Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics
Abstract
1. Introduction
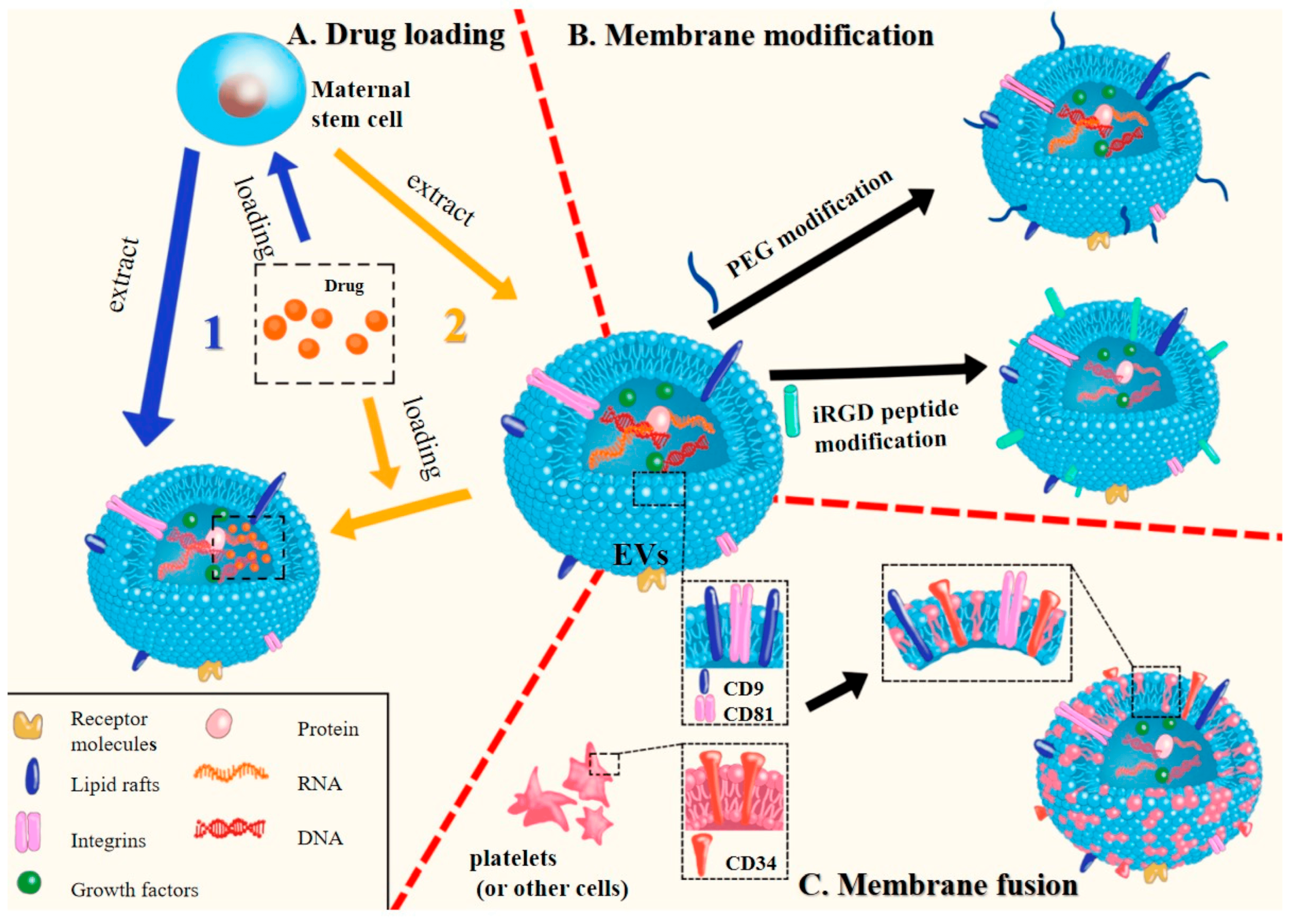
2. Preparation and Characterization of MSC Membrane-Coated Nanocarriers
2.1. Isolation of MSC Membrane
2.2. Coating of the Nanoparticle Cores by Membrane Nanovesicles
2.2.1. Extrusion
2.2.2. Sonication Method
2.2.3. Microfluidic Electroporation Method
2.2.4. Flash Nanocomplexation (FNC)
2.3. Characterization of Cell Membrane-Coated NPs
2.4. Effect of Cell Membrane Coating on Nanoparticle Properties
3. Classification of Mesenchymal Stem Cell Membrane-Based Nanocarriers
3.1. Lipid Based Nanocarriers
3.2. Polymeric Nanoformulations
3.3. Inorganic
4. Mesenchymal Stem Cell-Based Nanocarriers for Therapeutics and Regenerative Medicines
4.1. MSC-Based Nanocarriers in Regenerative Medicine
4.2. MSC-Based Nanocarriers in Anti-Cancer Medicine
5. Safety and Toxicity Implications of Mesenchymal Stem Cell-Based Nanocarriers In Vitro and In Vivo
6. Biotransformation Mechanisms and Clearance of Mesenchymal Stem Cell-Based Nanocarriers
7. Pharmacological and Immunological Barriers in Stem Cell Membrane-Based Nanocarriers
7.1. Challenges in Parenteral Delivery and Biodistribution
7.2. MSC-Based Nanocarriers’ Stabilities in Systemic Circulation and Their Clearance
7.3. Microenvironmental Heterogeneities and Nanoformualtion Uptake and Cellular Internalization
8. Future Perspective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedroza, M.; Gassaloglu, S.I.; Dias, N.; Zhong, L.; Hou, T.-C.J.; Kretzmer, H.; Smith, Z.D.; Sozen, B. Self-Patterning of Human Stem Cells into Post-Implantation Lineages. Nature 2023, 622, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.G.S.; Madsen, M.S.; Rauch, A.; Traynor, S.; Van Hauwaert, E.L.; Haakonsson, A.K.; Javierre, B.M.; Hyldahl, M.; Fraser, P.; Mandrup, S. Highly Interconnected Enhancer Communities Control Lineage-Determining Genes in Human Mesenchymal Stem Cells. Nat. Genet. 2020, 52, 1227–1238. [Google Scholar] [CrossRef]
- Saba, J.A.; Liakath-Ali, K.; Green, R.; Watt, F.M. Translational Control of Stem Cell Function. Nat. Rev. Mol. Cell Biol. 2021, 22, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, C.; Kuddannaya, S.; Zhang, J.; Arifin, D.R.; Han, Z.; Walczak, P.; Liu, G.; Bulte, J.W.M. In Vivo Tracking of Unlabelled Mesenchymal Stromal Cells by Mannose-Weighted Chemical Exchange Saturation Transfer MRI. Nat. Biomed. Eng. 2022, 6, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Gimple, R.C.; Yang, K.; Halbert, M.E.; Agnihotri, S.; Rich, J.N. Brain Cancer Stem Cells: Resilience through Adaptive Plasticity and Hierarchical Heterogeneity. Nat. Rev. Cancer 2022, 22, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Haakonsson, A.K.; Madsen, J.G.S.; Larsen, M.; Forss, I.; Madsen, M.R.; Van Hauwaert, E.L.; Wiwie, C.; Jespersen, N.Z.; Tencerova, M.; et al. Osteogenesis Depends on Commissioning of a Network of Stem Cell Transcription Factors That Act as Repressors of Adipogenesis. Nat. Genet. 2019, 51, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Mandrup, S. Transcriptional Networks Controlling Stromal Cell Differentiation. Nat. Rev. Mol. Cell Biol. 2021, 22, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Chen, H.; Ma, L.; E, W.; Wang, R.; Fang, X.; Zhou, Z.; Sun, H.; Wang, J.; Jiang, M.; et al. Systematic Identification of Cell-Fate Regulatory Programs Using a Single-Cell Atlas of Mouse Development. Nat. Genet. 2022, 54, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Q.; Zhu, J.; Ankrum, J.A. Manufacturing of Primed Mesenchymal Stromal Cells for Therapy. Nat. Biomed. Eng. 2019, 3, 90–104. [Google Scholar] [CrossRef]
- McGonagle, D.; Baboolal, T.G.; Jones, E. Native Joint-Resident Mesenchymal Stem Cells for Cartilage Repair in Osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 719–730. [Google Scholar] [CrossRef]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal Stem Cells Modified with Akt Prevent Remodeling and Restore Performance of Infarcted Hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, M.; Meng, R.; Zhao, Q.; Men, X.; Lan, Y.; Xu, H. Therapeutic Effects and Potential Mechanisms of Endoscopic Submucosal Injection of Mesenchymal Stem Cells on Chronic Atrophic Gastritis. Sci. Rep. 2023, 13, 20745. [Google Scholar] [CrossRef]
- Rui, K.; Hong, Y.; Zhu, Q.; Shi, X.; Xiao, F.; Fu, H.; Yin, Q.; Xing, Y.; Wu, X.; Kong, X.; et al. Olfactory Ecto-Mesenchymal Stem Cell-Derived Exosomes Ameliorate Murine Sjögren’s Syndrome by Modulating the Function of Myeloid-Derived Suppressor Cells. Cell Mol. Immunol. 2021, 18, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Hou, P.; Liu, S.; Zuo, M.; Liu, Z.; Chen, W.; Han, Y.; Li, Y.; Wang, T.; Feng, C.; et al. NAD+ Salvage Governs the Immunosuppressive Capacity of Mesenchymal Stem Cells. Cell Mol. Immunol. 2023, 20, 1171–1185. [Google Scholar] [CrossRef]
- Sazonovs, A.; Stevens, C.R.; Venkataraman, G.R.; Yuan, K.; Avila, B.; Abreu, M.T.; Ahmad, T.; Allez, M.; Ananthakrishnan, A.N.; Atzmon, G.; et al. Large-Scale Sequencing Identifies Multiple Genes and Rare Variants Associated with Crohn’s Disease Susceptibility. Nat. Genet. 2022, 54, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, Safety and Efficacy of iPSC-Derived Mesenchymal Stromal Cells in Acute Steroid-Resistant Graft versus Host Disease: A Phase I, Multicenter, Open-Label, Dose-Escalation Study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef]
- Kadri, N.; Amu, S.; Iacobaeus, E.; Boberg, E.; Le Blanc, K. Current Perspectives on Mesenchymal Stromal Cell Therapy for Graft versus Host Disease. Cell Mol. Immunol. 2023, 20, 613–625. [Google Scholar] [CrossRef]
- Fossati, V.; Peruzzotti-Jametti, L.; Pluchino, S. A Neural Stem-Cell Treatment for Progressive Multiple Sclerosis. Nat. Med. 2023, 29, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Sun, L. MicroRNAs in Mesenchymal Stem Cells: The Key to Decoding Systemic Lupus Erythematosus. Cell Mol. Immunol. 2021, 18, 2286–2287. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory Properties of Mesenchymal Stem Cells/Dental Stem Cells and Their Therapeutic Applications. Cell Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Liakouli, V.; Ciancio, A.; Del Galdo, F.; Giacomelli, R.; Ciccia, F. Systemic Sclerosis Interstitial Lung Disease: Unmet Needs and Potential Solutions. Nat. Rev. Rheumatol. 2023, 20, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Chen, B.; Lescoat, A.; Khanna, D.; Mu, R. Immune Cell Dysregulation as a Mediator of Fibrosis in Systemic Sclerosis. Nat. Rev. Rheumatol. 2022, 18, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Blanc, K.L.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of Severe Acute Graft-versus-Host Disease with Third Party Haploidentical Mesenchymal Stem Cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Ciccocioppo, R.; Bernardo, M.E.; Sgarella, A.; Maccario, R.; Avanzini, M.A.; Ubezio, C.; Minelli, A.; Alvisi, C.; Vanoli, A.; Calliada, F.; et al. Autologous Bone Marrow-Derived Mesenchymal Stromal Cells in the Treatment of Fistulising Crohn’s Disease. Gut 2011, 60, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Duijvestein, M.; Vos, A.C.W.; Roelofs, H.; Wildenberg, M.E.; Wendrich, B.B.; Verspaget, H.W.; Kooy-Winkelaar, E.M.C.; Koning, F.; Zwaginga, J.J.; Fidder, H.H.; et al. Autologous Bone Marrow-Derived Mesenchymal Stromal Cell Treatment for Refractory Luminal Crohn’s Disease: Results of a Phase I Study. Gut 2010, 59, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.-Q.; Luan, S.-L.; Altmann, D.R.; Thompson, A.J.; et al. Autologous Mesenchymal Stem Cells for the Treatment of Secondary Progressive Multiple Sclerosis: An Open-Label Phase 2a Proof-of-Concept Study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Cometa, A.M.; Locatelli, F. Mesenchymal Stromal Cells: A Novel and Effective Strategy for Facilitating Engraftment and Accelerating Hematopoietic Recovery after Transplantation? Bone Marrow Transpl. 2012, 47, 323–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koç, O.N.; Gerson, S.L.; Cooper, B.W.; Dyhouse, S.M.; Haynesworth, S.E.; Caplan, A.I.; Lazarus, H.M. Rapid Hema-Topoietic Recovery After Coinfusion of Autologous-Blood Stem Cells and Culture-Expanded Marrow Mesenchymal Stem Cells in Advanced Breast Cancer Patients Receiving High-Dose Chemotherapy. JCO 2000, 18, 307. [Google Scholar] [CrossRef]
- Mousaei Ghasroldasht, M.; Seok, J.; Park, H.-S.; Liakath Ali, F.B.; Al-Hendy, A. Stem Cell Therapy: From Idea to Clinical Practice. Int. J. Mol. Sci. 2022, 23, 2850. [Google Scholar] [CrossRef]
- Bai, X. Stem Cell-Based Disease Modeling and Cell Therapy. Cells 2020, 9, 2193. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem Cell-Based Therapy for Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Yadid, M.; Oved, H.; Silberman, E.; Dvir, T. Bioengineering Approaches to Treat the Failing Heart: From Cell Biology to 3D Printing. Nat. Rev. Cardiol. 2022, 19, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted Drug Delivery Strategies for Precision Medicines. Nat. Rev. Mater. 2021, 6, 351–370. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, L.; Lo, Y.; Shiu, S.C.-C.; Kinghorn, A.B.; Tanner, J.A. Aptamer-Enabled Nanomaterials for Therapeutics, Drug Targeting and Imaging. Cells 2022, 11, 159. [Google Scholar] [CrossRef]
- Mosallaei, M.; Simonian, M.; Ehtesham, N.; Karimzadeh, M.R.; Vatandoost, N.; Negahdari, B.; Salehi, R. Genetically Engineered Mesenchymal Stem Cells: Targeted Delivery of Immunomodulatory Agents for Tumor Eradication. Cancer Gene Ther. 2020, 27, 854–868. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Yu, M.; Serpooshan, V.; Wu, J.C.; Langer, R.; Lee, R.T.; Karp, J.M.; Farokhzad, O.C. Multiscale Technologies for Treatment of Ischemic Cardiomyopathy. Nat. Nanotech 2017, 12, 845–855. [Google Scholar] [CrossRef]
- Jovic, D.; Yu, Y.; Wang, D.; Wang, K.; Li, H.; Xu, F.; Liu, C.; Liu, J.; Luo, Y. A Brief Overview of Global Trends in MSC-Based Cell Therapy. Stem Cell Rev. Rep. 2022, 18, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, S.A.; McInnes, I.B. Reflections on ‘Older’ Drugs: Learning New Lessons in Rheumatology. Nat. Rev. Rheumatol. 2020, 16, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Allabun, S.; Ojo, S.; Alqahtani, M.S.; Shukla, P.K.; Abbas, M.; Wechtaisong, C.; Almohiy, H.M. Enhanced Drug Delivery System Using Mesenchymal Stem Cells and Membrane-Coated Nanoparticles. Molecules 2023, 28, 2130. [Google Scholar] [CrossRef]
- Auffinger, B.; Morshed, R.; Tobias, A.; Cheng, Y.; Ahmed, A.U.; Lesniak, M.S. Drug-Loaded Nanoparticle Systems And Adult Stem Cells: A Potential Marriage For The Treatment Of Malignant Glioma? Oncotarget 2013, 4, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, S.; Guo, J.; Alahdal, M.; Cao, S.; Yang, Z.; Zhang, F.; Shen, Y.; Sun, M.; Mo, R.; et al. Targeted Delivery of Doxorubicin by Nano-Loaded Mesenchymal Stem Cells for Lung Melanoma Metastases Therapy. Sci. Rep. 2017, 7, srep44758. [Google Scholar] [CrossRef] [PubMed]
- Thanuja, M.Y.; Anupama, C.; Ranganath, S.H. Bioengineered Cellular and Cell Membrane-Derived Vehicles for Actively Targeted Drug Delivery: So near and yet so Far. Adv. Drug Deliv. Rev. 2018, 132, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, B.; Ferrari, M. Nanotechnology for Mesenchymal Stem Cell Therapies. J. Control. Release 2016, 240, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef]
- García-Bernal, D.; García-Arranz, M.; Yáñez, R.M.; Hervás-Salcedo, R.; Cortés, A.; Fernández-García, M.; Hernando-Rodríguez, M.; Quintana-Bustamante, Ó.; Bueren, J.A.; García-Olmo, D.; et al. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy. Front. Cell Dev. Biol. 2021, 9, 650664. [Google Scholar] [CrossRef]
- Kolf, C.M.; Cho, E.; Tuan, R.S. Mesenchymal Stromal Cells: Biology of Adult Mesenchymal Stem Cells: Regulation of Niche, Self-Renewal and Differentiation. Arthritis Res. Ther. 2007, 9, 204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Almeida-Porada, G.; Atala, A.J.; Porada, C.D. Therapeutic Mesenchymal Stromal Cells for Immunotherapy and for Gene and Drug Delivery. Mol. Ther. Methods Clin. Dev. 2020, 16, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Mahindran, E.; Wan Kamarul Zaman, W.S.; Ahmad Amin Noordin, K.B.; Tan, Y.-F.; Nordin, F. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Hype or Hope for Skeletal Muscle Anti-Frailty. Int. J. Mol. Sci. 2023, 24, 7833. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Pegtel, D.M.; Baldini, N. Mesenchymal Stem Cell Secreted Vesicles Provide Novel Opportunities in (Stem) Cell-Free Therapy. Front. Physiol. 2012, 3, 359. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-G.; Wang, J.-L.; Zhang, Y.-X.; Li, L.; Reza, A.M.M.T.; Gurunathan, S. Biogenesis, Composition and Potential Therapeutic Applications of Mesenchymal Stem Cells Derived Exosomes in Various Diseases. Int. J. Nanomed. 2023, 18, 3177–3210. [Google Scholar] [CrossRef]
- Park, K.-S.; Bandeira, E.; Shelke, G.V.; Lässer, C.; Lötvall, J. Enhancement of Therapeutic Potential of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Stem Cell Res. Ther. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Y.; Li, N.; Hua, J. Interaction between Mesenchymal Stem Cells and Immune Cells during Bone Injury Repair. Int. J. Mol. Sci. 2023, 24, 14484. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Lam, E.W.-F.; Soeiro, I.; Tisato, V.; Bonnet, D.; Dazzi, F. Mesenchymal Stem Cells Inhibit Proliferation and Apoptosis of Tumor Cells: Impact on in Vivo Tumor Growth. Leukemia 2007, 21, 304–310. [Google Scholar] [CrossRef]
- Abbasi, B.; Shamsasenjan, K.; Ahmadi, M.; Beheshti, S.A.; Saleh, M. Mesenchymal Stem Cells and Natural Killer Cells Interaction Mechanisms and Potential Clinical Applications. Stem Cell Res. Ther. 2022, 13, 97. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Su, C.-Y.; Cheng, H.-W.; Chu, C.-Y.; Jeng, L.-B.; Chiang, C.-S.; Shyu, W.-C.; Chen, S.-Y. Stem Cell–Nanomedicine System as a Theranostic Bio-Gadolinium Agent for Targeted Neutron Capture Cancer Therapy. Nat. Commun. 2023, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Liu, L.; Moore, C.; Hsu, E.; Zhang, A.; Ren, Z.; Sun, Z.; Wang, X.; Zhu, J.; Shen, J.; et al. IL-2 Delivery by Engineered Mesenchymal Stem Cells Re-Invigorates CD8+ T Cells to Overcome Immunotherapy Resistance in Cancer. Nat. Cell Biol. 2022, 24, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Liam-Or, R.; Faruqu, F.N.; Walters, A.; Han, S.; Xu, L.; Wang, J.T.-W.; Oberlaender, J.; Sanchez-Fueyo, A.; Lombardi, G.; Dazzi, F.; et al. Cellular Uptake and in Vivo Distribution of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles Are Protein Corona Dependent. Nat. Nanotechnol. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xin, Y.; Cao, H.; Li, W.; Hua, Y.; Webster, T.J.; Zhang, C.; Tang, W.; Liu, Z. Recent Advances in Mesen-Chymal Stem Cell Membrane-Coated Nanoparticles for Enhanced Drug Delivery. Biomater. Sci. 2021, 9, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, Y. Cell Membrane-Engineered Nanoparticles for Cancer Therapy. J. Mater. Chem. B 2022, 10, 7161–7172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, X. Stem Cell Membrane-Camouflaged Targeted Delivery System in Tumor. Mater. Today Bio 2022, 16, 100377. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-H.; Zhou, Y.; Tabata, Y.; Gao, J.-Q. Mesenchymal Stem Cell-Based Drug Delivery Strategy: From Cells to Biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Wardhan, R.; Mudgal, P. Introduction to Biomembranes. In Textbook of Membrane Biology; Wardhan, R., Mudgal, P., Eds.; Springer: Singapore, 2017; pp. 1–28. ISBN 978-981-10-7101-0. [Google Scholar]
- Alonso-Goulart, V.; Ferreira, L.B.; Duarte, C.A.; de Lima, I.L.; Ferreira, E.R.; de Oliveira, B.C.; Vargas, L.N.; de Moraes, D.D.; Silva, I.B.B.; de O. Faria, R.; et al. Mesenchymal Stem Cells from Human Adipose Tissue and Bone Repair: A Literature Review. Biotechnol. Res. Innov. 2018, 2, 74–80. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, S.; Zhang, S.; Wang, L.; Yuan, H.; Hu, F. Cell Membrane Coated-Nanoparticles for Cancer Immunotherapy. Acta Pharm. Sin. B 2022, 12, 3233–3254. [Google Scholar] [CrossRef]
- Casula, E.; Traversari, G.; Fadda, S.; Klymenko, O.V.; Kontoravdi, C.; Cincotti, A. Modelling the Osmotic Behaviour of Human Mesenchymal Stem Cells. Biochem. Eng. J. 2019, 151, 107296. [Google Scholar] [CrossRef]
- Wright, A.; Snyder, L.; Knights, K.; He, H.; Springer, N.L.; Lillich, J.; Weiss, M.L. A Protocol for the Isolation, Culture, and Cryopreservation of Umbilical Cord-Derived Canine Mesenchymal Stromal Cells: Role of Cell Attachment in Long-Term Maintenance. Stem Cells Dev. 2020, 29, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wei, A.; Gao, Z.; Mu, X. Current Progress of Mesenchymal Stem Cell Membrane-Camouflaged Nanoparticles for Targeted Therapy. Biomed. Pharmacother. 2023, 161, 114451. [Google Scholar] [CrossRef] [PubMed]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation Techniques of Stem Cells Extracellular Vesicles: A Gate for Manufacturing of Clinical Grade Therapeutic Extracellular Vesicles and Long-Term Clinical Trials. Int. J. Vet. Sci. Med. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Piras, A.M.; Dash, M. Cell Membrane Coated Nanocarriers—An Efficient Biomimetic Platform for Targeted Therapy. J. Control. Release 2020, 327, 546–570. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, N.; Pishavar, E.; Baradaran, B.; Oroojalian, F.; Mokhtarzadeh, A. Stem Cell Membrane, Stem Cell-Derived Exosomes and Hybrid Stem Cell Camouflaged Nanoparticles: A Promising Biomimetic Nanoplatforms for Cancer Theranostics. J. Control. Release 2022, 348, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Cho, H.; Lee, S.; Kim, K. Biomimetic Nanoparticles Coated with Bacterial Outer Membrane Vesicles as a New-Generation Platform for Biomedical Applications. Pharmaceutics 2021, 13, 1887. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Faria, I.; Yousefiasl, S.; Macário-Soares, A.; Pereira-Silva, M.; Peixoto, D.; Zafar, H.; Raza, F.; Faneca, H.; Veiga, F.; Hamblin, M.R.; et al. Stem Cell Membrane-Coated Abiotic Nanomaterials for Biomedical Applications. J. Control. Release 2022, 351, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Borbolla, A.; García-Hevia, L.; Fanarraga, M.L. Cell Membrane-Coated Nanoparticles for Precision Medicine: A Comprehensive Review of Coating Techniques for Tissue-Specific Therapeutics. Int. J. Mol. Sci. 2024, 25, 2071. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, S.; Yu, Z.; Wang, Y.; Zhang, D.; Cao, C.; Wang, Z.; Cui, D.; Chen, D. Recent Advances in Microfluidic-Based Electroporation Techniques for Cell Membranes. Lab. Chip 2022, 22, 2624–2646. [Google Scholar] [CrossRef]
- Hu, H.; Yang, C.; Zhang, F.; Li, M.; Tu, Z.; Mu, L.; Dawulieti, J.; Lao, Y.-H.; Xiao, Z.; Yan, H.; et al. A Versatile and Robust Platform for the Scalable Manufacture of Biomimetic Nanovaccines. Adv. Sci. 2021, 8, 2002020. [Google Scholar] [CrossRef]
- Liu, L.; Yu, W.; Seitsonen, J.; Xu, W.; Lehto, V.-P. Correct Identification of the Core-Shell Structure of Cell Membrane-Coated Polymeric Nanoparticles. Chem. A Eur. J. 2022, 28, e202200947. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.H.; Hu, C.-M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, P.Y.; Deng, J.; Bady, S.C.; Cheng, H. Cell Membrane Coating for Reducing Nanoparticle-Induced Inflammatory Responses to Scaffold Constructs. Nano Res. 2018, 11, 5573–5583. [Google Scholar] [CrossRef]
- T. Luk, B.; Hu, C.-M.J.; H. Fang, R.; Dehaini, D.; Carpenter, C.; Gao, W.; Zhang, L. Interfacial Interactions between Natural RBC Membranes and Synthetic Polymeric Nanoparticles. Nanoscale 2014, 6, 2730–2737. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Meng, Q.-F.; Bu, L.-L.; Cai, B.; Huang, Q.; Sun, Z.-J.; Zhang, W.-F.; Li, A.; Guo, S.-S.; Liu, W.; et al. Erythrocyte Membrane-Coated Upconversion Nanoparticles with Minimal Protein Adsorption for Enhanced Tumor Imaging. ACS Appl. Mater. Interfaces 2017, 9, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pan, D.; Chen, S.; Martikainen, M.-V.; Kårlund, A.; Ke, J.; Pulkkinen, H.; Ruhanen, H.; Roponen, M.; Käkelä, R.; et al. Systematic Design of Cell Membrane Coating to Improve Tumor Targeting of Nanoparticles. Nat. Commun. 2022, 13, 6181. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Fang, Z.-S.; Chen, Y.-T.; Chen, Y.-I.; Yao, B.-Y.; Cheng, J.-Y.; Chien, C.-Y.; Chang, Y.-C.; Hu, C.-M.J. Targeting and Enrichment of Viral Pathogen by Cell Membrane Cloaked Magnetic Nanoparticles for Enhanced Detection. ACS Appl. Mater. Interfaces 2017, 9, 39953–39961. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ai, Y.; Wang, L.; Bu, P.; Sharkey, C.C.; Wu, Q.; Wun, B.; Roy, S.; Shen, X.; King, M.R. Targeted Drug Delivery to Circulating Tumor Cells via Platelet Membrane-Functionalized Particles. Biomaterials 2016, 76, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Su, Y.-Y.; Jiang, X.-C.; Gao, J.-Q. Cell Membrane-Coated Nanoparticles: A Novel Multifunctional Biomimetic Drug Delivery System. Drug Deliv. Transl. Res. 2023, 13, 716–737. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, D.; Wang, H.; Zhang, P. Recent Advances in Cell Membrane Coated-Nanoparticles as Drug Delivery Systems for Tackling Urological Diseases. Pharmaceutics 2023, 15, 1899. [Google Scholar] [CrossRef]
- Molinaro, R.; Evangelopoulos, M.; Hoffman, J.R.; Corbo, C.; Taraballi, F.; Martinez, J.O.; Hartman, K.A.; Cosco, D.; Costa, G.; Romeo, I.; et al. Design and Development of Biomimetic Nanovesicles Using a Microfluidic Approach. Adv. Mater. 2018, 30, 1702749. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red Blood Cell Membrane-Camouflaged Nanoparticles: A Novel Drug Delivery System for Antitumor Application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Jan, N.; Madni, A.; Khan, S.; Shah, H.; Akram, F.; Khan, A.; Ertas, D.; Bostanudin, M.F.; Contag, C.H.; Ashammakhi, N.; et al. Biomimetic Cell Membrane-coated Poly(Lactic- Co -glycolic Acid) Nanoparticles for Biomedical Applications. Bioeng. Transl. Med. 2022, 8, e10441. [Google Scholar] [CrossRef]
- Ma, Q.; Fan, Q.; Xu, J.; Bai, J.; Han, X.; Dong, Z.; Zhou, X.; Liu, Z.; Gu, Z.; Wang, C. Calming Cytokine Storm in Pneumonia by Targeted Delivery of TPCA-1 Using Platelet-Derived Extracellular Vesicles. Matter 2020, 3, 287–301. [Google Scholar] [CrossRef]
- Clavreul, A.; Montagu, A.; Lainé, A.-L.; Tétaud, C.; Lautram, N.; Franconi, F.; Passirani, C.; Vessières, A.; Montero-Menei, C.N.; Menei, P. Targeting and Treatment of Glioblastomas with Human Mesenchymal Stem Cells Carrying Ferrociphenol Lipid Nanocapsules. Int. J. Nanomed. 2015, 10, 1259–1271. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Misra, S.; Chopra, K.; Saikia, U.N.; Sinha, V.R.; Sehgal, R.; Modi, M.; Medhi, B. Effect of Mesenchymal Stem Cells and Galantamine Nanoparticles in Rat Model of Alzheimer’s Disease. Regen. Med. 2016, 11, 629–646. [Google Scholar] [CrossRef]
- Roger, M.; Clavreul, A.; Huynh, N.T.; Passirani, C.; Schiller, P.; Vessières, A.; Montero-Menei, C.; Menei, P. Ferrociphenol Lipid Nanocapsule Delivery by Mesenchymal Stromal Cells in Brain Tumor Therapy. Int. J. Pharm. 2012, 423, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bou Haidar, N.; Marais, S.; Dé, E.; Schaumann, A.; Barreau, M.; Feuilloley, M.G.J.; Duncan, A.C. Chronic Wound Healing: A Specific Antibiofilm Protein-Asymmetric Release System. Mater. Sci. Eng. C 2020, 106, 110130. [Google Scholar] [CrossRef]
- Choudhury, H.; Maheshwari, R.; Pandey, M.; Tekade, M.; Gorain, B.; Tekade, R.K. Advanced Nanoscale Carrier-Based Approaches to Overcome Biopharmaceutical Issues Associated with Anticancer Drug ‘Etoposide’. Mater. Sci. Eng. C 2020, 106, 110275. [Google Scholar] [CrossRef]
- Yang, N.; Ding, Y.; Zhang, Y.; Wang, B.; Zhao, X.; Cheng, K.; Huang, Y.; Taleb, M.; Zhao, J.; Dong, W.-F.; et al. Surface Functionalization of Polymeric Nanoparticles with Umbilical Cord-Derived Mesenchymal Stem Cell Membrane for Tumor-Targeted Therapy. ACS Appl. Mater. Interfaces 2018, 10, 22963–22973. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.; Ouyang, X.; Wang, J.; Sun, X.; Lv, Y. Mesenchymal Stem Cells Loaded with Paclitaxel-Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Glioma-Targeting Therapy. Int. J. Nanomed. 2018, 13, 5231–5248. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lin, Z.; Jurado-Sánchez, B.; Lin, X.; Wu, Z.; He, Q. Stem Cell Membrane-Coated Nanogels for Highly Effi-Cient in Vivo Tumor Targeted Drug Delivery. Small 2016, 12, 4056–4062. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, Y.; Kamareddine, M.H.; Tawk, A.; Elia, C.; El Mahmoud, A.; Terro, K.; El Harake, N.; El-Baba, B.; Makdessi, J.; Farhat, S. Inorganic Nanoparticles as Drug Delivery Systems and Their Potential Role in the Treatment of Chronic Myelogenous Leukaemia. Technol. Cancer Res. Treat. 2019, 18, 1533033819853241. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, C.; Liu, M.; Huang, J.; Jin, X.; Zhu, C.; Lv, M.; Yang, N.; Chen, S.; Shao, M.; et al. Nucleus-Targeting Manganese Dioxide Nanoparticles Coated with the Human Umbilical Cord Mesenchymal Stem Cell Membrane for Cancer Cell Therapy. ACS Appl. Mater. Interfaces 2023, 15, 10541–10553. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guan, Y.; Liu, H.; Hao, N.; Liu, T.; Meng, X.; Fu, C.; Li, Y.; Qu, Q.; Zhang, Y.; et al. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS Nano 2011, 5, 7462–7470. [Google Scholar] [CrossRef] [PubMed]
- Örgül, D.; Eroğlu, H.; Tiryaki, M.; Pınarlı, F.A.; Hekimoglu, S. In-Vivo Evaluation of Tissue Scaffolds Containing Simvastatin Loaded Nanostructured Lipid Carriers and Mesenchymal Stem Cells in Diabetic Wound Healing. J. Drug Deliv. Sci. Technol. 2021, 61, 102140. [Google Scholar] [CrossRef]
- Raghav, P.K.; Mann, Z.; Ahlawat, S.; Mohanty, S. Mesenchymal Stem Cell-Based Nanoparticles and Scaffolds in Regenerative Medicine. Eur. J. Pharmacol. 2022, 918, 174657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Lee, S.B.; Cho, S.-G. The Impact of Metallic Nanoparticles on Stem Cell Proliferation and Differentiation. Nanomaterials 2018, 8, 761. [Google Scholar] [CrossRef]
- Kwon, S.; Yoo, K.H.; Sym, S.J.; Khang, D. Mesenchymal Stem Cell Therapy Assisted by Nanotechnology: A Possible Combinational Treatment for Brain Tumor and Central Nerve Regeneration. IJN 2019, 14, 5925–5942. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Differentiation of Human Neural Stem Cells into Neural Networks on Graphene Nanogrids. J. Mater. Chem. B 2013, 1, 6291–6301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Cai, Y.; Jiang, Y.; Lin, X. Exosomes in Osteoarthritis and Cartilage Injury: Advanced Development and Potential Therapeutic Strategies. Int. J. Biol. Sci. 2020, 16, 1811. [Google Scholar] [CrossRef] [PubMed]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Kim, J. Mesenchymal Stem Cell-Derived Exosomes: Applications in Cell-Free Therapy. Korean J. Clin. Lab. Sci. 2018, 50, 391–398. [Google Scholar] [CrossRef]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. BioMed Res. Int. 2019, 2019, 6458237. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal Stem Cell Derived-Exosomes: A Modern Approach in Translational Medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, L.; Kelishadrokhi, A.F.; Soleimanizadeh, A.; Rahmati, S. Mesenchymal Stem Cell (MSC)-Derived Exosomes as a Cell-Free Therapy for Patients Infected with COVID-19: Real Opportunities and Range of Promises. Chem. Phys. Lipids 2021, 234, 105009. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fang, F.; Sun, M.; Zhang, Y.; Hu, M.; Zhang, J. Extracellular Vesicles as Bioactive Nanotherapeutics: An Emerging Paradigm for Regenerative Medicine. Theranostics 2022, 12, 4879–4903. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Huai, Q.; Zhu, C.; Zhang, X.; Dai, H.; Li, X.; Wang, H. Mesenchymal Stromal/Stem Cells and Their Extracellular Vesicles in Liver Diseases: Insights on Their Immunomodulatory Roles and Clinical Applications. Cell Biosci. 2023, 13, 162. [Google Scholar] [CrossRef]
- Abreu, S.C.; Lopes-Pacheco, M.; Weiss, D.J.; Rocco, P.R.M. Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Perspectives. Front. Cell Dev. Biol. 2021, 9, 600711. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, J.; Cai, J.; Xiao, J.; Sui, X.; Yuan, X.; Li, R.; Li, Y.; Yao, J.; Lv, G. Extracellular Vesicles Derived from Mesenchymal Stromal Cells as Nanotherapeutics for Liver Ischaemia–Reperfusion Injury by Transferring Mitochondria to Modulate the Formation of Neutrophil Extracellular Traps. Biomaterials 2022, 284, 121486. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-Y.; Yang, M.-Y.; Yeh, C.-A.; Yang, Y.-C.; Chang, K.-B.; Chen, K.-Y.; Liu, S.-Y.; Tang, C.-L.; Shen, C.-C.; Hung, H.-S. Therapeutic Applications of Mesenchymal Stem Cell Loaded with Gold Nanoparticles for Regenerative Medicine. Pharmaceutics 2023, 15, 1385. [Google Scholar] [CrossRef]
- D’Atri, D.; Zerrillo, L.; Garcia, J.; Oieni, J.; Lupu-Haber, Y.; Schomann, T.; Chan, A.; Cruz, L.J.; Creemers, L.B.; Machluf, M. Nanoghosts: Mesenchymal Stem Cells Derived Nanoparticles as a Unique Approach for Cartilage Regeneration. J. Control. Release 2021, 337, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Aravindhan, S.; Ejam, S.S.; Lafta, M.H.; Markov, A.; Yumashev, A.V.; Ahmadi, M. Mesenchymal Stem Cells and Cancer Therapy: Insights into Targeting the Tumour Vasculature. Cancer Cell Int. 2021, 21, 158. [Google Scholar] [CrossRef] [PubMed]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; De Pascalis, I.; Coccè, V.; Bonomi, A.; Pascucci, L.; Alessandri, G.; Pessina, A.; et al. Mesenchymal Stromal Cells Loaded with Paclitaxel Induce Cytotoxic Damage in Glioblastoma Brain Xenografts. Stem Cell Res. Ther. 2015, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Bhang, S.H.; Hwang, S.; Yoon, J.-K.; Song, J.; Jang, H.-K.; Kim, S.; Kim, B.-S. Mesenchymal Stem Cells Aggregate and Deliver Gold Nanoparticles to Tumors for Photothermal Therapy. ACS Nano 2015, 9, 9678–9690. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.J.; Cabaña-Muñoz, M.E. Nanoparticles and Mesenchymal Stem Cell (MSC) Therapy for Cancer Treatment: Focus on Nanocarriers and a Si-RNA CXCR4 Chemokine Blocker as Strategies for Tumor Eradication In Vitro and In Vivo. Micromachines 2023, 14, 2068. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Xiao, Y.; Xiao, Y.; Guo, Q.; Li, C.; Huang, Y.; Deng, Q.; Wen, J.; Zhou, F.; Luo, X.-H. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MiR-29b-3p Regulates Aging-Associated Insulin Resistance. ACS Nano 2019, 13, 2450–2462. [Google Scholar] [CrossRef]
- Liu, H.; Sun, R.; Wang, L.; Chen, X.; Li, G.; Cheng, Y.; Zhai, G.; Bay, B.-H.; Yang, F.; Gu, N.; et al. Biocompatible Iron Oxide Nanoring-Labeled Mesenchymal Stem Cells: An Innovative Magnetothermal Approach for Cell Tracking and Targeted Stroke Therapy. ACS Nano 2022, 16, 18806–18821. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lemaster, J.E.; Chen, F.; Li, J.; Jokerst, J.V. Photoacoustic Imaging of Human Mesenchymal Stem Cells Labeled with Prussian Blue–Poly(l-Lysine) Nanocomplexes. ACS Nano 2017, 11, 9022–9032. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, M.; Cui, X.; Liu, Y.; Liu, Y.; Deng, S.; Yuan, T.; Fan, Y.; Wang, Q.; Zhang, X. Cell-Free Osteoarthritis Treatment with Sustained-Release of Chondrocyte-Targeting Exosomes from Umbilical Cord-Derived Mesenchymal Stem Cells to Rejuvenate Aging Chondrocytes. ACS Nano 2023, 17, 13358–13376. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.R.; Jian, L.; Phua, L.C.; Ho, H.K.; Ren, Y.; Pastorin, G. Thin Films of Functionalized Multiwalled Carbon Nanotubes as Suitable Scaffold Materials for Stem Cells Proliferation and Bone Formation. ACS Nano 2010, 4, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug Through True Active Tumor Targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Gangadaran, P.; Rajendran, R.L.; Zhu, L.; Oh, J.M.; Lee, H.W.; Gopal, A.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; et al. A New Approach for Loading Anticancer Drugs Into Mesenchymal Stem Cell-Derived Exosome Mimetics for Cancer Therapy. Front. Pharmacol. 2018, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hong, W.; Ren, W.; Xu, T.; Qian, Z.; He, Z. Recent Progress in Targeted Delivery Vectors Based on Biomimetic Nanoparticles. Signal Transduct. Target. Ther. 2021, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic Roles of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Cao, Y.; Cao, M.; Wang, Y.; Cao, Y.; Gong, T. Nanomedicine in Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, T.; Xie, X.; Feng, Y.; Chen, Z.; Yang, H.; Wu, C.; Deng, S.; Liu, Y. PLGA-Based Drug Delivery Systems for Remotely Triggered Cancer Therapeutic and Diagnostic Applications. Front. Bioeng. Biotechnol. 2020, 8, 381. [Google Scholar] [CrossRef]
- Roger, M.; Clavreul, A.; Venier-Julienne, M.-C.; Passirani, C.; Sindji, L.; Schiller, P.; Montero-Menei, C.; Menei, P. Mesenchymal Stem Cells as Cellular Vehicles for Delivery of Nanoparticles to Brain Tumors. Biomaterials 2010, 31, 8393–8401. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liu, S.; Jia, S.; Xu, F. Emerging Frontiers in Drug Delivery with Special Focus on Novel Techniques for Targeted Therapies. Biomed. Pharmacother. 2023, 165, 115049. [Google Scholar] [CrossRef] [PubMed]
- Lalu, M.M.; McIntyre, L.; Pugliese, C.; Fergusson, D.; Winston, B.W.; Marshall, J.C.; Granton, J.; Stewart, D.J.; Group, C.C.C.T. Safe Ty of Cell Therapy with Mesenchymal Stromal Cells (Safecell): A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2012, 7, e47559. [Google Scholar] [CrossRef] [PubMed]
- Accomasso, L.; Gallina, C.; Turinetto, V.; Giachino, C. Stem Cell Tracking with Nanoparticles for Regenerative Medicine Purposes: An Overview. Stem Cells Int. 2016, 2016, 7920358. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.; Yin, L.; Liu, Z. The Roles of Nanoparticles in Stem Cell-Based Therapy for Cardiovascular Disease. Front. Bioeng. Biotechnol. 2020, 8, 947. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Jiang, X.; Li, Y.; Gao, J. Inorganic Nanoparticle-integrated Mesenchymal Stem Cells: A Potential Biological Agent for Multifaceted Applications. MedComm 2023, 4, e313. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Mesgin, R.M.; Nazari-Khanamiri, F.; Abdyazdani, N.; Imani, Z.; Talatapeh, S.P.; Nourmohammadi, A.; Nejati, V.; Rezaie, J. Mesenchymal Stem Cells-Derived Exosomes: Novel Carriers for Nanoparticle to Combat Cancer. Eur. J. Med. Res. 2023, 28, 579. [Google Scholar] [CrossRef] [PubMed]
- Satija, N.K.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, S.; Afrin, F.; Sharma, M.; Sharma, P.; Tripathi, R.P.; Gurudutta, G.U. Mesenchymal Stem Cell-Based Therapy: A New Paradigm in Regenerative Medicine. J. Cell Mol. Med. 2009, 13, 4385–4402. [Google Scholar] [CrossRef] [PubMed]
- Gallina, C.; Capelôa, T.; Saviozzi, S.; Accomasso, L.; Catalano, F.; Tullio, F.; Martra, G.; Penna, C.; Pagliaro, P.; Turinetto, V.; et al. Human Mesenchymal Stem Cells Labelled with Dye-Loaded Amorphous Silica Nanoparticles: Long-Term Biosafety, Stemness Preservation and Traceability in the Beating Heart. J. Nanobiotechnol 2015, 13, 77. [Google Scholar] [CrossRef]
- Pacheco-Herrero, M.; Soto-Rojas, L.O.; Reyes-Sabater, H.; Garcés-Ramirez, L.; de la Cruz López, F.; Villanueva-Fierro, I.; Luna-Muñoz, J. Current Status and Challenges of Stem Cell Treatment for Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 84, 917–935. [Google Scholar] [CrossRef]
- Dhada, K.S.; Hernandez, D.S.; Suggs, L.J. In Vivo Photoacoustic Tracking of Mesenchymal Stem Cell Viability. ACS Nano 2019, 13, 7791–7799. [Google Scholar] [CrossRef] [PubMed]
- Abudurexiti, M.; Zhao, Y.; Wang, X.; Han, L.; Liu, T.; Wang, C.; Yuan, Z. Bio-Inspired Nanocarriers Derived from Stem Cells and Their Extracellular Vesicles for Targeted Drug Delivery. Pharmaceutics 2023, 15, 2011. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Sun, J.; Ma, T.; Hua, F.; Shen, Z. A Brief Review of Cytotoxicity of Nanoparticles on Mesenchymal Stem Cells in Regenerative Medicine. IJN 2019, 14, 3875–3892. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.K.; Fitzpatrick, J.A.J.; Phillippi, J.A.; Andreko, S.; Waggoner, A.S.; Bruchez, M.P.; Ballou, B. Cholera Toxin B Conjugated Quantum Dots for Live Cell Labeling. Nano Lett. 2007, 7, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.S.; Clark, P.A.; Moioli, E.K.; Stroscio, M.A.; Mao, J.J. Labeling of Mesenchymal Stem Cells by Bioconjugated Quantum Dots. Nano Lett. 2007, 7, 3071–3079. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, G.; Wang, C.; Wang, H.; Yang, B.; Guan, F.; Li, D.; Feng, X. Biocompatibility of Quantum Dots (CdSe/ZnS) in Human Amniotic Membrane-Derived Mesenchymal Stem Cells in Vitro. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2015, 159, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-C.; Wang, F.-F.; Hung, S.-C.; Chen, Y.-J.; Wang, Y.-J. The Internalized CdSe/ZnS Quantum Dots Impair the Chondrogenesis of Bone Marrow Mesenchymal Stem Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79, 95–101. [Google Scholar] [CrossRef]
- Hsieh, S.-C.; Wang, F.-F.; Lin, C.-S.; Chen, Y.-J.; Hung, S.-C.; Wang, Y.-J. The Inhibition of Osteogenesis with Human Bone Marrow Mesenchymal Stem Cells by CdSe/ZnS Quantum Dot Labels. Biomaterials 2006, 27, 1656–1664. [Google Scholar] [CrossRef]
- Liu, H.; Tang, W.; Li, C.; Lv, P.; Wang, Z.; Liu, Y.; Zhang, C.; Bao, Y.; Chen, H.; Meng, X.; et al. CdSe/ZnS Quantum Dots-Labeled Mesenchymal Stem Cells for Targeted Fluorescence Imaging of Pancreas Tissues and Therapy of Type 1 Diabetic Rats. Nanoscale Res. Lett. 2015, 10, 265. [Google Scholar] [CrossRef]
- Yan, J.; Hou, S.; Yu, Y.; Qiao, Y.; Xiao, T.; Mei, Y.; Zhang, Z.; Wang, B.; Huang, C.-C.; Lin, C.-H.; et al. The Effect of Surface Charge on the Cytotoxicity and Uptake of Carbon Quantum Dots in Human Umbilical Cord Derived Mesenchymal Stem Cells. Colloids Surf. B Biointerfaces 2018, 171, 241–249. [Google Scholar] [CrossRef]
- Kim, J.; Ho Song, S.; Jin, Y.; Park, H.-J.; Yoon, H.; Jeon, S.; Cho, S.-W. Multiphoton Luminescent Graphene Quantum Dots for in Vivo Tracking of Human Adipose-Derived Stem Cells. Nanoscale 2016, 8, 8512–8519. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Li, D.; Mou, X.; Li, J.; Guo, W.; Wang, S.; Yu, X.; Ma, B.; Zhang, S.; Tang, W.; et al. Effects of Graphene Quantum Dots on the Self-Renewal and Differentiation of Mesenchymal Stem Cells. Adv. Healthc. Mater. 2016, 5, 702–710. [Google Scholar] [CrossRef]
- Popara, J.; Accomasso, L.; Vitale, E.; Gallina, C.; Roggio, D.; Iannuzzi, A.; Raimondo, S.; Rastaldo, R.; Alberto, G.; Catalano, F.; et al. Silica Nanoparticles Actively Engage With Mesenchymal Stem Cells in Improving Acute Functional Cardiac Integration. Nanomedicine 2018, 13, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Li, Y.; Huang, Q.; He, W.; Zhang, R.; Feng, Q.; Benayahu, D. The Negative Effect of Silica Nanoparticles on Adipogenic Differentiation of Human Mesenchymal Stem Cells. Mater. Sci. Eng. C 2017, 81, 341–348. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; He, W.; Zhang, R.; Feng, Q.; Benayahu, D. The Stimulatory Effect of Silica Nanoparticles on Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biomed. Mater. 2016, 12, 015001. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; de La Torre, P.; Manzano, M.; Cabañas, M.V.; Flores, A.I.; Vallet-Regí, M. Decidua-Derived Mesenchymal Stem Cells as Carriers of Mesoporous Silica Nanoparticles: In Vitro and in Vivo Evaluation on Mammary Tumors. Acta Biomater. 2016, 33, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-M.; Chung, T.-H.; Hung, Y.; Lu, F.; Wu, S.-H.; Mou, C.-Y.; Yao, M.; Chen, Y.-C. Internalization of Mesoporous Silica Nanoparticles Induces Transient but Not Sufficient Osteogenic Signals in Human Mesenchymal Stem Cells. Toxicol. Appl. Pharmacol. 2008, 231, 208–215. [Google Scholar] [CrossRef]
- Liu, H.-M.; Wu, S.-H.; Lu, C.-W.; Yao, M.; Hsiao, J.-K.; Hung, Y.; Lin, Y.-S.; Mou, C.-Y.; Yang, C.-S.; Huang, D.-M.; et al. Mesoporous Silica Nanoparticles Improve Magnetic Labeling Efficiency in Human Stem Cells. Small 2008, 4, 619–626. [Google Scholar] [CrossRef]
- Accomasso, L.; Rocchietti, E.C.; Raimondo, S.; Catalano, F.; Alberto, G.; Giannitti, A.; Minieri, V.; Turinetto, V.; Orlando, L.; Saviozzi, S.; et al. Fluorescent Silica Nanoparticles Improve Optical Imaging of Stem Cells Allowing Direct Discrimination between Live and Early-Stage Apoptotic Cells. Small 2012, 8, 3192–3200. [Google Scholar] [CrossRef]
- Huang, D.-M.; Hung, Y.; Ko, B.-S.; Hsu, S.-C.; Chen, W.-H.; Chien, C.-L.; Tsai, C.-P.; Kuo, C.-T.; Kang, J.-C.; Yang, C.-S.; et al. Highly Efficient Cellular Labeling of Mesoporous Nanoparticles in Human Mesenchymal Stem Cells: Implication for Stem Cell Tracking. FASEB J. 2005, 19, 2014–2016. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Xiao, J.-J.; Lu, Y.-T.; Li, W.; Duan, Y.-W.; Sheng, Z.-H.; Li, S.-L. Effect of superparamagnetic iron oxide on differentiation of rat bone marrow stem cells into chondrocytes in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2017, 37, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-L.; Zhang, J.-Z.; Lu, L.-J.; Mao, J.-J.; Cao, M.-H.; Mao, X.-H.; Zhang, F.; Duan, X.-H.; Zheng, C.-S.; Zhang, L.-M.; et al. Superparamagnetic Iron Oxide Nanoparticles-Complexed Cationic Amylose for In Vivo Magnetic Resonance Imaging Tracking of Transplanted Stem Cells in Stroke. Nanomaterials 2017, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ma, W.; Zhang, B.; Xie, Q. The Labeling of Stem Cells by Superparamagnetic Iron Oxide Nanoparticles Modified with PEG/PVP or PEG/PEI. Mater. Sci. Eng. C 2016, 62, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-L.; Zhang, Z.; Jiang, H.-S.; Chen, H.; Chen, Y.; Dai, Y.-T. Superparamagnetic Iron Oxide Nanoparticle Targeting of Adipose Tissue-Derived Stem Cells in Diabetes-Associated Erectile Dysfunction. Asian J. Androl. 2017, 19, 425. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Ku, J.H. Monitoring Transplanted Human Mesenchymal Stem Cells in Rat and Rabbit Bladders Using Molecular Magnetic Resonance Imaging. Neurourol. Urodyn. 2007, 26, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, G.J.-R.; Jacquart, M.; Lemaire, L.; Sindji, L.; Franconi, F.; Le Jeune, J.-J.; Montero-Menei, C.N. Mesenchymal and Neural Stem Cells Labeled with HEDP-Coated SPIO Nanoparticles: In Vitro Characterization and Migration Potential in Rat Brain. Brain Res. 2009, 1255, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Hsiao, J.-K.; Tai, M.-F.; Chen, S.-T.; Cheng, H.-Y.; Wang, J.-L.; Liu, H.-M. Direct Labeling of hMSC with SPIO: The Long-Term Influence on Toxicity, Chondrogenic Differentiation Capacity, and Intracellular Distribution. Mol. Imaging Biol. 2011, 13, 443–451. [Google Scholar] [CrossRef]
- Bulte, J.W.M.; Kraitchman, D.L.; Mackay, A.M.; Pittenger, M.F. Chondrogenic Differentiation of Mesenchymal Stem Cells Is Inhibited after Magnetic Labeling with Ferumoxides. Blood 2004, 104, 3410–3413. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Q.; Chi, C.-W.; Yang, C.-X.; Yan, X.-P. Penetrating Peptide-Bioconjugated Persistent Nanophosphors for Long-Term Tracking of Adipose-Derived Stem Cells with Superior Signal-to-Noise Ratio. Anal. Chem. 2016, 88, 4114–4121. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, Y.; Yu, J.; Chiu, D.T.; Wu, C. Purification of Semiconducting Polymer Dots by Size Exclusion Chromatography Prior to Cytotoxicity Assay and Stem Cell Labeling. Anal. Chem. 2018, 90, 5569–5575. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Q.; Meng, Z.; Guo, L.; Tang, Y.; Liu, Z.; Yin, S.; Qin, W.; Yuan, Z.; Zhang, X.; et al. Bright Polymer Dots Tracking Stem Cell Engraftment and Migration to Injured Mouse Liver. Theranostics 2017, 7, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.E.; Hernández-Rivera, M.; Zaibaq, N.G.; Ajala, A.; da Graça Cabreira-Hansen, M.; Mowlazadeh-Haghighi, S.; Willerson, J.T.; Perin, E.C.; Muthupillai, R.; Wilson, L.J. A New High-Performance Gadonanotube-Polymer Hybrid Material for Stem Cell Labeling and Tracking by MRI. Contrast Media Mol. Imaging 2018, 2018, e2853736. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Chen, H.; Yang, H.; Wu, H.; Zhao, X.; Wang, H.; Chour, T.; Neofytou, E.; Ding, D.; Daldrup-Link, H.; et al. Photoacoustic Imaging of Embryonic Stem Cell-Derived Cardiomyocytes in Living Hearts with Ultrasensitive Semiconducting Polymer Nanoparticles. Adv. Funct. Mater. 2018, 28, 1704939. [Google Scholar] [CrossRef] [PubMed]
- Ricles, L.M.; Nam, S.Y.; Sokolov, K.; Emelianov, S.Y.; Suggs, L.J. Function of Mesenchymal Stem Cells Following Loading of Gold Nanotracers. Int. J. Nanomed. 2011, 6, 407–416. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Thangaraj, M.; Kempen, P.J.; Sinclair, R.; Gambhir, S.S. Photoacoustic Imaging of Mesenchymal Stem Cells in Living Mice via Silica-Coated Gold Nanorods. ACS Nano 2012, 6, 5920–5930. [Google Scholar] [CrossRef] [PubMed]
- Ricles, L.M.; Nam, S.Y.; Treviño, E.A.; Emelianov, S.Y.; Suggs, L.J. A Dual Gold Nanoparticle System for Mesenchymal Stem Cell Tracking. J. Mater. Chem. B 2014, 2, 8220–8230. [Google Scholar] [CrossRef]
- Del Mar Encabo-Berzosa, M.; Sancho-Albero, M.; Crespo, A.; Andreu, V.; Sebastian, V.; Irusta, S.; Arruebo, M.; Martín-Duque, P.; Santamaria, J. The Effect of PEGylated Hollow Gold Nanoparticles on Stem Cell Migration: Potential Application in Tissue Regeneration. Nanoscale 2017, 9, 9848–9858. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Chen, Y.; Kawazoe, N.; Chen, G. TEMPO-Conjugated Gold Nanoparticles for Reactive Oxygen Species Scavenging and Regulation of Stem Cell Differentiation. ACS Appl. Mater. Interfaces 2017, 9, 35683–35692. [Google Scholar] [CrossRef]
- Lee, D.; Heo, D.N.; Nah, H.R.; Lee, S.J.; Ko, W.-K.; Lee, J.S.; Moon, H.-J.; Bang, J.B.; Hwang, Y.-S.; Reis, R.L.; et al. Injectable Hydrogel Composite Containing Modified Gold Nanoparticles: Implication in Bone Tissue Regeneration. Int. J. Nanomed. 2018, 13, 7019–7031. [Google Scholar] [CrossRef]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or Cell Membrane-Based Drug Delivery Systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, H.; Ke, S.; Zhuo, L.; Wang, H. Latest Advances in Biomimetic Nanomaterials for Diagnosis and Treatment of Cardiovascular Disease. Front. Cardiovasc. Med. 2023, 9, 1037741. [Google Scholar] [CrossRef]
- Guan, X.; Xing, S.; Liu, Y. Engineered Cell Membrane-Camouflaged Nanomaterials for Biomedical Applications. Nanomaterials 2024, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Huang, Y.; Zheng, J. Renal Clearable Nanocarriers: Overcoming the Physiological Barriers for Precise Drug Delivery and Clearance. J. Control. Release 2020, 322, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal Stem Cells-Derived Exosomes for Drug Delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Javius-Jones, K.; Hong, S.; Park, H. Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform. IJN 2023, 18, 509–525. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Litvinova, L.S.; Shupletsova, V.V.; Khaziakhmatova, O.G.; Daminova, A.G.; Kudryavtseva, V.L.; Yurova, K.A.; Malashchenko, V.V.; Todosenko, N.M.; Popova, V.; Litvinov, R.I. Human Mesenchymal Stem Cells as a Carrier for a Cell-Mediated Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 796111. [Google Scholar] [CrossRef]
- Guido, C.; Maiorano, G.; Gutiérrez-Millán, C.; Cortese, B.; Trapani, A.; D’Amone, S.; Gigli, G.; Palamà, I.E. Erythrocytes and Nanoparticles: New Therapeutic Systems. Appl. Sci. 2021, 11, 2173. [Google Scholar] [CrossRef]
- De la Torre, P.; Pérez-Lorenzo, M.J.; Alcázar-Garrido, Á.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715. [Google Scholar] [CrossRef]
- Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria de la Torre, R.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of Mesenchymal Stromal Cells after Administration in Animal Models and Humans: A Systematic Review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef]
- Colino, C.I.; Lanao, J.M.; Gutierrez-Millan, C. Targeting of Hepatic Macrophages by Therapeutic Nanoparticles. Front. Immunol. 2020, 11, 218. [Google Scholar] [CrossRef]
- Cai, D.; Gao, W.; Li, Z.; Zhang, Y.; Xiao, L.; Xiao, Y. Current Development of Nano-Drug Delivery to Target Macrophages. Biomedicines 2022, 10, 1203. [Google Scholar] [CrossRef]
- Yang, X.; Meng, Y.; Han, Z.; Ye, F.; Wei, L.; Zong, C. Mesenchymal Stem Cell Therapy for Liver Disease: Full of Chances and Challenges. Cell Biosci. 2020, 10, 123. [Google Scholar] [CrossRef]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Nold, P.; Hartmann, R.; Feliu, N.; Kantner, K.; Gamal, M.; Pelaz, B.; Hühn, J.; Sun, X.; Jungebluth, P.; del Pino, P.; et al. Optimizing Conditions for Labeling of Mesenchymal Stromal Cells (MSCs) with Gold Nanoparticles: A Prerequisite for in Vivo Tracking of MSCs. J. Nanobiotechnol. 2017, 15, 24. [Google Scholar] [CrossRef]
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-Neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748. [Google Scholar] [CrossRef]
- Alagesan, S.; Brady, J.; Byrnes, D.; Fandiño, J.; Masterson, C.; McCarthy, S.; Laffey, J.; O’Toole, D. Enhancement Strategies for Mesenchymal Stem Cells and Related Therapies. Stem Cell Res. Ther. 2022, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2022, 9, 797359. [Google Scholar] [CrossRef]
- Mehta, K.J. Iron Oxide Nanoparticles in Mesenchymal Stem Cell Detection and Therapy. Stem Cell Rev. Rep. 2022, 18, 2234–2261. [Google Scholar] [CrossRef]
- Li, X.; Dai, B.; Guo, J.; Zheng, L.; Guo, Q.; Peng, J.; Xu, J.; Qin, L. Nanoparticle–Cartilage Interaction: Pathology-Based Intra-Articular Drug Delivery for Osteoarthritis Therapy. Nano-Micro Lett. 2021, 13, 149. [Google Scholar] [CrossRef]
- Rice, O.; Surian, A.; Chen, Y. Modeling the Blood-Brain Barrier for Treatment of Central Nervous System (CNS) Diseases. J. Tissue Eng. 2022, 13, 20417314221095997. [Google Scholar] [CrossRef]
- Soland, M.A.; Bego, M.; Colletti, E.; Zanjani, E.D.; Jeor, S.S.; Porada, C.D.; Almeida-Porada, G. Mesenchymal Stem Cells Engineered to Inhibit Complement-Mediated Damage. PLoS ONE 2013, 8, e60461. [Google Scholar] [CrossRef]
- He, X.; Hong, W.; Yang, J.; Lei, H.; Lu, T.; He, C.; Bi, Z.; Pan, X.; Liu, Y.; Dai, L.; et al. Spontaneous Apoptosis of Cells in Therapeutic Stem Cell Preparation Exert Immunomodulatory Effects through Release of Phosphatidylserine. Signal Transduct. Target. Ther. 2021, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- Tissue Engineering and Regenerative Medicine International Society Asia-Pacific Chapter Conference 2022. Tissue Eng. Part A 2022, 28, 1. [CrossRef]
- Kidd, S.; Spaeth, E.; Dembinski, J.L.; Dietrich, M.; Watson, K.; Klopp, A.; Battula, V.L.; Weil, M.; Andreeff, M.; Marini, F.C. Direct Evidence of Mesenchymal Stem Cell Tropism for Tumor and Wounding Microenvironments Using in Vivo Bioluminescent Imaging. Stem Cells 2009, 27, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezanezhad, A.; Mirpour, S.; Bagheri, M.; Mohamadnejad, M.; Alimoghaddam, K.; Abdolahzadeh, L.; Saghari, M.; Malekzadeh, R. In Vivo Tracking of 111In-Oxine Labeled Mesenchymal Stem Cells Following Infusion in Patients with Advanced Cirrhosis. Nucl. Med. Biol. 2011, 38, 961–967. [Google Scholar] [CrossRef]
- Li, X.; An, G.; Wang, Y.; Liang, D.; Zhu, Z.; Tian, L. Targeted Migration of Bone Marrow Mesenchymal Stem Cells Inhibits Silica-Induced Pulmonary Fibrosis in Rats. Stem Cell Res. Ther. 2018, 9, 335. [Google Scholar] [CrossRef]
- Wang, H.; Liang, X.; Xu, Z.P.; Crawford, D.H.G.; Liu, X.; Roberts, M.S. A Physiologically Based Kinetic Model for Elucidating the in Vivo Distribution of Administered Mesenchymal Stem Cells. Sci. Rep. 2016, 6, 22293. [Google Scholar] [CrossRef]
- Wang, S.; Guo, L.; Ge, J.; Yu, L.; Cai, T.; Tian, R.; Jiang, Y.; Zhao, R.C.; Wu, Y. Excess Integrins Cause Lung Entrapment of Mesenchymal Stem Cells. Stem Cells 2015, 33, 3315–3326. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S. Pulmonary Passage Is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Kraitchman, D.L.; Tatsumi, M.; Gilson, W.D.; Ishimori, T.; Kedziorek, D.; Walczak, P.; Segars, W.P.; Chen, H.H.; Fritzges, D.; Izbudak, I.; et al. Dynamic Imaging of Allogeneic Mesenchymal Stem Cells Trafficking to Myocardial Infarction. Circulation 2005, 112, 1451–1461. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kong, W.H.; Kim, H.; Hahn, S.K. Targeted Systemic Mesenchymal Stem Cell Delivery Using Hyaluronate—Wheat Germ Agglutinin Conjugate. Biomaterials 2016, 106, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, I.; Thibaud, J.-L.; de Fornel, P.; Cassano, M.; Punzón, I.; Mauduit, D.; Vilquin, J.-T.; Devauchelle, P.; Sampaolesi, M.; Blot, S. In Vivo Stem Cell Tracking Using Scintigraphy in a Canine Model of DMD. Sci. Rep. 2020, 10, 10681. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, P.; Spriet, M.; Sole, A.; Walker, N.J.; Vaughan, B.; Galuppo, L.D. Scintigraphic Tracking of Allogeneic Mesenchymal Stem Cells in the Distal Limb After Intra-Arterial Injection in Standing Horses. Vet. Surg. 2016, 45, 619–624. [Google Scholar] [CrossRef]
- Sierra-Parraga, J.M.; Munk, A.; Andersen, C.; Lohmann, S.; Moers, C.; Baan, C.C.; Ploeg, R.J.; Pool, M.; Keller, A.K.; Møller, B.K.; et al. Mesenchymal Stromal Cells Are Retained in the Porcine Renal Cortex Independently of Their Metabolic State After Renal Intra-Arterial Infusion. Stem Cells Dev. 2019, 28, 1224–1235. [Google Scholar] [CrossRef]
- Li, Z.; Hu, X.; Mao, J.; Liu, X.; Zhang, L.; Liu, J.; Li, D.; Shan, H. Optimization of Mesenchymal Stem Cells (MSCs) Delivery Dose and Route in Mice with Acute Liver Injury by Bioluminescence Imaging. Mol. Imaging Biol. 2015, 17, 185–194. [Google Scholar] [CrossRef]
- Mäkelä, T.; Takalo, R.; Arvola, O.; Haapanen, H.; Yannopoulos, F.; Blanco, R.; Ahvenjärvi, L.; Kiviluoma, K.; Kerkelä, E.; Nystedt, J.; et al. Safety and Biodistribution Study of Bone Marrow–Derived Mesenchymal Stromal Cells and Mononuclear Cells and the Impact of the Administration Route in an Intact Porcine Model. Cytotherapy 2015, 17, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Scarfe, L.; Taylor, A.; Sharkey, J.; Harwood, R.; Barrow, M.; Comenge, J.; Beeken, L.; Astley, C.; Santeramo, I.; Hutchinson, C.; et al. Non-Invasive Imaging Reveals Conditions That Impact Distribution and Persistence of Cells after in Vivo Administration. Stem Cell Res. Ther. 2018, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Zaw Thin, M.; Ogunlade, O.; Comenge, J.; Patrick, P.S.; Stuckey, D.J.; David, A.L.; Lythgoe, M.F.; Beard, P.; Kalber, T.L. Stem Cell Delivery to Kidney via Minimally Invasive Ultrasound-Guided Renal Artery Injection in Mice. Sci. Rep. 2020, 10, 7514. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, F.; Pan, S.; Liu, Z. Stem Cells: A Potential Treatment Option for Kidney Diseases. Stem Cell Res. Ther. 2020, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, M.Y.; Eom, Y.W.; Baik, S.K. Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives. Gut Liver 2020, 14, 306–315. [Google Scholar] [CrossRef]
- Sun, H.; Shi, C.; Ye, Z.; Yao, B.; Li, C.; Wang, X.; Qian, Q. The Role of Mesenchymal Stem Cells in Liver Injury. Cell Biol. Int. 2022, 46, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A. Mesenchymal Stem Cell Delivery Routes and Fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Mesenchymal Stem/Stromal Cells as next-Generation Drug Delivery Vehicles for Cancer Therapeutics. Expert Opin. Drug Deliv. 2021, 18, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Yudintceva, N.; Lomert, E.; Mikhailova, N.; Tolkunova, E.; Agadzhanian, N.; Samochernych, K.; Multhoff, G.; Timin, G.; Ryzhov, V.; Deriglazov, V.; et al. Targeting Brain Tumors with Mesenchymal Stem Cells in the Experimental Model of the Orthotopic Glioblastoma in Rats. Biomedicines 2021, 9, 1592. [Google Scholar] [CrossRef]
- Do, A.D.; Kurniawati, I.; Hsieh, C.-L.; Wong, T.-T.; Lin, Y.-L.; Sung, S.-Y. Application of Mesenchymal Stem Cells in Targeted Delivery to the Brain: Potential and Challenges of the Extracellular Vesicle-Based Approach for Brain Tumor Treatment. Int. J. Mol. Sci. 2021, 22, 11187. [Google Scholar] [CrossRef]
- Xie, J.; Wang, B.; Wang, L.; Dong, F.; Bai, G.; Liu, Y. Intracerebral and Intravenous Transplantation Represents a Favorable Approach for Application of Human Umbilical Cord Mesenchymal Stromal Cells in Intracerebral Hemorrhage Rats. Med. Sci. Monit. 2016, 22, 3552–3561. [Google Scholar] [CrossRef] [PubMed]
- Benmelouka, A.Y.; Munir, M.; Sayed, A.; Attia, M.S.; Ali, M.M.; Negida, A.; Alghamdi, B.S.; Kamal, M.A.; Barreto, G.E.; Ashraf, G.M.; et al. Neural Stem Cell-Based Therapies and Glioblastoma Management: Current Evidence and Clinical Challenges. Int. J. Mol. Sci. 2021, 22, 2258. [Google Scholar] [CrossRef] [PubMed]
- Calinescu, A.-A.; Kauss, M.C.; Sultan, Z.; Al-Holou, W.N.; O’Shea, S.K. Stem Cells for the Treatment of Glioblastoma: A 20-Year Perspective. CNS Oncol. 2021, 10, CNS73. [Google Scholar] [CrossRef]
- Nakamizo, A.; Marini, F.; Amano, T.; Khan, A.; Studeny, M.; Gumin, J.; Chen, J.; Hentschel, S.; Vecil, G.; Dembinski, J.; et al. Human Bone Marrow–Derived Mesenchymal Stem Cells in the Treatment of Gliomas. Cancer Res. 2005, 65, 3307–3318. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hao, S.; Wang, B. Mesenchymal Stem Cells Transplantation in Intracerebral Hemorrhage: Application and Challenges. Front. Cell Neurosci. 2021, 15, 653367. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fan, X.; Mazhar, M.; Yang, S.; Xu, H.; Dechsupa, N.; Wang, L. Mesenchymal Stem Cell Application and Its Therapeutic Mechanisms in Intracerebral Hemorrhage. Front. Cell. Neurosci. 2022, 16, 898497. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; He, K.-J.; Zhang, J.-B.; Ma, Q.-H.; Wang, F.; Liu, C.-F. Advances in Intranasal Application of Stem Cells in the Treatment of Central Nervous System Diseases. Stem Cell Res. Ther. 2021, 12, 210. [Google Scholar] [CrossRef]
- Balyasnikova, I.V.; Prasol, M.S.; Ferguson, S.D.; Han, Y.; Ahmed, A.U.; Gutova, M.; Tobias, A.L.; Mustafi, D.; Rincón, E.; Zhang, L.; et al. Intranasal Delivery of Mesenchymal Stem Cells Significantly Extends Survival of Irradiated Mice with Experimental Brain Tumors. Mol. Ther. 2014, 22, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, L.; Zhang, G.-X.; Ma, C. Intranasal Delivery of Stem Cells as Therapy for Central Nervous System Disease. Exp. Mol. Pathol. 2015, 98, 145–151. [Google Scholar] [CrossRef]
- Meng, T.; Jiang, R.; Wang, S.; Li, J.; Zhang, F.; Lee, J.-H.; Jiang, J.; Zhu, M. Stem Cell Membrane-Coated Au-Ag-PDA Nanoparticle-Guided Photothermal Acne Therapy. Colloids Surf. B Biointerfaces 2020, 192, 111145. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Liu, J.; Liu, F.; Du, F.; Li, M.; Chen, A.T.; Bao, Y.; Suh, H.W.; Avery, J.; et al. Targeted Drug Delivery to Stroke via Chemotactic Recruitment of Nanoparticles Coated with Membrane of Engineered Neural Stem Cells. Small 2019, 15, 1902011. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, X.; Li, Y.; Zhou, Y.; Zhang, T.; Zhi, P.; Gao, J. Engineering Stem Cell Derived Biomimetic Vesicles for Versatility and Effective Targeted Delivery. Adv. Funct. Mater. 2020, 30, 2006169. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Kim, B.J.; Arai, Y.; Han, I.; Moon, J.J.; Paulmurugan, R.; Park, H.; Lee, S.-H. Bioengineered Stem Cell Membrane Functionalized Nanocarriers for Therapeutic Targeting of Severe Hindlimb Ischemia. Biomaterials 2018, 185, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wu, W.; Tang, H.; Jia, X.; Tang, J.; Ruan, X.; Li, F.; Leong, D.T.; Luo, D.; Yang, D. Self-Assembly of Stem Cell Membrane-Camouflaged Nanocomplex for microRNA-Mediated Repair of Myocardial Infarction Injury. Biomaterials 2020, 257, 120256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Jiang, Q.; Ding, X.; Li, Y.; Chen, C.; Yang, W.; Chen, S. Highly Biosafe Biomimetic Stem Cell Membrane-Disguised Nanovehicles for Cartilage Regeneration. J. Mater. Chem. B 2020, 8, 8884–8893. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, T.; Huang, T.; Gao, J. Current Advances and Challenges of Mesenchymal Stem Cells-Based Drug Delivery System and Their Improvements. Int. J. Pharm. 2021, 600, 120477. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Xavier, J.; Kumar, N.; Ahmad, M.Z.; Ranjan, O.P. Exosomes as Natural Nanocarrier-Based Drug Delivery System: Recent Insights and Future Perspectives. 3 Biotech 2023, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome Delivered Anticancer Drugs across the Blood-Brain Barrier for Brain Cancer Therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, X.; Liu, J.; Fan, D.; Zhou, Q.; Chen, C.; Wang, W.; Wang, G.; Wang, H.; Yuan, W.; et al. Effects of Exosomes on Pre-Metastatic Niche Formation in Tumors. Mol. Cancer 2019, 18, 39. [Google Scholar] [CrossRef]
- Mirzaaghasi, A.; Han, Y.; Ahn, S.-H.; Choi, C.; Park, J.-H. Biodistribution and Pharmacokinectics of Liposomes and Exosomes in a Mouse Model of Sepsis. Pharmaceutics 2021, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, X.; He, S.; Miao, Y.; Wu, N.; Li, J.; Gan, Y. Sphk2 RNAi Nanoparticles Suppress Tumor Growth via Downregulating Cancer Cell Derived Exosomal microRNA. J. Control. Release 2018, 286, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface Modification of Gold Nanoparticles with Neuron-Targeted Exosome for Enhanced Blood–Brain Barrier Penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome Engineering: Current Progress in Cargo Loading and Targeted Delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Pei, J.; Alugoju, P.; Anthikapalli, N.V.A.; Jayaraman, S.; Veeraraghavan, V.P.; Gopathy, S.; Roy, J.R.; Janaki, C.S.; Thalamati, D.; et al. New Strategies of Neurodegenerative Disease Treatment with Extracellular Vesicles (EVs) Derived from Mesenchymal Stem Cells (MSCs). Theranostics 2023, 13, 4138–4165. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Baldari, S.; Toietta, G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for in Vivo Tracking and Biodistribution Analysis. Stem Cells Int. 2016, 2016, 5029619. [Google Scholar] [CrossRef]
- Schneider, D.J.; Speth, J.M.; Penke, L.R.; Wettlaufer, S.H.; Swanson, J.A.; Peters-Golden, M. Mechanisms and Modulation of Microvesicle Uptake in a Model of Alveolar Cell Communication. J. Biol. Chem. 2017, 292, 20897–20910. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mäger, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular Vesicle in Vivo Biodistribution Is Determined by Cell Source, Route of Administration and Targeting. J. Extracell. Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S. Potential Role of Growth Factors Controlled Release in Achieving Enhanced Neuronal Trans-Differentiation from Mesenchymal Stem Cells for Neural Tissue Repair and Regeneration. Mol. Neurobiol. 2022, 59, 983–1001. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Cruz, C.M.; Shearer, J.J.; Figueiredo Neto, M.; Figueiredo, M.L. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells Int. 2017, 2017, 4015039. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.H.A.; Cruz, F.F.; Morales, M.M.; Weiss, D.J.; Rocco, P.R.M. Magnetic Targeting as a Strategy to Enhance Therapeutic Effects of Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2017, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Du, X.; He, Z.; Yan, Z.; Han, W. Nanoparticles for Stem Cell Tracking and the Potential Treatment of Cardiovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 662406. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, K.; Kumar, V.; Kandasamy, J.; RoyChoudhury, S. Regenerative Nanomedicine: Current Perspectives and Future Directions. Int. J. Nanomed. 2014, 9, 4153–4167. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, W.; Fang, Z.; Kienzle, A.; Feng, Q. Influence of Silver Nanoparticles on Osteogenic Differentiation of Human Mesenchymal Stem Cells. J. Biomed. Nanotechnol. 2014, 10, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver Nanoparticles Promote Osteogenic Differentiation of Human Urine-Derived Stem Cells at Noncytotoxic Concentrations. Int. J. Nanomed. 2014, 9, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- English, K.; Mahon, B.P. Allogeneic Mesenchymal Stem Cells: Agents of Immune Modulation. J. Cell. Biochem. 2011, 112, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Concise Review: Two Negative Feedback Loops Place Mesenchymal Stem/Stromal Cells at the Center of Early Regulators of Inflammation. Stem Cells 2013, 31, 2042–2046. [Google Scholar] [CrossRef]
- Caplan, A.I.; Sorrell, J.M. The MSC Curtain That Stops the Immune System. Immunol. Lett. 2015, 168, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of Culture-Expanded Mesenchymal Stem Cells in the Microvasculature. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Cobbs, C.; Jennings, M.; Bartholomew, A.; Hoffman, R. Mesenchymal Stem Cells Distribute to a Wide Range of Tissues Following Systemic Infusion into Nonhuman Primates. Blood 2003, 101, 2999–3001. [Google Scholar] [CrossRef] [PubMed]
- Galleu, A.; Riffo-Vasquez, Y.; Trento, C.; Lomas, C.; Dolcetti, L.; Cheung, T.S.; von Bonin, M.; Barbieri, L.; Halai, K.; Ward, S.; et al. Apoptosis in Mesenchymal Stromal Cells Induces in Vivo Recipient-Mediated Immunomodulation. Sci. Transl. Med. 2017, 9, eaam7828. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Son, J.G.; Song, N.W.; Kim, Y.-I.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Anti-Angiogenic Effect of Bare Titanium Dioxide Nanoparticles on Pathologic Neovascularization without Unbearable Toxicity. Nanomed. Nanotechnol. Biol. Med. 2014, 10, e1109–e1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, R.; Wu, H.; Jiang, X.; Gao, J. Mesenchymal Stem Cells: A Living Carrier for Active Tumor-Targeted Delivery. Adv. Drug Deliv. Rev. 2022, 185, 114300. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Qiu, W.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. The Achievements and Challenges of Mesenchymal Stem Cell-Based Therapy in Inflammatory Bowel Disease and Its Associated Colorectal Cancer. Stem Cells Int. 2020, 2020, 7819824. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Zouani, O.F. A Review of the Effects of the Cell Environment Physicochemical Nanoarchitecture on Stem Cell Commitment. Biomaterials 2014, 35, 5278–5293. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Liu, X.; He, W.; Huang, Q.; Feng, Q. Nanoparticles and Their Effects on Differentiation of Mesenchymal Stem Cells. Biomater. Transl. 2020, 1, 58–68. [Google Scholar] [CrossRef]
- Ko, E.; Cho, S.-W. Biomimetic Polymer Scaffolds to Promote Stem Cell-Mediated Osteogenesis. Int. J. Stem Cells 2013, 6, 87–91. [Google Scholar] [CrossRef][Green Version]
- Menale, C.; Campodoni, E.; Palagano, E.; Mantero, S.; Erreni, M.; Inforzato, A.; Fontana, E.; Schena, F.; van’t Hof, R.; Sandri, M.; et al. Mesenchymal Stromal Cell-Seeded Biomimetic Scaffolds as a Factory of Soluble RANKL in Rankl-Deficient Osteopetrosis. Stem Cells Transl. Med. 2018, 8, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the Promising Potential of Induced Pluripotent Stem Cells in Cancer Research and Therapy. Mol. Cancer 2023, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Park, E.; Yoo, H.S.; Park, J.; Jung, Y.M.; Park, J.H. Recent Advances in Monitoring Stem Cell Status and Differentiation Using Nano-Biosensing Technologies. Nanomaterials 2022, 12, 2934. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Compañ, A.; Guillén, M.I. Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases. Cells 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Subramaniyan, V.; Dahiya, R.; Dahiya, S.; Sudhakar, K.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Sekar, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology 2021, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.Á.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a Protein Corona on the Surface of Extracellular Vesicles in Blood Plasma. J. Extracell. Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Tekade, M.; Maheshwari, R.; Chourasiya, Y.; Hutcheon, G.A.; Tekade, R.K. Chapter 2—Engineered Mesenchymal Stem Cells as Nanocarriers for Cancer Therapy and Diagnosis. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; Advances in Pharmaceutical Product Development and Research; Academic Press: Cambridge, MA, USA, 2019; pp. 19–56. ISBN 978-0-12-814427-5. [Google Scholar]
- Mafi, P.; Hindocha, S.; Mafi, R.; Griffin, M.; Khan, W.S. Adult Mesenchymal Stem Cells and Cell Surface Characterization—A Systematic Review of the Literature. Open Orthop. J. 2011, 5, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.-Q.; Chen, K.-G.; Yu, X.-Y.; Zhao, G.; Shen, S.; Cao, Z.-T.; Luo, Y.-L.; Wang, Y.-C.; Wang, J. Promoting Tumor Penetration of Nanoparticles for Cancer Stem Cell Therapy by TGF-β Signaling Pathway Inhibition. Biomaterials 2016, 82, 48–59. [Google Scholar] [CrossRef]
- Salim, S.A.; Salaheldin, T.A.; Elmazar, M.M.; Abdel-Aziz, A.F.; Kamoun, E.A. Smart Biomaterials for Enhancing Cancer Therapy by Overcoming Tumor Hypoxia: A Review. RSC Adv. 2022, 12, 33835–33851. [Google Scholar] [CrossRef]
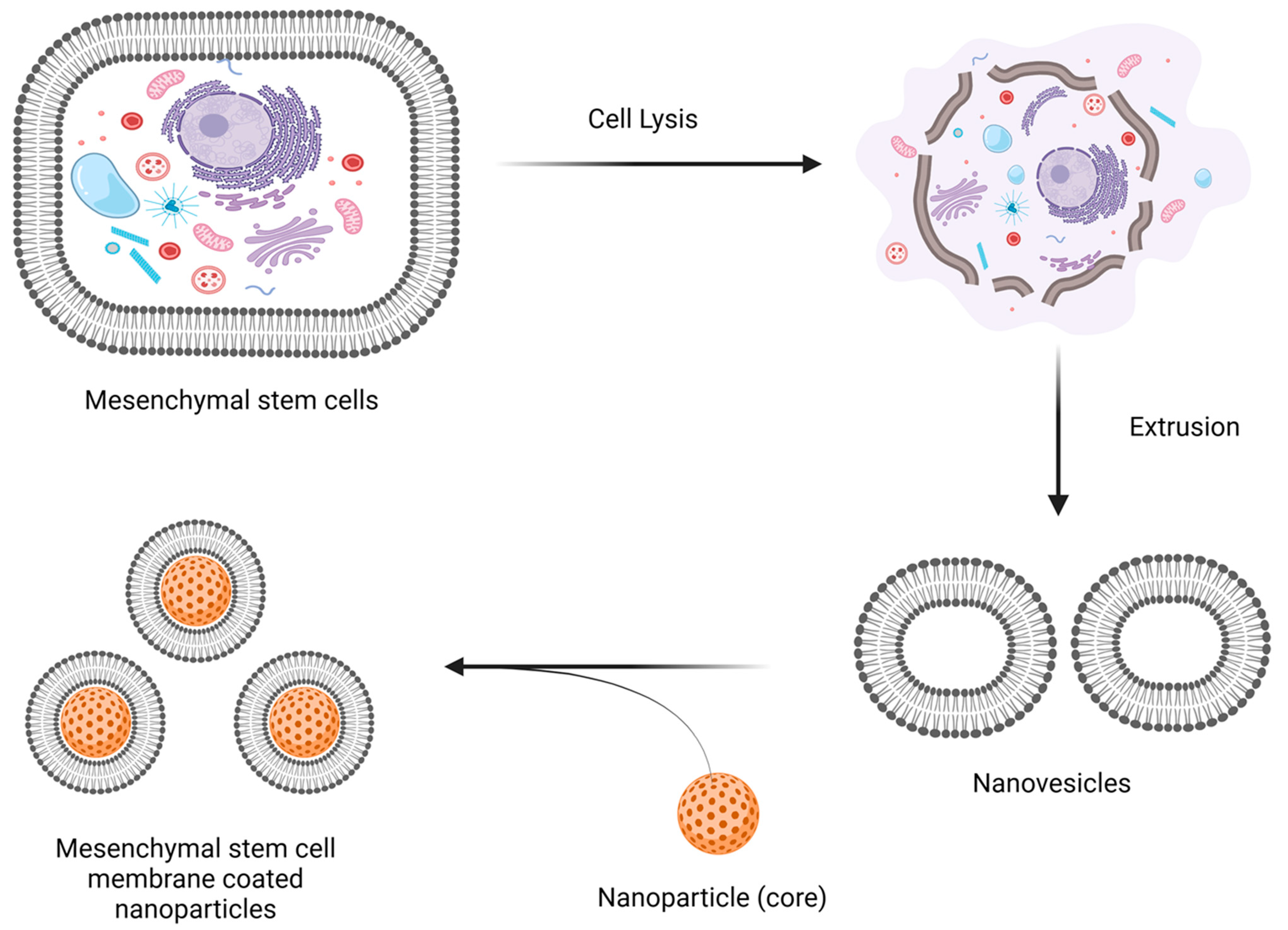
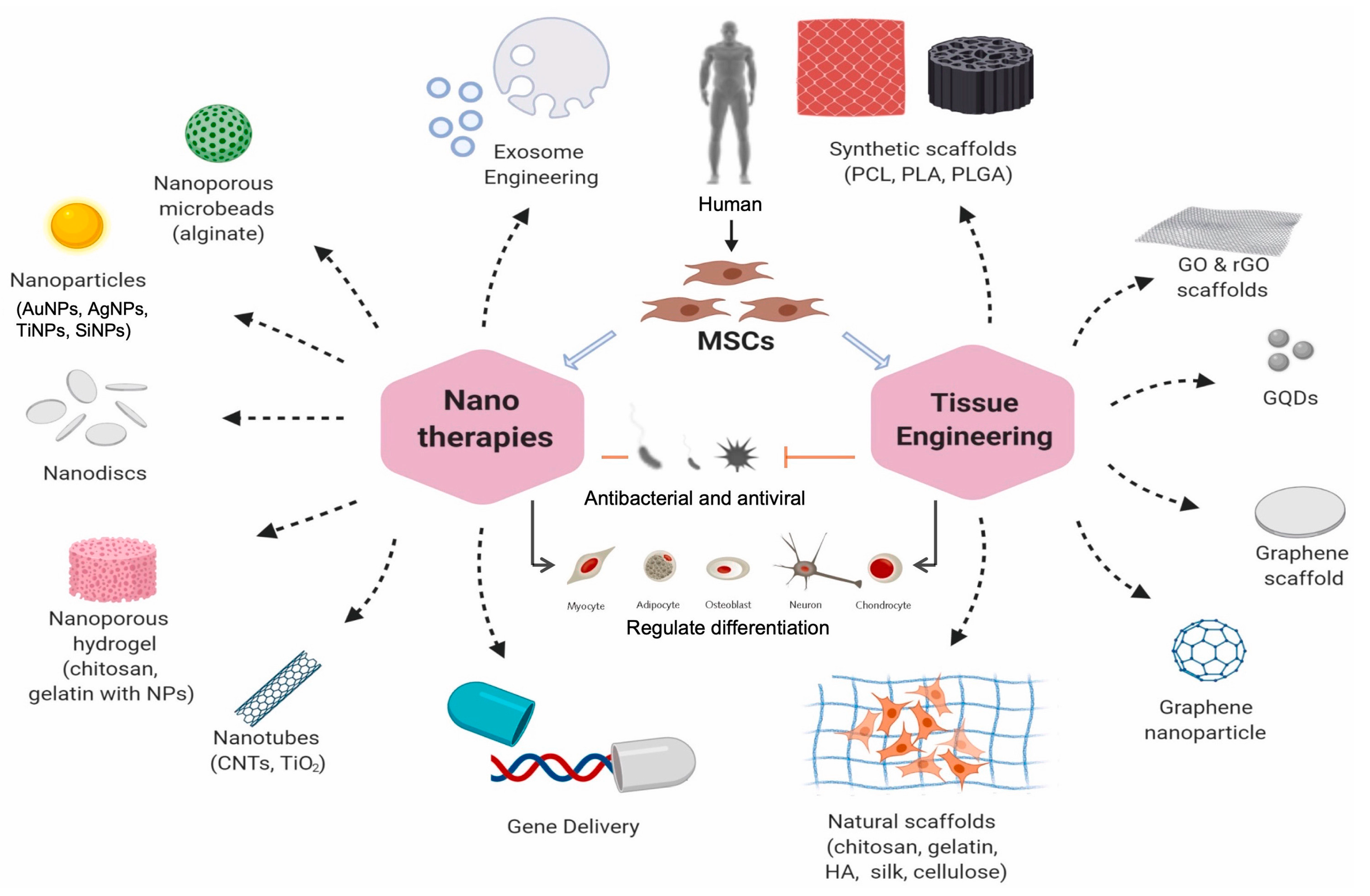
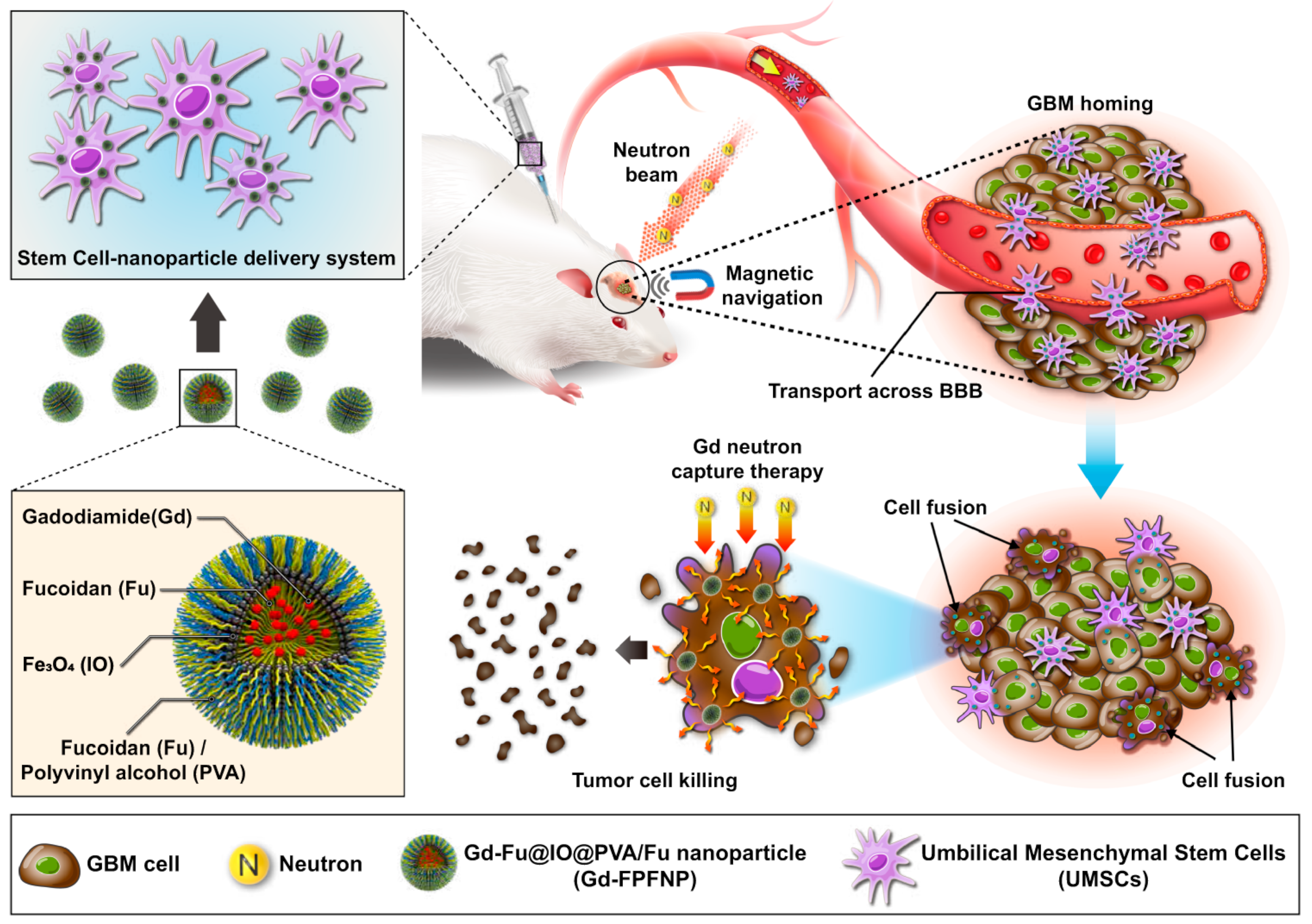
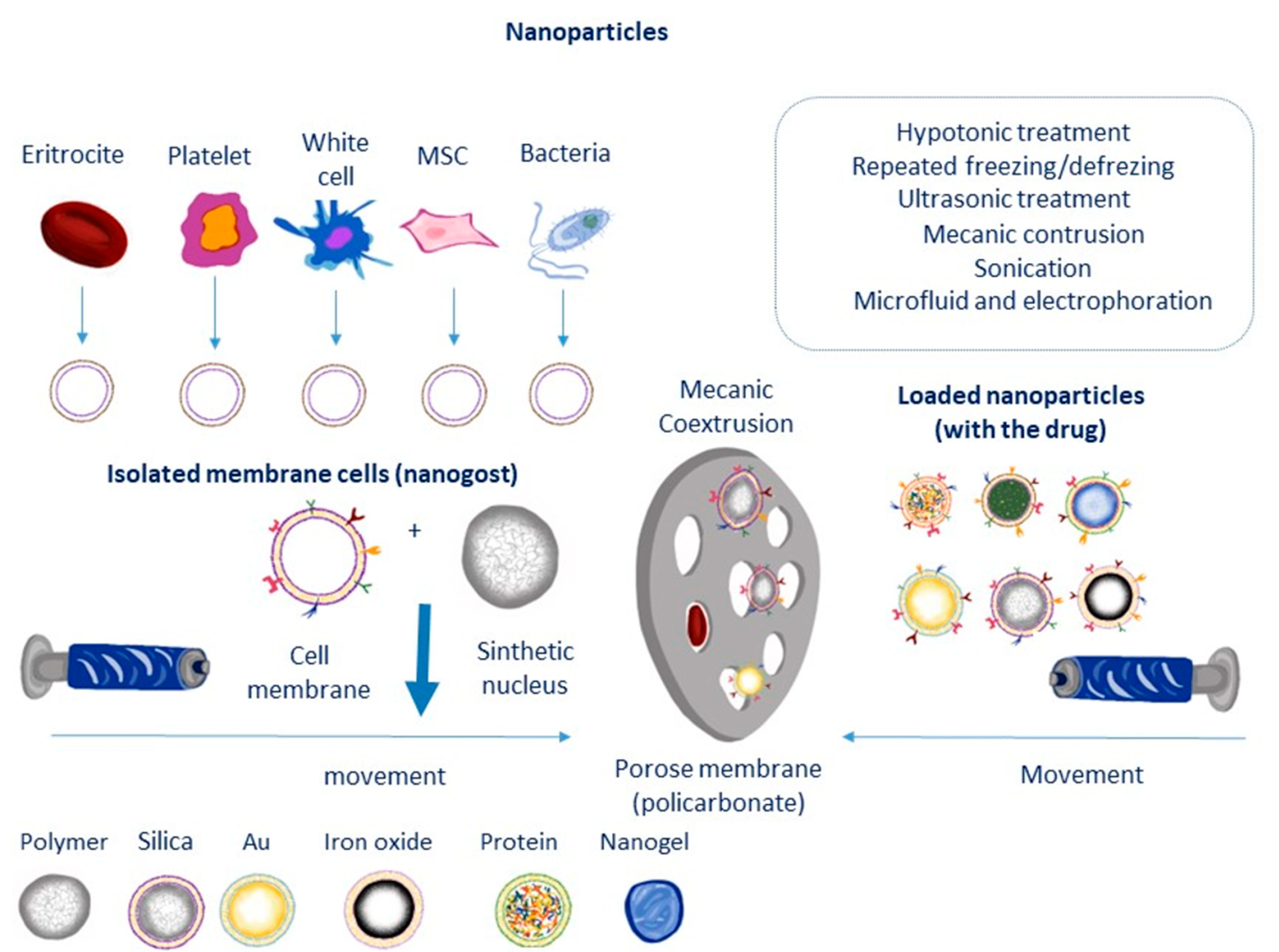
| Type of MSC | Type of Nanoparticle | Example of Drug | Method for Preparation | Disease/Disorder/Application | Reference |
|---|---|---|---|---|---|
| Lipid Nanoparticles | Lipid nanocapsules | Ferrociphenol | Phase inversion temperature method | Glioblastomas | [96] |
| Solid lipid Nanoparticle | Galantamine hydrobromide | Microemulsion method using hot homogenization | Alzheimer’s disease | [97] | |
| Lipid carrier nanoparticle | Ferrociphenol | Phase inversion temperature method | Brain tumor | [98] | |
| Nanostructured lipid carriers | Simvastatin | High shear homogenization | Diabetes | [107] | |
| Polymer Nanoparticle | PLGA nanoparticles | Doxorubicin | Double emulsion method | Tumor | [101] |
| Gelatin | Doxorubicin hydrochloride | Desolvation method | Human cervical cancer | [103] | |
| PLGA nanoparticles | Paclitaxel | Tumor | [102] | ||
| Inorganic Nanoparticle | Manganese Dioxide Nanoparticles | Paclitaxel | Coextrusion technique | Lung cancer | [105] |
| Silica nanorattle | Doxorubicin | Modified Stober reaction | Tumor | [106] |
| S. No. | Types of Stem Cells | Driven from Species | Types of Nanomaterials | Safety and Toxicity Analysis | Dose/Concentrations | Conclusions | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Mesenchymal stem cells | Human | Cholera toxin quantum dots | Assessment of the cell viabilities, morphological evaluation, proliferative and differentiationcapacities | 250 pm–16 nM | No deleterious outcomes were observed | [156] |
| 2 | Mesenchymal stem cells | Human | RGD peptide-conjugatedquantum dots | Assessment of proliferative and differentiation capacities | 20–50 nM | No deleterious outcomes were observed | [157] |
| 3 | Mesenchymal stem cells | Human | Cadmium selenium zinc sulfide quantum dots | Assessment of the cellular viabilities, and immune-phenotypic profiling | 0.75–3 μg/mL | No deleterious outcomes were observed | [158] |
| 4 | Mesenchymal stem cells | Human | Cadmium selenium zinc sulfide quantum dots | Evaluation of cellular viabilities, proliferative anddifferentiation capacities | 1.625 μg | Impaired chondrogenic differentiation was seen | [159] |
| 5 | Mesenchymal stem cells | Human | Cadmium selenium zinc sulfide quantum dots | Assessment of cellular viabilities, proliferative as well asdifferentiation capacities | 1.625 μg | Impaired chondrogenic differentiation was seen | [160] |
| 6 | Mesenchymal stem cells | Rat | Cadmium selenium zinc sulfide quantum dots | Cellular viabilities assessment, and evaluation of the differentiationcapacities | 16 μg/mL | No deleterious outcomes were observed | [161] |
| 7 | Mesenchymal stem cells | Human | Carbon quantum dots | Cellular viabilities evaluation, and differentiation capacities and capacities to for the single cell spheres | 50 μg/mL | No deleterious outcomes were observed | [162] |
| 8 | Adipocyte derived stem cells | Human | Graphene quantum dots | Cellular viabilities, and other metabolicactivities | 0.5, 1.0, and2.0 mg/mL | No deleterious outcomes were observed | [163] |
| 9 | Mesenchymal stem cells | Rat | Graphene quantum dots | Cellular viabilities, proliferative anddifferentiation capacities | 50 μg/mL | The osteogenic and adipogenicdifferentiation was enhanced | [164] |
| 10 | Mesenchymal stem cells | Human | Mesoporous silicananoparticles | Cellular adhesion capacities, immune-phenotypicprofiling | 50 μg/mL | The adhesion capacity was enhanced along with the increased expression ofConnexin-43 | [165] |
| 11 | Mesenchymal stem cells | Human | Spherical core-shellfluorescentsilica nanoparticles | Assessment of the cellular viabilities, and adipogenic differentiationcapacities | 100 μg/mL | Impaired adipogenic differentiationwas observed | [166] |
| 12 | Mesenchymal stem cells | Human | Core-shell fluorescentsilica nanoparticles | Evaluation of the cellular viabilities, osteogenic differentiationcapacities | 10 μg/mL | Enhanced osteogenic differentiation | [167] |
| 13 | Mesenchymal stem cells | Human | Mesoporous silicananoparticles | Assessment of the cellular viabilities, as well as the migrationcapacities | 100 and 200 μg/mL | No deleterious outcomes were observed | [168] |
| 14 | Mesenchymal stem cells | Human | Dye-loaded amorphoussilica nanoparticles | Evaluation of the cellular viabilities, proliferative as well asdifferentiation capacities | 50 μg/mL | No deleterious outcomes were observed | [150] |
| 15 | Mesenchymal stem cells | Human | Mesoporous silicananoparticles | Cellular viabilities evaluation, proliferative as well asdifferentiation capacities | 20 μg/mL | No deleterious outcomes were observed | [169] |
| 16 | Mesenchymal stem cells | Human | Mesoporous silicananoparticles | Measurements of the cellular viabilities, followed by the differentiationcapacities | 3–10 μg/mL | No deleterious outcomes were observed | [170] |
| 17 | Mesenchymal stem cells | Human | Mesoporous silicananoparticles | Evaluation of the cell viabilities, morphologies, Immuno-phenotypic profiles, proliferative as well as differentiationcapacities | 20 μg/mL | No deleterious outcomes were observed | [171] |
| 18 | Mesenchymal stem cells | Human | Mesoporous silicananoparticles | Cellular viabilities assessment, immuno-phenotypicprofiling, proliferative as well asdifferentiation capacities | 20 μg/mL | No deleterious outcomes were observed | [172] |
| 19 | Mesenchymal stem cells | Rat | Superparamagnetic iron-oxide nanoparticles | Cell viabilities assessment, and then differentiationcapacities | 1, 5 μg/mL | Increment chondrogenic differentiationwas observed | [173] |
| 20 | Mesenchymal stem cells | Rat | Superparamagnetic iron-oxide nanoparticles complexed amylose cationized with spermin | Evaluation of the cell viabilities, rate of apoptosis, levels of the intracellular reactive oxygen species, measurements of the mitochondrialtransmembranepotentials, and differentiationcapacities | 30 μg/mL | No deleterious outcomes were observed | [174] |
| 21 | Adipocyte-derived stem cells | Rat | Polyethylene glycol/poly vinyl pyrrolidone—Superparamagnetic iron-oxide nanoparticlesand Polyethylene glycol/polyethylene imine Superparamagnetic iron-oxide nanoparticles | Assessment of the cellular viabilities, followed by the assessment of the morphologies | 12, 25, and 50 μg/mL | No deleterious outcomes were observed | [175] |
| 22 | Adipocyte derived stem cells | Rat | Superparamagnetic iron-oxide nanoparticles | Assessment of cellular viabilities, cellular morphologies, proliferative capacities | 50 μg/mL | No deleterious outcomes were observed | [176] |
| 23 | Mesenchymal stem cells | Human | Superparamagnetic iron-oxide nanoparticles | Evaluations of cellular viabilities, as well as differentiationcapacities | 25 μg/mL | No deleterious outcomes were observed | [177] |
| 24 | Mesenchymal stem cells | Rat | 1-hydroxyethylidene-1.1-bisphosphonic acid coated Superparamagnetic iron-oxide nanoparticles | Assessment of cellular viabilities, cellular morphologies, differentiationcapacities | 25 μg/mL | No deleterious outcomes were observed | [178] |
| 25 | Mesenchymal stem cells | Human | Superparamagnetic iron-oxide nanoparticles | Cellular viability evaluations, and assessment of cell morphologies, and differentiationcapacities | 1, 10, and 100 μg Fe/mL | No deleterious outcomes were observed | [179] |
| 26 | Mesenchymal stem cells | Human | Superparamagnetic iron-oxide nanoparticles | Assessment of cellular viabilities, cellular morphologies, and differentiationcapacities | 13–16 pg Fe/cell | Impaired chondrogenic differentiation was seen | [180] |
| 27 | Adipocyte-derived stem cells | Mouse | Penetrating peptide-bioconjugate-persistentluminescent nanoparticles | Cellular viabilities assessments, and evaluations of differentiation capacity | 50 μg/mL | No deleterious outcomes were observed | [181] |
| 28 | Mesenchymal stem cells | Human | Purified polymernanoparticles | Assessments of cell viability, and proliferativecapacities | 0, 5, 10, 20, 40 μg/mL | No deleterious outcomes were observed | [182] |
| 29 | Mesenchymal stem cells | Human | R8-Polymer nanoparticles | Cellular viabilities measurements, proliferative as well asdifferentiation capacities, tumorigenic index assessments, and immunophenotypicprofiling | 10 μg/mL | No deleterious outcomes were observed | [183] |
| 30 | Mesenchymal stem cells | Porcine | Gadonanotubes; polymer nanoparticles | Cell viability measurements | 1014 Gd3+ ions/cell | No deleterious outcomes were observed | [184] |
| 31 | hESC-CM | Human | Polymer nanoparticles | Cell viabilities assessments, and immunophenotypicprofiling | 0, 2, 4, 8 × 10−9 M | No deleterious outcomes were observed | [185] |
| 32 | Mesenchymal stem cells | Human | Gold nanoparticles | Measurements of cellular viabilities, proliferative index anddifferentiation capacities | 1012 NPs/mL | No deleterious outcomes were observed | [186] |
| 33 | Mesenchymal stem cells | Human | Silica-coated goldnanoparticles | Measurements of cellular viabilities, proliferative indices anddifferentiation capacities | 0.0–0.14 nM | No deleterious outcomes were observed | [187] |
| 34 | Mesenchymal stem cells | Rat | Silica-coated goldnanoparticles | Cellular viability assessment, and proliferativecapacities | 1012 NPs/mL | No deleterious outcomes were observed | [188] |
| 35 | Mesenchymal stem cells | Mouse | PEGylated goldnanoparticles | Assessments of cellular viabilities, migration capacities, proliferative indices, differentiation capabilities andcapacities for the colonization of the scaffolds | 100 μg/mL | Increased migration capacities, increased differentiationof osteoclasts, and increased capacities for thescaffolds colonization | [189] |
| 36 | Mesenchymal stem cells | Human | 2,2,6,6-tetramethylpiperidine-N-oxyl ConjugatedGold nanoparticles | Measurements of cell viabilities, proliferative indices anddifferentiation capacities | 0.05–1.00 mM | Increment in chondrogenic differentiation, while decreased adipogenic differentiation | [190] |
| 37 | Adipocyte-derived stem cells | Human | N-acetyl cysteine modifiedgold nanoparticles | Assessments of cellular viabilities, as well as ALP activities | 20 μM | Increased cell viabilities | [191] |
| S. No | Inner Nanoparticle Core | Outer Coating Membrane | Active Pharmaceutical Compound | Method of Preparation | Animal Model | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Polydopamine-coated gold silver nanoparticles | Mesenchymal stem cell membrane | - |
| Male golden hamsters injected with Propionibacterium acnes | Enhanced photothermal conversion efficiency, increased efficiency of cellular uptake, and increased anti-proliferative effects | [251] |
| 2 | Poly (lactic-co-glycolic acid) nanoparticles | Neural stem cell membranes overexpressing the CXC receptors | Glyburide | Freeze–thaw cycles and sonication and co-extrusion | Middle cerebral artery occlusion mice | For enhancing the therapeutic effect of glyburide | [252] |
| 3 | Liposomes | Mesenchymal stem cell membrane | Curcumin | Freeze–thaw cycles and sonication | Middle cerebral artery occlusion mice | For increasing the survival rate and prevention of the weight loss tendency | [253] |
| 4 | Poly (lactic-co-glycolic acid) nanoparticles | Adipose-derived stem cell membranes overexpressing the CXCR4 receptors | Vascular endothelial growth factor | Hypotonic lysis and sonication | Female C57BL/6 mice with hindlimb ischemia | Decreasing the uptake by macrophages, and to enhance the targeting of ischemic tissues | [254] |
| 5 | Mesoporous silica nanoparticles | Mesenchymal stem cell membrane | microRNA21 | Sonication | Mice with myocardial infarction | Increasing the targeting of infarcted myocardium, and inhibition of the apoptosis of the cardiomyocytes | [255] |
| 6 | Iron oxide nanoparticles | Mesenchymal stem cell membrane | Kartogenin | Hypotonic lysis and sonication | Rats with osteochondral autograft transplantation | To increase the cartilage regeneration activity, and for enhancing the biosafety profiles | [256] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, M.S.; Ahmad, A.; Pathan, R.R.; Mishra, R.K. Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics. J. Xenobiot. 2024, 14, 827-872. https://doi.org/10.3390/jox14030047
Baig MS, Ahmad A, Pathan RR, Mishra RK. Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics. Journal of Xenobiotics. 2024; 14(3):827-872. https://doi.org/10.3390/jox14030047
Chicago/Turabian StyleBaig, Mirza Salman, Anas Ahmad, Rijawan Rajjak Pathan, and Rakesh Kumar Mishra. 2024. "Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics" Journal of Xenobiotics 14, no. 3: 827-872. https://doi.org/10.3390/jox14030047
APA StyleBaig, M. S., Ahmad, A., Pathan, R. R., & Mishra, R. K. (2024). Precision Nanomedicine with Bio-Inspired Nanosystems: Recent Trends and Challenges in Mesenchymal Stem Cells Membrane-Coated Bioengineered Nanocarriers in Targeted Nanotherapeutics. Journal of Xenobiotics, 14(3), 827-872. https://doi.org/10.3390/jox14030047







