka, A.; Leung, P.S.C.; Gershwin, M.E. Evolution of our understanding of PBC. Best. Pract. Res. Clin. Gastroenterol. 2018, 34, 3–9. [Google Scholar] [CrossRef]
- Cheng, J.S.; Chen, W.T.; Ku, H.P.; Chien, R.N.; Chang, M.L. Characteristic geoepidemiology of primary biliary cholangitis in Taiwan: A nationwide population-based study. Hepatol. Res. 2023, 53, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Wolbold, R.; Klein, K.; Burk, O.; Nüssler, A.K.; Neuhaus, P.; Eichelbaum, M.; Schwab, M.; Zanger, U.M. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 2003, 38, 978–988. [Google Scholar] [CrossRef]
- Thangavel, C.; Boopathi, E.; Shapiro, B.H. Inherent sex-dependent regulation of human hepatic CYP3A5. Br. J. Pharmacol. 2013, 168, 988–1000. [Google Scholar]


| CYP | Inhibitor Concentration | References/Comments |
|---|---|---|
| CYP2A6 | Tranylcypromine 5 μM | The (R)-enantiomer in human liver microsomes inhibits CYP2A6 at Ki values of 0.04–0.2 μM [11,12,13]. |
| CYP2B6 | Clopidogrel 5 μM | Clopidogrel inhibits CYP2B6 at IC50 values of 0.0146–0.046 μM [14,15,16]. |
| CYP2D6 | Quinidine 5 μM | This inhibits CYP2D6 at Ki values of 0.03–0.4 μM [17,18]. |
| CYP2E1 | Pyrazole 2 mM | This inhibits CYP2E1 (Ki of 35.5 μM). It also inhibits ADH [19,20]. |
| CYP3A4/3A5/3A7/3A43 | Ketoconazole 100 nM–1 μM | Using recombinant enzymes, Ki values of 0.0267 and 0.109 μM for CYP3A4 and CYP3A5, respectively [21]. Ketoconazole is a very selective inhibitor of CYP3A when used at sub-micromolar concentrations [22]. |
| CYP4A11 | Epalrestat 5 μM | Using recombinant enzymes, a Ki value of 1.82 μM for CYP4A11. The most potent inhibition out of 17 other recombinant CYPs [23]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitch, A.C.; Abdelghany, T.M.; Charlton, A.; Cooke, M.; Wright, M.C. Ionic Liquid 1-Octyl-3-Methylimidazolium (M8OI) Is Mono-Oxygenated by CYP3A4 and CYP3A5 in Adult Human Liver. J. Xenobiot. 2024, 14, 907-922. https://doi.org/10.3390/jox14030050
Leitch AC, Abdelghany TM, Charlton A, Cooke M, Wright MC. Ionic Liquid 1-Octyl-3-Methylimidazolium (M8OI) Is Mono-Oxygenated by CYP3A4 and CYP3A5 in Adult Human Liver. Journal of Xenobiotics. 2024; 14(3):907-922. https://doi.org/10.3390/jox14030050
Chicago/Turabian StyleLeitch, Alistair C., Tarek M. Abdelghany, Alex Charlton, Martin Cooke, and Matthew C. Wright. 2024. "Ionic Liquid 1-Octyl-3-Methylimidazolium (M8OI) Is Mono-Oxygenated by CYP3A4 and CYP3A5 in Adult Human Liver" Journal of Xenobiotics 14, no. 3: 907-922. https://doi.org/10.3390/jox14030050
APA StyleLeitch, A. C., Abdelghany, T. M., Charlton, A., Cooke, M., & Wright, M. C. (2024). Ionic Liquid 1-Octyl-3-Methylimidazolium (M8OI) Is Mono-Oxygenated by CYP3A4 and CYP3A5 in Adult Human Liver. Journal of Xenobiotics, 14(3), 907-922. https://doi.org/10.3390/jox14030050






